Abstract
Graphene-based materials have gained significant attention due to their unique mechanical, chemical, thermal, and optical properties. Among these, Graphene oxide (GO) is one of the promising materials extensively studied. Unlike other graphene derivatives, GO is chemically modified with diverse oxygenated functional groups, rendering it more hydrophilic. It serves as a precursor for graphene synthesis. Notably, recent researchers have focused on synthesising GO using alternative low-cost carbon-rich materials such as coconut shells, sugarcane bagasse, tea, pine leaves and scrap tyres instead of graphite. These non-conventional carbon sources decrease the demand for costly, non-renewable graphite, increase reliability, and offer an eco-friendly approach to waste management. This comprehensive review aims to explore accessible methods for synthesising graphene oxide and highlight various alternative feedstocks utilising agricultural, industrial, and plastic waste as precursors. Furthermore, a comparative assessment of various production methods and their performance in different applications is outlined to provide insights for the commercialisation of GO in future applications.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Novel materials are key drivers of scientific and technological advancements, leading researchers to explore materials with exceptional properties. Graphene and related materials have garnered extensive attention and emerged as highly researched materials in the global. Graphene is often called a “material of the future”, because of its exceptional properties (Rümmeli et al. 2011). Since it was first separated from graphite through a simple mechanical peeling method in 2004 by Novoselov and Geim (Novoselov et al. 2004), graphene has significantly impacted various industries, including transparent conductive films (Zheng et al. 2014), flexible electronics (Stanford et al. 2020), energy storage (Wang et al. 2020), optoelectronics (Bonaccorso et al. 2010), robotics (Yang et al. 2019), biosensing (Jiang et al. 2020), water purification (Safarpour and Khataee 2019), textile industry (Karim et al. 2017) and more. Graphene comprises a two-dimensional hexagonal skeleton made of a single layer of sp2 hybridised carbon atoms with exceptional electrical, mechanical, thermal, and physicochemical properties (Li et al. 2017). Despite its numerous unique characteristics, graphene has several drawbacks, including low electrochemical activity, easy agglomeration and complex and expensive processing, which significantly limit its application in certain fields including medical and biological (Geim and Novoselov 2007). To overcome these limitations, researchers have employed functional modifications to transform graphene into graphene oxide, expanding its potential applications.
Graphene oxide (GO) is a layered carbon structure with oxygen-containing functional groups distributed across its basal planes and edges. This configuration gives a hybrid system comprising a mixture of sp3 and sp2 hybridised carbon atoms (Shin et al. 2017). GO can exist as a mono- or multi- layer stacked structure, with a monolayer referred to as graphene oxide, two layers known as two-layered GO, and GO possessing more than two layers but fewer than five layers referred to as a few-layered GO. Multilayered GO comprises five to ten layers, while material with eleven or more stacked layers is denoted as graphite oxide (Dreyer et al. 2010; Kumar et al. 2021). GO has outstanding properties, notably its hydrophilic nature, which allows it to disperse in numerous solvents, especially water (Klechikov et al. 2015). Additionally, graphene-based composites can be synthesised by modifying the oxygen-functionalized groups of GO (Zheng et al. 2022). GO has found applications in diverse fields such as desalination (Ali et al. 2016), drug delivery (Sun et al. 2008), oil–water separation (Alammar et al. 2020), immobilization catalysis (Li et al. 2013), solar cells (Jiao et al. 2014), energy storage (Ambrosi and Pumera 2016), healthcare (Hai et al. 2023) and construction materials (Zaid et al. 2022). The production of graphene has seen development in numerous ways recently. The methods reported for graphene synthesis can be categorised into top-down and bottom-up approaches (Tour 2014). In the top-down approach, graphite is separated to yield graphene, whereas the bottom-up approach involves synthesising graphene from a carbon precursor (Bhuyan et al. 2016; Mbayachi et al. 2021). The predominant method for producing GO is the top-down approach, which involves obtaining GO by exfoliating graphite oxide (Anwar et al. 2022). To promote environmental sustainability, researchers have explored novel production methods for synthesising GO, offering economic and environmental benefits (Grace and Prabha Littis Malar 2020; Amir Faiz et al. 2020). By using waste-based carbon and transforming it into graphene and GO, numerous applications can be realised (Sujiono et al. 2020; Amir Faiz et al. 2020; Sarhan et al. 2022).
The latest review authored by Torres et al. (2021) concentrated on examining the production, performance, and diverse applications of graphene-derived nanomaterials sources from waste biomass. However, it is worth noting that the study did not sufficiently concentrate on the production of GO from waste sources. The synthesis of GO from waste sources remains a persistent challenge, necessitating further research and improvement. These challenges include optimising synthesis parameters, managing variable carbon content, addressing contaminations, assessing environmental impact, and considering factors such as cost, scalability, and reproducibility (Sujiono et al. 2020; Wachid et al. 2014). Therefore, it is crucial to focus on the development of novel methods that enable cost-effective, environmentally friendly, and scalable production of GO, while also exploring its diverse applications. This comprehensive review article aims to provide an extensive overview of the existing methods employed in synthesising GO from waste materials and investigates its wide-ranging applications. By addressing these challenges and exploring new avenues, this review aims to contribute to advancing GO synthesis and its utilisation in sustainable applications.
Structure and Properties of GO
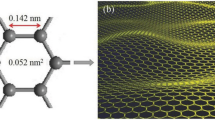
GO represents the oxidised counterpart of graphene, a concept dating back to the pioneering work of the English chemist Brodie in 1859, who first documented graphite oxidation. The term “graphene” was officially coined about thirty years ago to describe the single atom-thin carbon layer of graphite (Boehm et al. 1986). Graphene is a material composed of carbon atoms bonded to each other in a repeating pattern of hexagons, as depicted in Fig. 1a. Graphene is recognised as a fundamental building block for all two-dimensional material made of sp2 graphitic structures, which includes graphite oxide (Novoselov et al. 2004). Compared to graphene, graphite oxide possesses a more complicated structure due to numerous oxygenated functional groups. Studying the structure of graphite oxide is fundamental to comprehending GO. Despite the production of graphite oxide in the mid-1800s, ongoing debate persists regarding its composition and structure due to its non-stoichiometric composition, complexity, and sensitivity to preparation methods (Dimiev et al. 2013; Sun 2019). GO comprises a monolayer of graphite with carboxyl, hydroxyl, and epoxy oxygen functionalities distributed across its basal plane and edges, leading to a combination of sp2 and sp3 hybridised carbon atoms, as depicted in Fig. 1b (Nováček et al. 2017).
The schematic illustrations of (a) the hexagonal arrangement of sp2 hybridised carbon atoms in graphene, and (b) GO, where oxygen and hydrogen atoms are incorporated into the hexagonal network (Gutiérrez-Cruz et al. 2022)
Graphene exhibits remarkable mechanical, electrical, thermal and optical properties attributed to its distinct structural and morphological characteristics (Li et al. 2017). Despite these properties, the practical applications of purely single-layer graphene are hindered by its hydrophobic nature and the challenges associated with mass scale production (Chung et al. 2013; Padmajan Sasikala et al. 2018). However, the precursor GO has advanced much in large-scale production by exfoliating bulk graphite oxide. Consequently, the proportion of defects in the graphene structure significantly impacts its physical and mechanical properties (Ibrahim et al. 2021). Conversely, GO exhibits higher chemical activity than pristine graphene due to the abundance of oxygen-containing functional groups and structural defects (Dreyer et al. 2010). GO can undergo reduction processes to yield graphene, which can be achieved through different methods such as treatment with reducing agents (Park et al. 2011), thermal annealing (Yang et al. 2009), or electrochemical techniques (Chen et al. 2012). Furthermore, GO has exhibited strong oxidising properties that can be used for a diverse set of reactions such as oxidation of olefins, methyl benzenes, and various dehydrogenations (Jia et al. 2011). Another reactivity of GO is its ability for chemical functionalisation. The functionalisation involves introducing carboxylic acid, hydroxyl, and epoxy groups to GO through chemical reactions (Dreyer et al. 2010). In addition, GO can be functionalised by activating it with specially selected polymers or small molecules (Fang et al. 2009). This functionalisation has a notable effect on enhancing the dispersibility of GO in organic solvents, such as N,N-dimethylformamide (DMF), N-methyl-2-pyrrolidone (NMP), and dimethyl sulfoxide (DMSO) (Johnson et al. 2015). In contrary to graphene, GO exhibits hydrophilicity. This hydrophilicity facilitates the incorporation of GO into aqueous systems and improves its compatibility with other materials, enabling diverse applications in fields such as nanocomposite, drug delivery, and biosensing (Gui et al. 2014; Chung et al. 2013). These unique features of GO unlock a broad range of applications in different industries, offering enhanced performance and improved functionality in numerous technological and scientific endeavours.
Synthesis methods of Graphene Oxide
There are several conventional and modern methods to prepare graphite oxide. Table 1 provides a concise summary highlighting the key differences among these methods.
The initial endeavour to synthesise graphite oxide was undertaken by British chemist B. C. Brodie, who explored the chemical reactivity of flake graphite. The GO synthesis generally involves two main steps: oxidation by an oxidising agent and subsequent exfoliation of the sheets. In his experiment, Brodie introduced a mixture of KClO3 into a graphite slurry in fuming HNO3 acid and maintained it at a constant temperature of 60 °C for four days. This process led to the formation of Brodie’s graphene oxide (BR-GO). The composition of the resulting material was determined as C11H4O5 (C/O ratio 2.2). This product exhibited solubility in distilled H2O and tended to clump in more acidic environments. Staudenmaier (1898) modified Brodie’s procedure using conc. H2SO4 acid, fuming HNO3 acid, and the gradual addition of KClO3 during the reaction, leading to Staudenmaier’s graphene oxide (ST-GO). The increased acidity in this modified process resulted in a reduced reaction time, while maintaining similar properties to BR-GO. In 1937, Hofmann (Hofmann and König 1937) synthesised Hofmann’s graphene oxide (HO-GO) employing KClO3 and non-fuming HNO3 acid, resulting in a lower oxygen content with a C/O ratio 2.5. Their investigation revealed that the conc. HNO3 acid played a significantly impacted the extent of oxidation during the synthesis process, with lower concentrations leading to higher levels of graphene oxide oxidation.
The well-established “Hummers method”, pioneered by William S. Hummers Jr. and Richard E. Offeman in 1958, involved oxidising graphite to graphitic oxide through a process employing a mixture of H2SO4, NaNO3 and KMnO4 (Fig. 2). Notably, this method substantially decreased the reaction time from several days, as observed in previous methods to only a few hours, ranging from 8 and 12 h. The replacement of KClO3 with KMnO4 enhanced the safety of the reaction by eliminating the use of explosive chlorate compounds. At the same time, the inclusion of NaNO3 helped to eliminate the release of toxic fumes from HNO3. The initial step in the synthesis of graphitic oxide involved mixing 100 g of graphite powder with 50 g of NaNO3 in 2.3 L of H2SO4. To ensure safety, the mixture was then chilled to 0 °C in an ice bath. With continuous vigorous stirring, 300 g of KMnO4 was cautiously introduced to the mixture to avoid the temperature exceeding 20 °C. Following the removing of the ice bath, the temperature was maintained at 35 °C for 30 min. During this time, the mixture thickened gradually with a decreasing effervescence, forming a brownish-grey coloured paste with the release of minimal gas. After 30 min, 4.6 L of H2O was added gradually to the paste, resulting vigorous effervescence, and raising the temperature to 98 °C. The resulting brown solution was then kept at this temperature for an additional 15 min. Following the dilution of the suspension with 14 L of warm water, a 3% H2O2 treatment was added to remove any remaining MnO4- and MnO2, yielding a bright yellow solution. Subsequently, the warm mixture was filtered to prevent precipitation as a secondary reaction. The resulting GO residue was dispersed to around 0.5% solids after washing the yellowish-brown filter cake three times with warm water. Resinous anion and cation exchangers were used to remove residual salt impurities. The dry form of GO was achieved through centrifugation and subsequent dehydration at 40 °C using phosphorus pentoxide under vacuum conditions. The resulting product from the Hummers method, known as Hummers’ graphene oxide (HU-GO) displayed a comparable C/O ratio of 2.25 to that of BR-GO (2.2) (Hummers and Offeman 1958). Despite its promising results, the Hummers method suffers from time-consuming separation and purification, environmental concerns due to NOx generation, scalability issues and large amounts of acid consumption.
A schematic representation of Hummers method (Torres et al. 2021)
Over time and through further experimentation, the Hummers method underwent notable changes, leading to what is now known as the "Modified Hummers" (Zaaba et al. 2017). Various modifications have been proposed, including two-step, nitrate-free, co-oxidant, and room- and low-temperature methods (Akhavan and Ghaderi 2009; Sim et al. 2014). These modifications involve adjustments to the quantities of KMnO4 and the proportion of the reactants as recommended by different research groups. These alternations aim to optimise the synthesis procedure and increase the efficiency and properties of the resulting graphitic oxide materials (Akhavan and Ghaderi 2009; Lingappan et al. 2013; Sim et al. 2014). In 2010, Marcano and his associates developed a novel approach known as the “Improved Hummers” method. This method involves mixing H3PO4 and H2SO4 in a ratio of 1:9, followed by the introduction of KMnO4 and graphite in a ratio of 6:1 ratio within an ice bath. Subsequently, the mixture underwent heating to 50 °C and was stirred for a duration of 12 h. To remove the excess of KMnO4, the product was cooled and poured onto ice containing 30% H2O2 (3 mL). Phosphoric acid serves dual roles as a dispersing and etching agent and acts as a stabiliser throughout the oxidation process in the GO synthesis, ensuring safety. This method results in a greater yield of GO with enhanced oxidation and a more regular structure (Marcano et al. 2010; Zaaba et al. 2017). However, this process is highly time-consuming with the purification and separation, large amounts of acids consumed. Modified and Improved Hummers methods have been used to synthesise carbonous waste sources into GO. Many researchers have explored various waste sources such as coconut shells (Sujiono et al. 2020), tea (Amir Faiz et al. 2020), rice straw (Goswami et al. 2017), electronic scraps (Siaw et al. 2020) and scrap tyres (Anuar et al. 2023) to synthesise GO using the Hummers methods. In 2017, Nasir et al. (Nasir et al. 2017) synthesised GO from carbonised oil palm including leaves, kernel shells and empty fruit bunches using the improved synthesis method. However, synthesising carbonous materials from the Modified and Improved Hummers methods has certain limitations, including time-consuming, non- eco-friendly, complex separation, extensive purification, and low yield etc. Therefore, converting waste products sustainably to produce GO is still challenging. The chemical routes for preparing GO often result in a structural damage due to the strongly acidic conditions and impurities. These characteristics make the material less suitable for electronic applications. Apart from the chemical methods, electrochemical synthesis offers a promising alternative for the bulk production of GO and is more suitable for electronic applications (Liu et al. 2019; Kakaei and Hasanpour 2014). This method is more eco-friendly, as it allows the reuse of the electrolyte and reduce the need for extensive washing of equipment. In contrast to standard procedures, the better quality of electrochemical GO can be attributed to using aqueous electrolytes and the absence of oxidising agents, thereby avoiding impurities (Liu et al. 2019; Kakaei and Hasanpour 2014). Furthermore, the versatility of experimental setups allows for precise modulation of the degree of oxidation and density of defects in the synthesised GO (Liu et al. 2019; Kakaei and Hasanpour 2014; Md Disa et al. 2015). However, the cost of the electrolyte can increase expenses when scaling up the process. The simple electrochemical exfoliation techniques provide minimal control over the structures of the resultant materials (Liu et al. 2019).
Recently, numerous researchers (Li et al. 2012; Long et al. 2015) have proposed thermochemical techniques to synthesise graphene derivatives using biomasses. Among these techniques, pyrolysis is a prominent bottom-up method to synthesise GO. The term “pyrolysis” combines “pyro”, meaning fire, and “lysis”, referring to separation, representing the process under high temperature and non-oxidising atmosphere (Mbayachi et al. 2021). The implementation of pyrolysis techniques on diverse waste precursors has demonstrated the successful production of graphene-based materials (Kong et al. 2020). Mainly, carbon precursors are pyrolyzed into biochar, bio-oil, gas, and other various valuable products. The pyrolysis involves various reactions, including depolymerisation, decarboxylation, dehydration, intermolecular condensation and aromatisation at different temperatures (Liu et al. 2015a, b). The classification of pyrolysis into two main types includes conventional pyrolysis (CP) and microwave-assisted pyrolysis (MAP), depending on the heating method utilised. Conventional pyrolysis utilises electric heating, while microwave-assisted pyrolysis relies on the microwave heating. For example, Thangaraj et al. (2023) have synthesised GO from sugarcane bagasse using ferric citrate as a catalyst in two steps of conventional pyrolysis. However, conventional pyrolysis is often inefficient and energy-intensive compared to microwave-assisted pyrolysis. Therefore, microwave pyrolysis has garnered significant attention in the research community due to its faster, more energy-efficient process and the production of more desired products than conventional pyrolysis (Du et al. 2011; Fodah et al. 2021). Nevertheless, a significant challenge associated with this method lies in the complexity of regulating particle size and incomplete understanding of the mechanism of microwave pyrolysis. There is insufficient study of GO synthesis using only microwave pyrolysis technique.
Characterisation of Synthesised Graphene Oxide
The characterisation of synthesised GO involves a comprehensive analysis utilising a range of analytical techniques such as X-Ray Diffraction Analysis (XRD), Raman Spectroscopy, Fourier Transform – Infrared Spectroscopy (FT-IR), Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), X-ray Photoelectron Spectroscopy (XPS) etc.
XRD, widely used for crystalline material characterisation, reveals crucial information about the average spacing between layers. The XRD pattern for the GO synthesised through the Modified Hummers method is illustrated in Fig. 3a (Paulchamy et al. 2015). The diffraction peak of pure graphite is observed at approximately 26°, indicative of its well organised layered structure with an interlayer spacing of 0.34 nm, as illustrated in the insert of Fig. 3a. The absence of the peak at 26° and the emergence of a peak at 10° signify complete oxidation of graphite into GO, resulting in an increased d-spacing from 0.34 nm to 0.82 nm (Paulchamy et al. 2015).
(a) XRD pattern of GO (insert shows XRD of graphite) (Paulchamy et al. 2015), (b) Raman spectrum of GO (Shahriary and Athawale 2014), (c) FT-IR of GO, (d) SEM images of Graphite (i), GO (ii), FESEM images of GO (iii,iv) (Paulchamy et al. 2015), (e) HRTEM of GO (i)-(iii), SAED pattern of GO (iv) (Syed et al. 2017), (f) XPS spectra in the C1s region (Ren et al. 2011)
Raman spectroscopy proves to be a non-destructive technique offering valuable structural insights into carbon-based materials. Key features observed in Raman spectra include the G and D peaks, occurring around 1580 cm−1 and 1350 cm−1, respectively. The G peak corresponds to the stretching bond of sp2 carbon pairs in chains, and rings. The D peak represents the breathing mode of aromatic rings, indicative of structural defects. The intensity of the D peak serves as an indicator of the degree of disorder, while the presence of the overtone 2D peak at 2680 cm−1, correlates with the number of graphene layers (N) (Shahriary and Athawale 2014). The Raman spectrum of GO, obtained under excitation at 532 nm, is illustrated in Fig. 3b. The pronounced D peak at 1348 cm−1, exhibiting intensity comparable to that of the G peak at 1592 cm−1, and a bandwidth indicate significant structural disorder of GO. Moreover, the presence of weak and broad 2D peaks further indicate disorder within the material. Additionally, a defect-activated peak labelled D + G is visibly present near 2950 cm−1 (Shahriary and Athawale 2014). This Raman spectroscopy analysis contributes valuable insights into the structural characteristics and disorder level of synthesised GO.
The FT-IR spectrum of GO, depicted in Fig. 3c, exhibits distinctive features providing insights into its properties. In the high-frequency range of the FT-IR spectrum, a broad peak ranging from 3000 to 3700 cm−1, accompanied by a sharp peak at 1635 cm−1, signifies the stretching and bending vibrations of OH groups from H2O molecules adsorbed on GO (Paulchamy et al. 2015), indicating strong hydrophilicity. Further analysis reveals absorption peaks at 2930 cm−1 and 2850 cm−1, corresponding to symmetric and anti-symmetric stretching vibrations of CH2. Within the medium frequency range, peaks observed at 1630 cm−1 and 740 cm−1 are attributed to the stretching vibrations of C = O and C = C, originating from carbonyl and carboxylic acid groups present at the edges of GO. The absorption peaks at 1110 cm−1 and 1385 cm−1 are correspond to the stretching vibration of C–OH (alcohol), and C-O (carboxylic acid), respectively (Shahriary and Athawale 2014). These peaks indicate the presence of oxygen-containing group, revealing the oxidation of graphite.
SEM images offer valuable insights into the structure and morphology of nanomaterials. In Fig. 3d-(i), the SEM image of typical graphite illustrates how sheets are stacked together. The SEM image of Fig. 3d-(ii) displays exfoliated GO, clearly showcasing the process of graphene sheets exfoliation. Observing the SEM images of GO reveals 2D nanosheet morphologies characterised by folded and wrinkled textures, occasional rough surfaces, irregular edges, and crumpling due to scrolling. Further analysis of grain size and surface morphology was conducted using Field Emission Scanning Electron Microscopy (FESEM). The obtained FESEM images reveal distinct and interconnected three-dimensional graphene sheets, creating a porous network that resembles a loosely woven sponge-like structure, as illustrated in Fig. 3d-(iii,iv) (Paulchamy et al. 2015). Notably, SEM has provided insights into the size range of GO sheets, revealing lateral dimensions ranging from as large as 200 µm to as small as a few microns within the sample (Zhang et al. 2019). This comprehensive morphological examination contributes to a deeper understanding of the structural characteristics of GO.
TEM stands as a valuable instrument for the identification of single layer and few layer GO sheets in research studies. The technique utilises electron passing through the sample, revealing contrast based on variations in electron density. Consequently, single sheets appear brighter compared to multiple sheets due to their reduced thickness. TEM images (Fig. 3e) of ultrasonically exfoliated GO showcase a unique wrinkled morphology, indicating the successful exfoliation of GO into single or very few layers. These distinctive wrinkles and folds within the exfoliated GO sheets serve as prominent indicators characteristic features of single-layer structures. The GO nanosheets, whether single- or few-layered, displayed a flat morphology with a width exceeding 1.5 µm. The selected area electron diffraction (SAED) pattern of GO represents a polycrystalline nature (Syed et al. 2017; Zhang et al. 2019; Fu et al. 2013).
XPS involves directing X-rays onto the surface of a sample to measure the number and kinetic energy of emitted electrons. This process widely used for analysing the elemental composition of the surface. In XPS analysis of GO in Fig. 3f, the C1s peak undergoes deconvolution into five distinct components: sp2 carbons from graphite (284.6 eV), carboxylic acid (289.1 eV), carbonyls (288.0 eV), C atoms attached to hydroxyl groups (285.7 eV), and epoxy/ether groups (286.7 eV), (Zhang et al. 2019). Within the O1s spectra of GO, two main components are observed: C-O (hydroxyl and epoxy, 532.5 eV) and C = O (531.6 eV), accompanied by two minor components attributed to O–H (533.5 eV) and quinones (530.5 eV) (Zhang et al. 2019). XPS analysis is utilised to ascertain both the atomic composition and the C/O ratio of GO. The obtained C/O atomic ratio of 3.0 indicates a substantial introduction of oxygen-containing groups during the oxidation of graphite (Ren et al. 2011).
Synthesis of GO from Waste Materials and its Characterisations
Managing and utilising waste pose substantial global challenges. Researchers worldwide are currently focusing on converting waste into value-added products, with a particular focus on carbon-based nanomaterials (Asif and Saha 2023). The synthesis of GO from waste has gained significant recognition and value owing to its exceptional properties. The use of natural graphite and synthetic graphite as precursors for GO synthesis is hindered by various limitations. Natural graphite availability is restricted in certain countries, while the production of synthetic graphite demands exceedingly high temperatures (≥ 2500 °C) and incurs significant costs (Li et al. 2015). Conversely, carbon residues and biomass are increasingly proposed as promising precursors for graphene material synthesis. This is attributed to their high carbon content, environmental-friendly nature, lower processing temperatures, widespread availability and cost-effectiveness (Yap et al. 2023). Numerous studies have explored the utilisation of different carbon precursors and biomass sources, including coconut shells (Sujiono et al. 2020), sugarcane bagasse (Somanathan et al. 2015), tea (Amir Faiz et al. 2020), oil palm leaves (Fathy et al. 2019), pine leaves (Singhal et al. 2022), rice straw (Goswami et al. 2017) and industrial scraps such as tyres (Anuar et al. 2023), bio-soot (Kumar Sahoo et al. 2020), Zn-C batteries (Hassanin et al. 2022) for synthesising GO (Fig. 4).
Agricultural wastes, comprising residues from the initial processing of crops, fruits, and vegetables, pose significant environmental challenges globally. Recycling these wastes into valuable products offers bio-based approach to addressing both local and global environmental challenges. However, high-temperature methods are typically required due to the abundance of cellulose, hemicellulose, and lignin in the presence of agricultural waste (Hashmi et al. 2020). The production of carbon-based materials presents an alternative approach to reduce and elucidate issues associated with waste-related biomass.
In recent decades, synthetic polymers such as polyethylene terephthalate (PET) and electronic products such as batteries and electronic devices have become integral to our community. The exponential growth in industrial waste production has resulted in significant environmental issues. However, industrial waste is rarely repurposed for useful applications due to its unfavourable mechanical characteristics, thermal stability, and low electrical conductivity (Mallakpour and Behranvand 2016). Consequently, the appropriate management of this waste presents a considerable global issue. One common method utilised for the disposal of industrial scraps is through their incineration within designated landfill areas. It has long been a standard procedure for managing industrial waste. However, this method can have adverse environmental impacts, such as soil and groundwater contamination, release heat and volatile compounds, and cause climate change (Saha and Ghoshal 2005). The next approach to eliminate industrial waste is incineration with energy recovery, feedstock or chemical recycling to generate liquid products and gaseous along with carbon-enriched materials (Mishra et al. 2003). Carbon waste is a promising precursor for synthesising carbon-based materials, including microspheres, nanotubes, and graphene derivatives (Mendoza-Carrasco et al. 2016). Most industrial scraps are rich in carbon and contain minimal mineral impurities in their chemical composition, making them suitable for synthesising carbon materials (Nakagawa et al. 2004). Therefore, a current issue is finding efficient waste utilisation techniques that can convert waste into value-added materials like GO while eliminating impurities. However, there are still limitations with the existing methods for synthesising GO using carbon and biomass sources.
The advancement of carbon-based materials derived from renewable resources such as agricultural, industrial, and plastic is paramount. However, several challenges are linked to the process of producing graphite from carbonous and biomass wastes, such as processing conditions, impurities, and production costs. The primary motivation behind utilising these sources is to offer “green and sustainable” alternatives, utilising cost-effective raw materials for the mass production of carbon-based materials. Recycling carbon precursors and biomass to produce GO offers substantial economic and environmental advantages, unlocking a broad range of applications. Table 2 illustrates the summary of the synthesis of GO from different carbon precursors and biomasses and their possible applications.
GO from Agricultural Residues
Agricultural residues and raw biomass offer an abundant, carbon–neutral, cost-effective source for bulk scale production of valuable carbonaceous materials (Filho et al. 2019; Bazaka et al. 2016). Various types of agricultural waste, including coconut shells, sugarcane bagasse, tea and rice husk, have been investigated for their potential in graphite production (Amir Faiz et al. 2020). The main precursor for synthesising GO is graphite powder. Given the escalating demand for commercial graphite, there is an exploration of alternative raw materials that are cost-effective and environmentally friendly. Graphite occurs naturally in three forms: crystalline flakes (90–98%), crystalline lumps (90–99%) and amorphous (70–80%), (Yang et al. 2017). It can be further categorised as natural or synthetic graphite, depending on the process of graphitisation utilising heat-induced hydrocarbon precursors. There were reported different methods for the graphitisation of carbon precursors and biomass, including pyrolysis, the template method, foaming technique, and catalytic graphitisation (Kim et al. 2016). Agricultural bio-waste generally contains many volatile compounds and impurities, posing a potential disruption to the production method and compromising the quality of the resultant graphitic carbon. Additionally, achieving optimal conditions for converting agricultural biomass into graphitic carbon presents considerable challenges. Precise control of pressure, temperature, catalyst, and other parameters is essential to achieve the intended product. Utilising agricultural biomass as raw materials for graphitic carbon synthesis holds the potential for cost reduction. However, optimising the cost-effectiveness of this method involves considering factors such as feedstock availability, processing expenses, and the market demand for the end product.
Coconut Shell and Husk
Hydrocarbon compounds are naturally found in various materials, and one such material is coconut shell charcoal (Sujiono et al. 2020), stands out with its substantial carbon content, recorded at 74.3% (Bledzki et al. 2010). In a study conducted in 2020, Sujiono and colleagues employed a modified Hummers method to synthesise GO from coconut shell. Simultaneously, graphite powder was derived from the coconut shell through the process of pyrolysis for graphitisation. The thermally dried coconut shell sample was carbonised at 600 °C for 3 h to synthesis charcoal with a significant carbon content. In a previous research endeavour, Wachid et al. (2014) experimented with different heating temperatures (400, 800 and 1000 °C) to generate nanocrystalline graphite from coconut shells. Their findings indicated that the sample heated at 1000 °C with a holding time of 3 h exhibited the highest crystalline percentage. Compared with the Sujiono and Wachid works, nanocrystalline graphite was obtained when the sample was heated at the most elevated temperatures (1000 °C).
Apart from coconut shell, coconut husk, which contains a significant amount of silica and carbon, was used by Grace and Colleagues (Grace and Prabha Littis Malar 2020) to synthesise graphite. The carbonised sample underwent grinding and sieving to obtain a powdered form, followed by treatment with hydrofluoric acid (HF) to eliminate silica impurities. The resulting solution underwent further washing with deionized H2O and NaOH until it reached a pH level between 6–7. Finally, the sample was subjected to oven dry at 110 °C for a duration of 12 h. The resulting graphite powder was characterised by XRD, Raman, FTIR, SEM.
Sujiono and the research team (Sujiono et al. 2020) obtained in two distinct forms of GO samples: as a colloid solution and as powder. In XRD analysis (Fig. 5a) revealed that the GO sample exhibited a significant presence of graphite 2H phase, accounting for 71.53% of the observed structure. This observation indicated the tendency of the GO sample to undergo a transformation into a reduced graphene oxide (rGO) phase (Compton and Nguyen 2010). This occurs due to the utilisation of an amorphous material as the precursor in the synthesis process and the alternations in the oxidation degree throughout this procedure. While graphite possesses a high oxidation rate, it was noted that certain samples experienced reduction when subjected to oven dry. This case to the separation of oxygen compound groups from the GO bonding layer (Liu et al. 2015a, b). The D and G bands in Raman spectrum (Fig. 5b) correspond to the stretching vibrations of sp2 and sp3 carbon, respectively, with the D band originating from carbon defect sites and the G band representing sp2 carbon. The ID/IG intensity ratio in high-quality samples is typically below 2. The ID/IG intensity ratio of GO derived from coconut shell waste was found to be 0.89, indicating good quality. FTIR spectroscopy analysis (Fig. 5c) indicated the presence of different oxygen-containing functional groups, such as carboxyl, hydroxyl, epoxy, and alcohol, thereby confirming its hydrophilic nature. Furthermore, the SEM image (Fig. 5d) revealed a surface morphology abundant with granular particles, exhibiting varied size distributions ranging from 4.26 µm to 47 µm, alongside pores measuring 2.71 µm in size.
The characterization of GO samples derived from coconut shell (a) XRD pattern, (b) Raman spectrum, (c) FTIR spectrum, (d) SEM image with scale 100 µm (Sujiono et al. 2020)
Similarly, Wachid’s research (Wachid et al. 2014) showed that the development of graphite in their study had an intermediate structure between crystalline and amorphous, referred to as turbostratic. The existence of the non-crystalline phase in the sample was attributed to the utilisation of raw materials sourced from natural resources. Moreover, Tamilselvi et al. (2020) conducted the direct production of rGO from coconut coir and shells through carbonisation in a muffled furnace with ferrocene as an oxidising catalyst. This approach utilised oxidising agents instead of wet chemical methods such as thermal decomposition and microwave-assisted synthesis to prevent contamination and by-product formation in the rGO production process (Rivero et al. 2018).
Sugarcane Bagasse and Lignocellulosic Materials
In 2014, Akhavan and colleagues (Akhavan et al. 2014) conducted a study on the synthesis of GO and graphene derived from natural and industrial wastes, followed by the modified Hummers method. This research used vegetations such as bagasse, fruit, wood, leaves, and animal bones, cow dung for the carbonisation process. Based on Raman, XPS, atomic force microscopy (AFM), and current–voltage characteristics, the properties of the synthesised graphene sheets, including single-layer and multi-layer structures, composition, carbonaceous structure, and electrical properties were investigated. The results indicated that these properties were largely independent of the starting materials, demonstrating comparable characteristics to high-quality graphene sheets that were achieved using highly pure graphite.
In 2015, Somanathan et al. conducted a study focusing on GO synthesis from sugarcane bagasse via the direct oxidation within a muffled atmosphere that was called “SOMA-GO” method. The method involved crushing the bagasse fibre into a fine powder after juice extraction. This powdered sugarcane bagasse was then combined with ferrocene and introduced directly into a muffle furnace at 300 °C for 10 min under atmospheric conditions. The XRD pattern (Fig. 6a) obtained in this study exhibited well-graphitized two-dimensional structures composed of GO sheets. The presence of a peak at 2θ = 11.6° indicated the complete oxidation of agricultural sugarcane bagasse into GO with an interlayer distance of 0.79 nm. The Raman spectrum (Fig. 6b) demonstrated an ID/IG intensity ratio of 0.76 for GO, aligning with findings from previous investigations. The FTIR spectroscopy (Fig. 6c) confirmed the introduction of oxygen-containing functional groups, including carboxylic, hydroxyl, and epoxy groups, resulting from the oxidation of sugarcane bagasse. The surface morphology analysis (Fig. 6d) of the sugarcane bagasse-derived GO revealed sheet-like (Fig. 6d-(i,iii)) and flake-like structures (Fig. 6d-(ii,iv), consistent with observations reported by previous researchers. High-resolution Transmission electron microscopy (HRTEM) analysis (Fig. 6e) exhibited wrinkles, bends, and edges on GO nanosheets in multiple locations. The selected area (Fig. 6e-(iv)) of the electron diffraction pattern confirmed the existence of a polycrystalline structure indicative of GO. Compared with other methods, the SOMA-GO method employed in this study offers environmental advantages as it eliminates the emission of toxic gases such as NO2, N2O4, and ClO2 during the synthesis. Additionally, this method effectively converted solid sugarcane bagasse into value-added GO simply and rapidly, yielding a well-graphitized GO structure.
The characterisation of GO samples based on sugarcane bagasse (a) XRD pattern, (b) Raman spectrum, (c) FTIR spectrum, (d) FESEM images, (e) HRTEM images (Somanathan et al. 2015)
Thangaraj et al. (2023) employed a two-step pyrolysis process with utilising ferric citrate as a catalyst and subsequent treatment with conc. H2SO4 to synthesise GO from sugarcane dry leaves (Fig. 7). The synthesised GO was confirmed by a dominant peak at 12.47° in XRD (Fig. 8a) with an interlayer distance of 0.71 nm.
The schematic representation of the two step pyrolysis utilised for producing GO from dried sugarcane leaves (Thangaraj et al. 2023)
The characterisation of GO samples based on sugarcane dry leaves (a) XRD pattern, (b) Raman spectrum, (c) FTIR spectrum, (d) SEM images, (e) TEM images (Thangaraj et al. 2023)
The Raman spectrum (Fig. 8b) exhibited distinct D and G bands, signifying the presence of both sp2 and sp3 hybridized carbon atoms in the synthesised sample. Furthermore, the FTIR spectrum (Fig. 8c) identified various oxygen-containing functional groups in the GO structure. Morphology analysis (Fig. 8d) revealed sheet-like structures of prepared GO. TEM images (Fig. 8e) are shown few wrinkled textures throughout the GO sheets. The SADE pattern (Fig. 8e) analysis of GO sheets revealed diffused concentric diffraction rings, indicating the semi amorphous nature of the material, and confirming its poor crystallinity. The prepared GO exhibited numerous oxygenated functional groups covalently attached to a graphene basal plane, resulting in C/O atomic ratio of 4.46. Notably, the pyrolysis approach employed in this study stands out for its simplicity, reliability, efficiency, and cost-effectiveness compared to other methods. However, no applications were explored in this study.
In 2020, Tohamy and his research group (Tohamy et al. 2020a) conducted a study in which GO was synthesised from four different agricultural residues: sugarcane bagasse, pinewood sawdust, rice straw, and lignocellulosic materials using the SOMA-GO method. Their findings revealed that GO derived from sugarcane bagasse exhibited higher hydrophilicity and better water dispersibility, attributed to the presence of numerous hydrophilic functional groups, as confirmed by FTIR analysis (Tohamy et al. 2020a). Furthermore, in the same year, the research group (Tohamy et al. 2020b) examined the thermal properties of GO synthesised from these various agricultural waste sources. The thermal decomposition process of these four various agricultural residues with the presence of ferrocene exhibited the three main reaction steps, consistent with GO synthesised from pure graphite. In 2022, the same research group (Sarhan et al. 2022) introduced a novel magnetic hydrogel loaded with a nanocomposite of GO for Ni(II) removal from wastewater. The GO nanocomposite was prepared using a single-step method, involving the oxidation of sugarcane bagasse using ferrocene, following the same procedure as SOMA-GO synthesis. The characteristics of the nanocomposite were comparable to those of the SOMA-GO product, with one exception: the XRD pattern analysis. The Raman spectra exhibited a smaller ID/IG peak intensity ratio (0.59), indicating reduced defects and disorders within the material. However, the XRD analysis of GO exhibited signals at 2θ = 9.3 and 21.8°, corresponding to the (001) and (002) plans, suggesting incomplete oxidation of sugarcane bagasse. It is worth noting that GO is a non-stoichiometric compound, and the ratio of carbon to oxygen is influenced by both the preparation technique and the extent of material oxidation.
In 2020, Hashmi and colleagues (Hashmi et al. 2020) conducted a research focusing on the fabrication of GO using various agricultural wastes including sugarcane bagasse, orange peel, rice bran and a combination of these wastes referred to as the tri-composite. The method employed for GO synthesis was like the SOMA-GO approach and used individual agricultural wastes and the tri-composite forms. Comparative analysis revealed that successful synthesis of GO was achieved only when using the tri-composite agricultural waste. The presented results highlight a distinct diffraction peak in the XRD pattern at 2θ = 12.70°, indicating a corresponding d-spacing of 0.70 nm. Additionally, the average particle size, as determined through SEM analysis, is reported as 2.04 nm. The intense and sharp peak observed in the GO sample indicated good crystallinity, confirming the successful synthesis of GO from the tri-composite agricultural waste. This sustainable, time-efficient, cost-effective method holds significant potential for industrial-scale GO fabrication. Moreover, this approach is environmental friendliness and biocompatible. It enables the utilisation of various types of solid agriculture waste containing carbon, eliminating the need for sanitation as feedstock. Consequently, this method allows to produce high-quality and value-added GO.
Faghiri and Ghorbani (2020) produced GO using sugar beet bagasse through a modified Hummers method, which was further employed for the stabilising silver nanoparticles (AgNPs). The process of converting carbonaceous waste into graphite powder involved a two-stage approach. The initial step encompassed carbonisation, followed by utilising FeCl3 as a catalyst in the second stage. Subsequently, the Hummers method was adapted to attain a high-quality GO product. The synthesised GO was characterised through XRD, FTIR, SEM, TEM, and UV–vis spectroscopy. The UV–vis spectrum of GO showed that carbon-based materials display distinctive features with a peak in the range of 200–300 cm−1, which is related to sp2 hybridisation (Soltani and Lee 2017). The spectrum of the synthesised GO exhibited an absorbance peak at approximately 230 nm, representing the π → π* transition and a weaker shoulder peak at 300 nm, indicating the n → π* transition. All the analyses were confirmed the successful synthesis of GO.
In 2017, Goswami and associates (Goswami et al. 2017) introduced the pioneering use of rice straw biomass as a sustainable precursor for the cost-effective and scalable GO production via the Hummers method. This method initially subjected the oven-dried straw to carbonisation in a muffle furnace under a nitrogen atmosphere at 450 °C for 45 min, forming biochar. Afterwards, the biochar underwent a series of steps, including crushing and sieving, resulting in a homogeneous powder. This powder was then utilised as a replacement for graphite in the synthesis of GO via the Hummers method. The resulting GO nanoplatelets were characterised using XRD, FTIR, AFM, SEM and TEM. The comprehensive analysis conclusively established structural and functional equivalence to those traditionally prepared from graphite.
Tea and Coffee
Azurahanim and colleagues (Amir Faiz et al. 2020) studied converting tea waste into GO by carbonisation at high temperatures, followed by the modified Hummers method. This research highlights the potential of tea as a low-cost and temperature-effective starting material for GO synthesis. The process of preparing chemically modified graphitised biomass followed a standard route of modified Hummers method. The tea residues revealed its potential in producing GO at a low cost and temperature. Increasing the oxidation time, a similar GO structure derived from tea was achieved, comparable to that prepared from commercial graphite. The tea waste samples were carbonised at three specific temperatures: 650 °C (S1), 750 °C (S2) and 850 °C (S3). However, the carbonisation yield remained consistent across all the samples. The XRD studies indicated that the prominent peak (2θ = 26°) width narrowed as the carbonisation temperature increased, indicating increased structural ordering and graphitisation of the carbonised tea residues. Raman analysis demonstrated ID/IG ratio for S1 = 0.84, S2 = 0.92, S3 = 0.96, signifying increased defects in the carbon structure and greater disorder in sp2 carbon formation with rising carbonation temperature. The connection between the ID/IG ratio and heating temperature indicates that elevated temperatures affect the defects in the sp2 carbon structure. Subsequently, GO was produced through the modified Hummers method, with the S1 carbonised sample as the precursor. The synthesised GO was confirmed through FTIR and SEM analysis. Furthermore, the synthesised GO underwent loading with titanium dioxide (TiO2) through the hydrothermal method, forming a nanocomposite of rGO/TiO2.
Challa et al. (2023) investigated the synthesis of GO nanoparticles using spent coffee grounds, an abundantly discarded carbonaceous source. The oven-dried ground coffee waste was carbonised in a tube furnace at 700 °C for 4 h under argon gas flow. The resultant black char served as a precursor for GO production employing the Modified Hummers method. The synthesised GO was confirmed through the Raman spectroscopy, FTIR, and SEM analysis. In the Raman spectra (Fig. 9a), the D and G bands appeared at 1339 cm−1 and 1598 cm−1 respectively, characteristic of GO, while the presence of the 2D band at 2770 cm −1 indicated the multi-layer stacking of graphene sheets. FTIR (Fig. 9b) confirmed the formation of GO, while SEM (Fig. 9c) revealed a reduction in particle sizes to the nanoscale following increased ultrasonication. The produced GO was then used in electrospun cellulose acetate scaffolds, indicating potential applications in tissue engineering.
The characterisation of GO samples synthesised from ground coffee (a) Raman spectrum, (b) FTIR spectrum, (c) SEM images (Challa et al. 2023)
Oil Palm Waste and Pine Leaves
Nasir et al. (2017) introduced a novel approach for producing rGO from GO utilising oil palm waste, including leaves, empty fruit bunches, and kernel shells. Before synthesis GO and rGO, they investigated the impact of heating temperature on the production yield. The waste materials were carbonised in a furnace under N2 gas for 3 h at 400 °C to 900 °C with a heating rate of 10 °C/min. Following carbonisation, the GO was synthesised using an improved Hummers method. Raman, XRD, FTIR, SEM, TGA and BET analysis were used to analyse the products. Subsequently, the GO underwent reduction through low temperature annealing at 300 °C in a furnace under N2 gas for 1 h. The Raman analysis revealed an increase in the IG/ID ratio, indicating enhanced graphitisation in the rGO samples derived from different oil palm waste components. This method offers scalability and cost-efficiency in producing carbon nanostructured materials and needs to further investigate into potential applications.
Fathy et al. (2019) produced GO from oil palm leaves via the catalytic acid spray (CAS) process, employing Co silicate as a catalyst for Cu (II) ion removal from wastewater. In this method, pre-treated microcrystalline cellulose fraction from oil palm leaves were subjected to mixing with conc. H2SO4 for 10 min, followed by thorough washing and drying at 40 °C for 6 h. Subsequently, the material underwent decantation, cobalt silicate addition, and heating at 40 °C for 30 min. Finally, the resulting GO was then cooled and dehydrated. The synthesised GO was characterised using Raman, XRD, and FTIR. According to the Raman spectroscopy, GO has been synthesised as a single sheet with a broader 2D band, between 2650 cm−1 and 2700 cm−1. However, the intensity ratio of IG/ID is 4, indicating low-quality GO. In XRD, the broadband was indicated of GO synthesis, possibly due to combining GO sheets with silicate content catalysts or cellulosic precursors. The FTIR showed that GO comprises carboxylic acids, carbonyl, and hydroxyl groups. This study pioneers the use of the CAS method for GO synthesis, which is a simple, low-cost, scalable method. However, compared with other methods, synthesised GO has low quality due to the catalyst effect.
In 2022, Singhal et al. investigated using pine leaves as a sustainable and environmentally friendly source for producing biochar, which was further transformed into GO (Fig. 10). The process commenced with the preparation of biochar, achieved through pyrolysis of pine leaves at 750 °C for 3 h in an N2 environment. The subsequent conversion of biochar to GO was facilitated using a modified Hummers method. The XRD, Raman, FTIR, SEM and elemental analysis, confirmed the biochar and respective GO. The XRD analysis revealed a decrease in the crystallite size of biochar after its conversion into GO. The Raman spectra analysis of GO exhibited a notable blue shift in the G band at 1595 cm−1, along with a clearly defined broad D band at 1380 cm−1, indicative of the presence of epoxy and hydroxyl groups. Both biochar and the corresponding GO were examined for their potential in long-term electrochemical energy storage and resistance to corrosion in a potassium hydroxide solution (KOH). However, it is worth noting that a significant limitation of this synthesis pathway was the utilisation of the modified Hummers method.
Schematic representation of Biochar and GO synthesis from Pine Leaves (Singhal et al. 2022)
Biological Source
Roy et al. (2014) introduced an innovative and cost-effective method for synthesising GO by pyrolyzing Tasar silk cocoons followed by oxidation using conc. HNO3 (Fig. 11). This work opened new possibilities for utilising Tasar silk cocoon as a magnetic-fluorescent biomaterial. Initially, Tasar silk cocoons were ground and pyrolyzed in a muffle furnace at 400 °C for 2 h under an argon atmosphere. After purification, the carbonised cocoon underwent oxidation through treatment with conc. HNO3 for 24 h. The final product was analysed using Raman, FTIR, SEM and TEM analysis. Raman analysis (Fig. 12a) compared the position and intensity of the D band and G band for both raw and synthesised GO samples. In the synthesised GO, two prominent bands appeared at 1592 cm−1 (G band) and 1359 cm−1 (D band), showcasing a blue shift compared to the raw carbon. This shift was attributed to the conversion of graphite crystals into graphene sheet and the presence of more defects. FTIR spectroscopy (Fig. 12b) revealed the various oxygen functionalities present in the synthesised GO. The SEM images provided insights into the surface morphology changes induced by oxidation, with the raw carbon (Fig. 12c-(i)) displaying layered sheets with carbon particulates with its amorphous nature, while the oxidised carbon exhibited multilayer GO sheets with a width of 2.6 nm (Fig. 12c-(ii-iv)). TEM images (Fig. 12d-(i-iii)) displayed oxidised carbon, indicating the presence of multilayered of GO. The SAED (Fig. 12d-(iv)) confirmed a hexagonal lattice with typical sixfold symmetry for GO. These characterisations demonstrated similarities with commercial GO. The synthesised water-soluble GO exhibited remarkable fluorescence properties, expanding its potential applications.
The schematic diagram of GO synthesis from Tasar silk cocoon (Roy et al. 2014)
The characterization of GO synthesised from Tasar silk cocoon and raw carbon (a) Raman spectrum (b) FTIR spectrum (c) SEM images (i) raw carbon (ii-iv) GO sheets (d) TEM images (i-iii) GO (iv) SAED pattern of GO (Roy et al. 2014)
GO from Industrial Scarps
Industries produce large quantities of scarps, promoting exploration into utilising these waste materials for the synthesis of GO – a concept offering both environmental and resource conservation advantages. Various waste sources such as soot (Kumar Sahoo et al. 2020), newspapers (Akhavan et al. 2014), scarp tyres (Anuar et al. 2023), electronic components (Siaw et al. 2020), and used batteries (Siaw et al. 2020) have been explored for their viability in GO synthesis. Researchers are employing innovative methodologies to convert suitable constituents from industrial waste into GO. The resulting GO exhibits distinctive properties that render it suitable for diverse applications.
Soot Powder and Newspapers
In 2014, Akhavan (Akhavan et al. 2014), mentioned earlier in the context of GO synthesis from agricultural residues, explored the synthesis of GO using semi-industrial and industrial waste such as newspaper, soot powders (generated from diesel vehicle exhaust), respectively. The industrial waste was initially carbonised within the temperature range of 400–500 °C and used a Hummers method was employed for chemical exfoliation of the graphitised materials. The surface morphology, including chemical composition, sheet thickness, carbonaceous structure, and electrical properties of the sheets synthesised from different sources were observed to be nearly identical and comparable to those of the graphene sheets derived from highly pure graphite.
In 2020, Kumar Sahoo et al. synthesised GO from bio-soot (diesel engines) to adsorb the highly noxious Congo red dye. The investigation of the morphological structure of the bio-soot revealed a higher degree of graphitisation compared to carbon black or activated charcoal. The process of preparing (Fig. 13) GO involved oxidising the bio-soot powder using the modified Hummers method. The resulting GO was characterised through XRD, Raman, FTIR, BET, FESEM and HRTEM. The XRD (Fig. 14a) study showed a strong peak at 10.8° (002) corresponding to an interlayer spacing of 0.86 nm. This observation suggests the existence of oxygen-containing groups within the graphitic layers.
Synthetic procedure for GO from bio-soot (Kumar Sahoo et al. 2020)
(a) XRD pattern of GO, bio-soot and waste, (b) Raman spectra of GO and bio-soot, (c) FTIR spectra of GO and bio-soot, (d) FESEM image, (e) TEM image, and (f) EDX Spectra of GO derived from bio-soot (Kumar Sahoo et al. 2020)
In the Raman spectra of GO (Fig. 14b), the observation of the D band at 1360 cm−1 and the G band at 1610 cm−1 confirmed the successful production of GO from bio-soot. This formation was further validated by FTIR (Fig. 14c). The FESEM image of GO (Fig. 14d) showed a few flaky layered and wrinkled large sheet-like structures with smooth surface, and the TEM images of GO (Fig. 14e) represented a single sheet-like structures. The EDX spectra (Fig. 14f) of GO exhibited the presence of carbon and oxygen, with weight ratios of 94.34 and 5.66 receptively. The advantageous aspect of bio-soot lies in its readily available as a by-product of diesel engine exhaust, thus converting an environmental pollutant into a valuable product.
Scrap Tyres
Bonnia et al. and Anuar et al. in their respective studies (Bonnia et al. 2021; Anuar et al. 2023) have been investigated the synthesis and characterisation of GO derived from discarded tyres using a modified Hummers method. The study aimed to convert regenerated carbon black from tyres into GO, turning waste into a valuable resource. The presence of GO was confirmed through analysis using Raman, XRD, FTIR, and FESEM. The study revealed an ID/IG ratio of 0.82 for GO-1% carbon waste tyres (CWT), indicating the successful production of high-quality GO. The presence of defects in the GO structure was significantly influenced by the acids employed as oxidation reagents during the synthesis, with the weight percent of the precursor playing a role. The XRD analysis exhibited a broad and wide peak, indicating that the synthesised GO was in the amorphous phase. Furthermore, the FTIR results further supported the presence of hydroxyl and epoxy groups within the GO structure, contributing to its hydrophilic properties.
Tatrari et al. (2022) introduced an innovative methodology for the mass-scale production of zinc doped rGO spheres (ZGS) from scrap tyres using an environmentally friendly double step pyrolysis technique. Comprehensive characterisation of the prepared samples was conducted using advanced spectroscopic techniques including XRD, Raman spectroscopy and FTIR. The XRD results exhibited two broad peaks at 2θ = 25° and 2θ = 44°, indicating the graphitic nature of the sample and the transformation of amorphous structure into reduced carbonic moieties. The Raman analysis revealed well-defined D, G and 2D bands at 1366 cm−1, 1560 cm−1, and 2791 cm−1, respectively, confirming the graphitic nature of the product. Furthermore, FT-IR data revealed evidence of partial oxidation at the ZGS, which could potentially be attributed to interactions with ZnO and acid treatment during the synthesis process. Finally, the synthesised ZGS demonstrated promising applications in supercapacitors.
Electronic Scarps and Other Industrial Waste
Siaw et al. 2020 investigated a synthesis of GO from various industrial waste, including electronic scraps, used batteries, metal finishing scraps, and spent petroleum catalysts. This was achieved through a combination of leaching and modified Hummers method. During the leaching step, impurities were efficiently eliminated by dissolution in 6 M HCl at 70 °C for 210 min, resulting in a graphite residue with a purity of 92.28%. Following this, GO was synthesised via a modified Hummers method using conc. H2SO4 and KMnO4. The KMnO4 ratio was adjusted from 1:3 to 1:5 to enhance the oxidation process, eliminating the need for NaNO3. Additionally, the production time for each GO synthesis step was reduced from 330 min (using the Hummers method) to 150 min. The elemental analysis revealed that heavy metals constituted the predominant impurities in industrial waste. The leaching process effectively removed most impurities, particularly when employing 6 M HCl at temperature 70 °C, a liquid-to-solid ratio of 1:10 and a leaching duration of 210 min. The XRD and FTIR analyses confirmed the synthesis of GO. Initially, the XRD analysis of the graphite present in the industrial scraps exhibited a diffraction peak at 2θ = 26.6°, indicating an interlayer distance of 0.34 nm. The conversion of graphite into GO was evidenced by a shift in the XRD peak to 2θ = 10.35°, indicating a d-spacing of 0.73 nm. Additionally, TEM observations revealed the presence of both monolayer and multilayer GO with wrinkles surface, indicating the characteristic morphology of GO.
Hassanin et al. (2022) synthesised GO through a modified Hummers method, using Zn-C batteries as precursors, which reacted with SiO2 sourced from rice husk. The products were characterised using Raman, XRD, BET, SEM, EDX, and FTIR. The Raman spectrum exhibited distinctive peaks at 1585 cm−1 for the G band and 1355 cm−1 for the D band. The XRD analysis (Fig. 15a) revealed a broad peak at 2θ = 14°, and the FTIR (Fig. 15b) was confirmed the oxygenated functional groups on its surface. The SEM image (Fig. 15c) demonstrated definite shapes and included distinct irregular composition. The C to O atomic ratio was shown in EDX spectrum (Fig. 15d) as 91.84 and 6.59 respectively. The BET analysis (Fig. 15e) highlighted its high surface area as 37.37 m2/g. All analytical results collectively confirmed the successful synthesis of GO, although the method relied on a modified Hummers approach. Moreover, the study showcased the utilisation of available carbon waste to produce a silica/GO composite as a sorbent material.
(a) XRD pattern, (b) FTIR spectrum, (c) SEM image, (d) EDX Spectra, (e) BET Analysis of GO synthesised from Zn-C batteries (Hassanin et al. 2022)
Loudiki et al. (2022) developed a synthesis method for GO using carbon rods extracted from used ZnC batteries, employing a newly optimised electrochemical approach. The method incorporates sonication for the recycling of carbon rods, providing a rapid and cost-effective technique. The study aimed to establish a simple synthesis process incorporating ultrasonication with standard Hummer’s reagents to produce GO, utilising carbon rods from ZnC batteries. The standard Hummers reagents were used for graphite oxidation and exfoliation while the sonication favoured the reaction. The proposed method prevented explosion problems and energy reaction time. The GO derived from carbon rods was characterised by XRD, FTIR, UV–Vis and SEM. The XRD analysis indicated a highly crystalline structure in the hexagonal carbon graphite powder from the battery rods, exhibiting a peak resembling that of graphite at 26.5°. However, the synthesised GO were exhibited a broad peak at 2θ extending from 10 to 45°, indicating an amorphous structure due to the presence of impurities in the carbon rods powder. FTIR was used to compare the different functional groups of the ZnC based graphite and synthesised GO. The SEM images showed that the two-dimensional flat morphology of the ZnC material, while the GO has randomly oriented sheets indicating the high degree of exfoliation. Then the electrochemical performances of GO were investigated and compared to the Hummers GO.
GO from Plastic Waste
Research on GO synthesis from plastic waste is limited; however, El Essawy et al. (2017) reported on the sustainable synthesis approach for graphene production using recycled PET bottle for dye adsorption in aqueous solutions. The research focused on the production of graphene through the thermal dissociation of Poly-ethylene-terephthalate (PET). The crushed PET was inserted into a sealed stainless-steel reactor, heated to 800 °C with a rate of 8 °C min −1, and maintained at this temperature for 1 h. The resulting dark products were then cooled and analysed. Characterisation techniques such as Raman, SEM, TEM, BET, TGA, and FTIR were used to analyse the synthesised graphene. The elemental analysis of PET indicated its potential as a precursor for graphene synthesis owing to its notable carbon amount and lack of inorganic constituents and impurities. SEM images showed a fibrous network morphology with void spaces, indicative of porosity, while TEM image displayed relatively transparent graphene layers entangled and rippled with observed graphite deposits. The XRD analysis revealed broad peaks corresponding to disordered graphite like carbons. The intensity ratio of Raman D and G bands indicated the degree of disorder in the prepared graphene. BET surface area measurements indicated significantly higher surface area and micropore volumes for the synthesised graphene compared to PET, suggesting its potential as an effective adsorbent for dye removal from aqueous solutions. Overall, the study demonstrated the feasibility of using graphene synthesised from PET waste as a low-cost and efficient adsorbent for removing methylene blue (MB) and acid blue (AB25) from aqueous solutions. However, further research is needed to explore the production of GO from plastic waste.
GO has emerged as a central focus of research due to its diverse applications across various fields. However, the reliance on conventional and limited graphite as a raw material may not be sustainable in the near future. GO synthesised from waste and other organic precursors displays properties akin to conventionally derived GO. Nonetheless, further studies are necessary to identify suitable waste precursors, considering factors such as availability, processing conditions, and product quality. The present study identifies Agro waste as a promising carbon-rich precursor, particularly sugarcane-derived GO, which stands out among agricultural wastes. The findings show that GO derived from sugarcane bagasse exhibits higher hydrophilicity and better water dispersibility, attributed to numerous hydrophilic functional groups. The SOMA-GO method and direct ferric citrate pyrolysis prove to be the best methods for synthesising GO from sugarcane bagasse, offering simplicity, reliability, efficiency, cost-effectiveness, and environmental friendliness compared to the Modified Hummers method. While waste-derived GO may contain impurities and dispensable elements, the necessity for additional purification processes raises economic challenges. For instance, tyre-derived char used for synthesising GO contains significant impurities such as Zn and S, highlighting purity issues that need resolution before adapting tyre char as a raw material. On the other hand, bio soot emerges as a promising industrial precursor for GO synthesis compared to other industrial wastes.
To ensure a selective and sustainable approach for GO production, extensive research is required to identify the best methods for producing GO with desired characteristics. Typically, GO has a carbon–oxygen ratio of 1.5 to 2.5, and various chemical methods can produce it, with the Hummers method being the most common. However, none of the waste materials in this study fall within this range, except for sugarcane dry leaves derived GO from direct pyrolysis from ferric citrate, which exhibited a C/O atomic ratio of 4.46. Moreover, the average particle size of GO derived from these waste precursors ranged from 200 µm to nanometres. Despite addressing environmental concerns and offering a sustainable and cost-effective method for GO production, the unique properties of resulting GO from waste materials introduce in its applications.
Applications of GO from Waste Materials
The innovative application of GO derived from waste has garnered significant attention in recent years. GO, a remarkable two-dimensional carbon-based nanomaterial, exhibits a diverse range of exceptional properties that render it highly versatile for various applications. Numerous researchers have explored the applications of GO derived from waste material such as coconut shells (Sujiono et al. 2020), sugarcane bagasse (Somanathan et al. 2015), rice straw (Goswami et al. 2017), tea (Amir Faiz et al. 2020), pine leaves (Singhal et al. 2022) etc.
The synthesis of GO from coconut shells, as investigated by Sujiono et al. 2020, was analysed using the Tauc plot method, revealing a band gap energy value of 4.38 eV, indicative its semiconductor properties. Initially, coconut shell is an insulator material (Saithanu and Suksri 2018), characterised by a band gap energy ≥ 5 eV. However, after being synthesised into GO material, it transforms into a semiconductor with a bandgap energy of less than 5 eV (Quaschning 2016). This reduction in bandgap energy is attributed to the exfoliation of graphite powder and the sonication of the sample throughout the synthesis method (Yu et al. 2016). In 2020, Tamilselvi and the research team (Tamilselvi et al. 2020) reported an effective method for rapidly synthesising high-quality rGO with properties well-suited for high-performance flexible supercapacitor devices. Additionally, Mohan, and his team (Mohan et al. 2019) presented a scalable strategy for synthesising GO and graphene-tin oxide from coconut shells and wood. Their study highlighted the potential of the graphene-tin oxide nanocomposite derived from charcoal of coconut shell as an antimicrobial agent in various applications. Further investigations are required to explore its potential applications in sanitary agent manufacturing, sewage treatments, and food packaging.
In 2015, Somanathan and his team proposed a simple and cost-effective method called SOMA-GO for synthesising GO from sugarcane bagasse. This method presents promising opportunities for economical production of graphene-based materials used in energy storage, gas sensors, and other functional devices (Somanathan et al. 2015). Building upon the SOMA-GO approach, Sarhan and his team (2022) synthesised GO and developed magnetite GO hydrogels. These magnetite hydrogels exhibited remarkable enhancement in Ni (II) adsorption from wastewater, with a removal efficiency of 98.82% (Sarhan et al. 2022). Despite various adsorbents being explored for Ni (II) removal, including green algae, aerobic activated sludge, fly ash, activated carbon, and crab shell, their limited adsorption efficiency hinders their widespread application (Sarhan et al. 2022). Consequently, there is a growing demand for novel adsorbents with higher capacity. Carbon-based nano sorbents like graphene (G), graphene oxide (GO), and carbon nanotubes (CNTs) have emerged as effective usage for wastewater purification (Arora and Attri 2020). Additionally, using sustainable composites offers numerous advantages including affordability, biodegradability, low density, eco-friendliness, and reduced energy consumption, in contrast to synthetic materials. However, despite the potential of adsorbents derived from sugarcane bagasse, particularly in Ni (II) adsorption, further optimisation is required to enhance their effectiveness.
In 2020, Hashmi and colleagues introduced the synthesis of GO using a combination of agricultural waste, including sugarcane bagasse, orange peel and rice bran, under a muffled atmosphere. They suggested that this straightforward technique leads to new opportunities for various applications such as energy devices, dye degradation, wastewater treatment and biomedical purposes. In a parallel study, Faghiri and Ghorbani (2020) conducted the production of GO from sugar beet bagasse employing a modified Hummers method. Subsequently, they employed the stabilization of silver nanoparticles (AgNPs) for the colorimetric and visual detection of trace amounts of Hg2+ in environmental water samples. The optimisation results suggested that Ag-GO exhibited high selectivity for detecting Hg2+ at a pH of 5.0 and a response time of 2 min, with a detection limit of 0.64 nM. This approach proved effective in quantifying Hg2+ in environmental samples, highlighting the promising application potential and significant market value of Ag-GO in environmental studies.
In 2017, Goswami and associates introduced an innovative study where biochar derived from rice straw biomass served as a sustainable precursor for the cost-effective and large-scale production of GO. This novel GO synthesis method was applied for the crystal violet (CV) adsorption from an aqueous solution. The experimental findings demonstrated that the produced GO nanoplatelets achieved a remarkable 90–99.8% CV removal. The sustainable synthesis procedure, affordability, minimal energy consumption and high treatment efficiency marked this method as highly effective for removing dyes from solutions. Furthermore, the study has shown the possible use of these GO nanoplatelets in the real-time treatment of both industrial and domestic wastewater.
Amir Faiz et al. (2020) prepared graphitized tea waste and oxidized it through the modified Hummers method. The resulting GO was then combined with titanium dioxide (TiO2) through hydrothermal processes, resulting in a nanocomposite of rGO/TiO2. The photocatalytic efficiency of the rGO/TiO2 nanocomposite was assessed through the degradation of methyl orange (MO) under ultraviolet (UV) light exposure. Their findings indicated that incorporating GO at a concentration of 11.11% of the total weight of the rGO/TiO2 nanocomposite significantly inhibited electron recombination, thereby enhancing its photocatalytic efficiency compared to using TiO2 solely. This work contributes to preparing low-cost photocatalytic nanocomposite materials for the degradation of various water pollutants. Challa et al. (2023) synthesised GO from spent coffee grounds for tissue engineering applications (Fig. 16). The investigation demonstrated that incorporating GO as a matrix modifier is non-toxic and promotes cell growth. The biocompatibility of the resulting fibres is influenced by the oxygen-rich and hydrophilic properties of GO. Utilising Agro-residual biomass, such as spent coffee, for the production and modification of biomaterials is highlighted. These findings contribute to the exploration of sustainable bio-composites and align with efforts in environmental conservation.
The schematic diagram of synthesised GO from spent coffee grounds for the application of tissue engineering (Challa et al. 2023)
Singhal et al. (2022) synthesised of GO using biochar derived from pine leaves, following a subsequent modified Hummers method. The resulting pine biochar and corresponding GO were examined for their potential in long-term electrochemical energy storage and corrosion protection in a 2.0 M KOH solution (Fig. 17). The working electrodes (WE) were prepared using biochar (WEBC) and GO (WEGO) with hydroxy methyl propyl cellulose serving as a binder. WEGO exhibited a 24.49% increase in supercapacitance compared to WEBC under identical electrochemical conditions, showcasing the capacitive behaviour of GO. Electrochemical impedance spectra (EIS) revealed stability of WE over 24 h, exhibiting potentiodynamic polarisation within the ranging -1.5 to 1.0 @ 0.005 V/s. This indicated a corrosion rate (mm/year) of WEGO @ 4.69 × 10–4, that was much reduced over WEBC (2.27 × 10–3) under equivalent conditions. Notably, GO displayed superior corrosion protection performance (4.69 × 10–4 mm/year) compared to bare electrodes during the potentiodynamic polarisation. This research introduces a sustainable approach to fabricating working electrodes using biochar-derived GO, resulting in enhanced electrochemical supercapacitance, stability, and corrosion resistance.
The schematic diagram of sustainable synthesis of GO from pine leaves for applications in energy storage and corrosion protection (Singhal et al. 2022)
Research conducted by Roy et al. (2014), GO was produced from Tasar silk cocoon through a process involving pyrolysis followed by treatment with conc. HNO3. The oxidised sample showed remarkable fluorescence, magnetic properties, and multi-photon imaging. Notably, an increase in the excitation wavelength corresponded to a rise in the fluorescence emission intensity of the GO, peaking at a wavelength of 380 nm. These observed magnetic properties and fluorescence were attributed to defects induced within the GO structure during oxidation, resulting in the incorporation of oxygen containing hydrophilic groups. Moreover, the GO displayed soft ferromagnetic behaviour along with single and two-photon fluorescence features, suggesting its potential applications in spintronics, magneto-optics, carbon-based magneto resistance, and magnetic memory devices. This research not only presents a novel synthesis approach, but also highlights the multifaceted properties for innovative applications.
The research by Kumar Sahoo et al. (2020) focused on synthesising GO from bio-shoot, specifically utilising biodiesel soot powder obtained from automobile. They utilised the Modified Hummers method to oxidise the extracted biodiesel soot and produce GO. The synthesised GO was then employed as an adsorbent for the effective removal of Congo red dye, a highly noxious substance, as illustrated in Fig. 18. Congo red (CR) dyes, known for their anionic properties, served as model organic pollutants to evaluate the absorption capabilities of GO. Studies were conducted on varying parameters such as adsorbent dosages, pH levels of the solution, contact time and concentration. Additionally, isotherm studies and kinetic were investigated to gain insights into adsorption process of CR. The result indicated that the adsorption process adhered to both the Langmuir isotherm model and pseudo-second-order kinetic. The GO surface exhibited a maximum adsorption capacity of 120.20 mg/g, a significant improvement compared to bio-soot, which was 70.23 mg/g. This highlights the superior adsorption capabilities of GO due to its increased amount of adsorption sites. The adsorption process of CR onto GO was observed to occur spontaneously and endothermic.
The schematic diagram of preparation of GO from bio-soot and its utilisation as an adsorbent (Kumar Sahoo et al. 2020)
In 2014, Akhavan and colleagues synthesised high-quality GO and rGO sheets using industrial carbonaceous and various natural residues as starting materials and investigated the electronic properties. Their findings revealed that the electrical resistance of both GO or rGO sheets remained consistent regardless of the starting materials utilised, with values of approximately 105 MΩ sq−1 for GO and 1 MΩ sq−1 for rGO sheets (Akhavan et al. 2014). The most recent work by Bonnia et al. (2021) and Anuar et al. (2023) synthesised GO from scrap tyres. Despite this achievement, the practical applications of this synthesised GO have not been implemented yet. However, Tatrari and colleagues (Tatrari et al. 2022) used synthesised zinc-doped graphene spheres to supercapacitor application. These spheres were synthesised on a large scale using a cost-effective modified pyrolysis method. Density Functional Theory (DFT) analysis revealed a significant increase in the quantum capacitance of the graphene spheres, approximately 5.5 times higher than pristine graphene, upon zinc incorporation. The electrochemical performance of the supercapacitor device based on these zinc-doped spheres exhibited exceptional power density and energy density.
In 2022, Hassanin et al. prepared silica/GO composite from environmental solid waste and applied it for U(VI) sorption (Fig. 19). The SiO2/GO adsorbent was derived from GO via the Hummers method using spent carbon rod batteries, while the silica component was sourced from rice husk. The study considered various factors influencing U(VI) sorption on SiO2/GO, such as sorption time, pH, SiO2/GO dosage, temperature, and concentration of U(VI) ions. The experimental data revealed that the U(VI) uptake capacity of SiO2/GO is 145.0 mg/g at a pH of 4.0. Kinetic analysis aligned well with the pseudo second order model, and the sorption isotherm exhibited consistency with the Langmuir model. The sorption processes were found to be spontaneous. random, and exothermic in nature. Moreover, SiO2/GO was effectively regenerated using 0.8 M H2SO4 and a 1:50 solid: liquid phase ratio after 60 min of agitation. Furthermore, seven cycles of sorption and elution confirmed the persistent effectiveness of SiO2/GO in U(VI) adsorption from aqueous sources. In a separate study, Loudiki et al. (2022) employed a novel optimised approach to synthesise GO from carbon rods extracted from used (ZnC) batteries for electrochemical applications. The electrochemical characteristics of the synthesised GO was assessed through cyclic voltammetry (CV) and current–voltage techniques. The findings indicate that sonication-based GO exhibits superior electrocatalytic properties compared to GO obtained through the standard Hummers method. Oxygen functionalities in GO allow for easy dispersal in organic solvents and water (Johnson et al. 2015). This unique property makes GO a valuable additive in ceramic and polymer manufacturing processes, enhancing their electrical and mechanical properties, including tensile strength, elasticity and conductivity (Sun et al. 2022; Han et al. 2015). Furthermore, GO and its composites offer diverse applications in the fields of energy storage/conversion (Wang et al. 2020) and environmental protection (Alammar et al. 2020). These include applications in hydrogen storage materials (Singh and De 2020), air pollutant removal (Joel and Lujanienė 2022), photocatalysis (Sharma et al. 2018) for water splitting and purification, and electrode materials (Wu et al. 2012) for various supercapacitors and lithium batteries. Additionally, GO finds utility in the oil and gas industries as an additive for drilling fluid (Kosynkin et al. 2012), cementing (Pan et al. 2015), enhanced oil recovery (Arenas-Blanco et al. 2021), desalination (Ali et al. 2016), oil spill clean-up (Alammar et al. 2020), and emulsion stabilization (He et al. 2013). It can also improve fluid loss control, lubrication, rheology, and shale stability (Neuberger et al. 2018). The current body of literature on the applications of waste-derived GO is limited. Therefore, there are still many ways to use waste-based GO materials that have yet to be tried, offering exciting opportunities for the future.
The schematic representation of (a) silica synthesis from rice husk, (b) GO synthesis from spent Zn-C batteries, (c) SiO2/GO synthesis, (d) U(VI) sorption at SiO2/GO (Hassanin et al. 2022)
Conclusions and Perspectives
This review has underscored the significance of innovative research in the synthesis of GO using diverse waste, including agricultural, industrial, and plastic, while also exploring their potential applications. An issue associated with graphite, especially highly pure graphite (HPG), as the main starting material of GO synthesis is its high cost. To overcome this challenge, it is worth considering the production of suitable and cost-effective graphitic materials derived from carbonaceous waste. Recent studies have proposed using agricultural residues such as coconut shells, sugarcane bagasse, orange peel, tea, oil palm and industrial wastes such as soot powders, scrap tyres and electronic scraps for GO synthesis. However, the choice of carbon precursor significantly influences key properties of the resulting GO. For instance, GO derived from sugarcane bagasse exhibits enhanced hydrophilicity and superior water dispersibility due to the abundance of hydrophilic functional groups. Yet, challenges arise from unwanted elements, impurities, and contaminants in waste materials, necessitating pre-treatment processes for high-quality GO production. The utilisation of industrial residues for GO synthesis introduces notable impurities like Zn, and S that must be addressed before considering them as a viable raw material. Conversely, sugarcane bagasse and bio soot emerge as promising agricultural and industrial precursor for GO synthesis, displaying favourable characteristics compared to other alternatives.
While most efforts have revolved carbonising biomass or carbonaceous waste and utilising the Modified Hummers method for GO synthesis, only a few researchers have used the alternative routes. For instance, Optimal techniques, such as the SOMA-GO and direct ferric citrate pyrolysis have been used for synthesising GO from sugarcane bagasse. A comparative analysis has revealed that the Modified Hummers method exhibits significant limitations, including long synthesis time, complex separation and purification steps, high toxicity, limited environmental friendliness, excessive oxidant use, and scalability issues. In contrast, the pyrolysis and catalytic processes are straightforward, rapid, reliable, efficient, cost-effective, and eco-friendly alternatives. However, there needs to be more exploration of GO synthesis utilising exclusive pyrolysis techniques, especially microwave assisted. Microwave-assisted pyrolysis offers distinct advantages, including enhanced efficiency, rapidity, simplicity, and cost-effectiveness. This approach represents a promising avenue for advancing GO synthesis methods, emphasizing the need for further exploration.
The practical utility of these materials remains somewhat limited compared to conventional GO and are mainly focused on water purification and biomedical. However, the widespread application of GO derived from waste materials still needs to be improved, leaving opportunities for further exploration in areas such as composite material, solar cells, cementing and desalination. It is crucial to standardise the characteristics and sources of the carbon precursors, as well as the methods used, to produce GO with optimal properties while maintaining eco-friendliness. Reproducing waste materials into GO is a critical challenge, requiring a comprehensive optimisation of method variables and techniques to control the quality, size, and morphology of produced GO. Integrating improved morphological properties into scalable and affordable procedures is essential for advancing the field. While research has explored waste precursors, none have transitioned into commercially viable products. Future investigations are anticipated to focus on developing sustainable and scalable methods for the mass production of GO. To secure sustainable production, it is imperative to select starting materials and processes based on life cycle assessments and economic analyses. This might produce more waste-based GO and uncover novel applications in emerging technologies.
Data Availability
Data will be made available on request.
References
Akhavan O, Ghaderi E (2009) Photocatalytic Reduction of Graphene Oxide Nanosheets on TiO2 Thin Film for Photoinactivation of Bacteria in Solar Light Irradiation. The J Phys Chem C 113:20214–20220
Akhavan O, Bijanzad K, Mirsepah A (2014) Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv 4:20441–20448
Alammar A, Park S-H, Williams CJ, Derby B, Szekely G (2020) Oil-in-water separation with graphene-based nanocomposite membranes for produced water treatment. J Membr Sci 603:118007
Ali MEA, Wang L, Wang X, Feng X (2016) Thin film composite membranes embedded with graphene oxide for water desalination. Desalination 386:67–76
Ambrosi Adriano, Pumera Martin (2016) Electrochemically Exfoliated Graphene and Graphene Oxide for Energy Storage and Electrochemistry Applications. Chem - A Eur J 22:153–59
Amir Faiz MS, Che Azurahanim CA, Yazid Y, Suriani AB, Siti Nurul Ain MJ (2020) Preparation and characterization of graphene oxide from tea waste and it’s photocatalytic application of TiO2/graphene nanocomposite. Mater Res Express 7:015613
Anuar, AU, Bonnia NN, Jamil NM, Affandi NDN (2023) Graphene oxide based regenerated carbon waste tyre (rCB): Synthesis by modified Hummers method and characterization, Materials Today: Proceedings. https://doi.org/10.1016/j.matpr.2023.02.280
Anwar A, Chang T-P, Chen C-T (2022) Graphene oxide synthesis using a top–down approach and discrete characterization techniques: a holistic review. Carbon Letters 32:1–38
Arenas-Blanco BA, Pérez-Rodríguez EM, Hernández RC, Santos-Santos N, Mejía-Ospino E (2021) Sulfonated Graphene Oxide Nanofluid: Potential Applications for Enhanced Oil Recovery. Energy Fuels 35:20071–20078
Arora B, Attri P (2020) Carbon Nanotubes (CNTs): A Potential Nanomaterial for Water Purification. J Composites Sci 4:135
Asif, FC, Saha GC (2023) Graphene-like Carbon Structure Synthesis from Biomass Pyrolysis: A Critical Review on Feedstock -;Process -;Properties Relationship, C, 9: 31. https://doi.org/10.3390/c9010031
Bazaka K, Jacob MV, Ostrikov K (2016) Sustainable Life Cycles of Natural-Precursor-Derived Nanocarbons. Chem Rev 116:163–214
Bhuyan Md SA, Nizam Md U, Islam Md M, Bipasha FA, Hossain SS (2016) Synthesis of graphene. Int Nano Lett 6:65–83
Bledzki AK, Mamun AA, Volk J (2010) Barley husk and coconut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Compos Sci Technol 70:840–846
Boehm HP, Setton R, Stumpp E (1986) Nomenclature and terminology of graphite intercalation compounds. Carbon 24:241–245
Bonaccorso F, Sun Z, Hasan T, Ferrari AC (2010) Graphene photonics and optoelectronics. Nat Photonics 4:611–622
Bonnia, NN, Ain ZZ, Mohd MM, Engku ZEZ (2021) Graphene Oxide from Recycle Carbon of Waste Tyre Using Modified Hummer’s Method. In Recent Trends in Manufacturing and Materials Towards Industry 4.0: Selected Articles from iM3F 2020, Malaysia, 171–78. Springer. https://doi.org/10.1007/978-981-15-9505-9_17
Brodie BC (1859) On the Atomic Weight of Graphite. Philosophical Trans Royal Soc London Series I 149:249–259
Challa AA, Saha N, Szewczyk PK, Karbowniczek JE, Stachewicz U, Ngwabebhoh FA, Saha P (2023) Graphene oxide produced from spent coffee grounds in electrospun cellulose acetate scaffolds for tissue engineering applications. Mater Today Commun 35:105974
Chen Da, Feng H, Li J (2012) Graphene Oxide: Preparation, Functionalization, and Electrochemical Applications. Chem Rev 112:6027–6053
Chung C, Kim Y-K, Shin D, Ryoo S-R, Hong BH, Min D-H (2013) Biomedical Applications of Graphene and Graphene Oxide. Acc Chem Res 46:2211–2224
Compton OC, Nguyen ST (2010) Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 6:711–723
Dimiev AM, Alemany LB, Tour JM (2013) ’Graphene Oxide Origin of Acidity, Its Instability in Water, and a New Dynamic Structural Model. ACS Nano 7:576–588
Disa Md, Nurhafizah SA, Bakar SA, Mohamed A, Isa IM, Kamari A, Hashim N, Aziz AA, Mahmood MR (2015) The Synthesis of Graphene Oxide via Electrochemical Exfoliation Method. Advanced Materials Research 1109:55–59
Dreyer DR, Park S, Bielawski CW, Ruoff RS (2010) The chemistry of graphene oxide. Chem Soc Rev 39:228–240
Du Z, Li Y, Wang X, Wan Y, Chen Q, Wang C, Lin X, Liu Y, Chen P, Ruan R (2011) Microwave-assisted pyrolysis of microalgae for biofuel production. Biores Technol 102:4890–4896
El Essawy NA, Ali SM, Farag HA, Konsowa AH, Elnouby M, Hamad HA (2017) Green synthesis of graphene from recycled PET bottle wastes for use in the adsorption of dyes in aqueous solution. Ecotoxicol Environ Saf 145:57–68
Faghiri F, Ghorbani F (2020) Synthesis of graphene oxide nanosheets from sugar beet bagasse and its application for colorimetric and naked eye detection of trace Hg2+ in the environmental water samples. Microchem J 152:104332
Fang M, Wang K, Hongbin Lu, Yang Y, Nutt S (2009) Covalent polymer functionalization of graphene nanosheets and mechanical properties of composites. J Mater Chem 19:7098–7105
Fathy M, Hosny R, Keshawy M, Gaffer A (2019) Green synthesis of graphene oxide from oil palm leaves as novel adsorbent for removal of Cu(II) ions from synthetic wastewater. Graphene Technol 4:33–40
Filho V, Batistella L, Alves JL, da Silva J, Althoff C, Moreira R, José H (2019) Evaluation of Gaseous Emissions from Thermal Conversion of a Mixture of Solid Municipal Waste and Wood Chips in a Pilot-Scale Heat Generator. Renewable Energy 141:402–410
Fodah A, Ghosal M, Behera D (2021) Microwave-assisted pyrolysis of agricultural residues: current scenario, challenges, and future direction. Int J Environ Sci Technol 19
Fu C, Zhao G, Zhang H, Li S (2013) Evaluation and Characterization of Reduced Graphene Oxide Nanosheets as Anode Materials for Lithium-Ion Batteries. Int J Electrochem Sci 8:6269–6280
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6:183–191
Goswami S, Banerjee P, Datta S, Mukhopadhayay A, Das P (2017) Graphene oxide nanoplatelets synthesized with carbonized agro-waste biomass as green precursor and its application for the treatment of dye rich wastewater. Process Saf Environ Prot 106:163–172
Grace AS, Prabha Littis Malar GS (2020) Synthesis and characterization of graphene oxide from coconut husk ash. Orient J Chem 36:348
Gui D, Liu C, Chen F, Liu J (2014) Preparation of polyaniline/graphene oxide nanocomposite for the application of supercapacitor. Appl Surf Sci 307:172–177
Gutiérrez-Cruz A, Ruiz-Hernández AR, Vega-Clemente JF, Luna-Gazcón DG, Campos-Delgado J (2022) A review of top-down and bottom-up synthesis methods for the production of graphene, graphene oxide and reduced graphene oxide. J Mater Sci 57:14543–14578
Hai ND, Dat NM, Nam NTH, An H, Tai LT, Huong LM, Cong CQ, Giang NTH, Tinh NT, Hieu NH (2023) A review on the chemical and biological synthesis of silver nanoparticles@graphene oxide nanocomposites: A comparison. Materials Today Sustainability 24:100544
Han W, Zhao G, Zhang X, Zhou S, Wang P, An Y, Baosheng Xu (2015) Graphene oxide grafted carbon fiber reinforced siliconborocarbonitride ceramics with enhanced thermal stability. Carbon 95:157–165
Hashmi A, Singh A, Jain B, Singh A (2020) Muffle atmosphere promoted fabrication of graphene oxide nanoparticle by agricultural waste. Fullerenes, Nanotubes, Carbon Nanostruct 28:1–10
Hassanin MA, Negm SH, Youssef MA, Sakr AK, Mira HI, Mohammaden TF, Al-Otaibi JS, Hanfi MY, Sayyed MI, Cheira MF (2022) Sustainable Remedy Waste to Generate SiO2 Functionalized on Graphene Oxide for Removal of U(VI) Ions. Sustainability 14:2699
He Y, Fei Wu, Sun X, Li R, Guo Y, Chuanbao Li Lu, Zhang FX, Wang W, Gao J (2013) Factors that Affect Pickering Emulsions Stabilized by Graphene Oxide. ACS Appl Mater Interfaces 5:4843–4855
Hofmann U, König E (1937) Untersuchungen über Graphitoxyd. Z Anorg Allg Chem 234:311–336
Hummers WS Jr, Offeman RE (1958) Preparation of Graphitic Oxide. J Am Chem Soc 80:1339–1439
Ibrahim A, Klopocinska A, Horvat K, Abdel Hamid Z (2021) Graphene-based nanocomposites: synthesis, mechanical properties, and characterizations Polymers 13(17):2869
Jia H-P, Dreyer DR, Bielawski CW (2011) C–H oxidation using graphite oxide. Tetrahedron 67:4431–4434
Jiang Z, Feng Bo, Jin Xu, Qing T, Zhang P, Qing Z (2020) Graphene biosensors for bacterial and viral pathogens. Biosens Bioelectron 166:112471
Jiao K, Wang X, Wang Y, Chen Y (2014) Graphene oxide as an effective interfacial layer for enhanced graphene/silicon solar cell performance. J Mater Chem C 2:7715–7721
Joel EF, Lujanienė G (2022) Progress in Graphene Oxide Hybrids for Environmental Applications. Environments 9:153
Johnson DW, Dobson BP, Coleman KS (2015) A manufacturing perspective on graphene dispersions. Curr Opin Colloid Interface Sci 20:367–382
Kakaei K, Hasanpour K (2014) Synthesis of graphene oxide nanosheets by electrochemical exfoliation of graphite in cetyltrimethylammonium bromide and its application for oxygen reduction. J Mater Chem A 2:15428–15436
Karim N, Afroj S, Tan S, He P, Fernando A, Carr C, Novoselov KS (2017) Scalable Production of Graphene-Based Wearable E-Textiles. ACS Nano 11:12266–12275
Kim T, Jo C, Lim W-G, Lee J, Lee J, Lee K-H (2016) Facile conversion of activated carbon to battery anode material using microwave graphitization. Carbon 104:106–111
Klechikov A, Junchun Yu, Thomas D, Sharifi T, Talyzin AV (2015) Structure of graphene oxide membranes in solvents and solutions. Nanoscale 7:15374–15384
Kong X, Zhu Y, Lei H, Wang C, Zhao Y, Huo E, Lin X, Zhang Q, Qian M, Mateo W (2020) Synthesis of graphene-like carbon from biomass pyrolysis and its applications. Chem Eng J 399:125808
Kosynkin DV, Ceriotti G, Wilson KC, Lomeda JR, Scorsone JT, Patel AD, Friedheim JE, Tour JM (2012) Graphene oxide as a high-performance fluid-loss-control additive in water-based drilling fluids. ACS Appl Mater Interfaces 4:222–227
Kumar V, Kumar A, Lee D-J, Park S-S (2021) Estimation of Number of Graphene Layers Using Different Methods: A Focused Review. Materials 14:4590
Lavin-Lopez MDP, Romero A, Garrido J, Sánchez-Silva L, Valverde JL (2016) Influence of different improved hummers method modifications on the characteristics of graphite oxide in order to make a more easily scalable method. Ind Eng Chem Res 55(50):12836–12847
Li X-H, Kurasch S, Kaiser U, Antonietti M (2012) Synthesis of Monolayer-Patched Graphene from Glucose. Angew Chem Int Ed 51:9689–9692
Li Q, Fan F, Wang Y, Feng W, Ji P (2013) Enzyme Immobilization on Carboxyl-Functionalized Graphene Oxide for Catalysis in Organic Solvent. Ind Eng Chem Res 52:6343–6348
Li F, Jiang X, Zhao J, Zhang S (2015) Graphene oxide: A promising nanomaterial for energy and environmental applications. Nano Energy 16:488–515
Li Y, Zhang H, Porwal H, Huang Z, Bilotti E, Peijs T (2017) Mechanical, electrical and thermal properties of in-situ exfoliated graphene/epoxy nanocomposites. Compos A Appl Sci Manuf 95:229–236
Lingappan N, Gal YS, Lim KT (2013) Synthesis of reduced graphene oxide/polypyrrole conductive composites. Mol Cryst Liq Cryst 585(1):60–66
Liu G, Wang L, Wang Bo, Gao T, Wang D (2015a) A reduced graphene oxide modified metallic cobalt composite with superior electrochemical performance for supercapacitors. RSC Adv 5:63553–63560
Liu W-J, Jiang H, Han-Qing Yu (2015b) Development of biochar-based functional materials: toward a sustainable platform carbon material. Chem Rev 115:12251–12285
Liu, Fei, Chaojun Wang, Xiao Sui, Adil Riaz, Meiying Xu, Li Wei, and Yuan Chen. 2019. 'Synthesis of graphene materials by electrochemical exfoliation: Recent progress and future potential', Carbon Energy, 1. https://doi.org/10.1002/cey2.14
Long C, Chen X, Jiang L, Zhi L, Fan Z (2015) Porous layer-stacking carbon derived from in-built template in biomass for high volumetric performance supercapacitors. Nano Energy 12:141–151
Loudiki A, Matrouf M, Azriouil M, Laghrib F, Farahi A, Bakasse M, Lahrich S, El Mhammedi MA (2022) Graphene oxide synthesized from zinc-carbon battery waste using a new oxidation process assisted sonication: Electrochemical properties. Mater Chem Phys 275:125308
Mallakpour S, Behranvand V (2016) Manufacture and characterization of nanocomposite materials obtained from incorporation of d-glucose functionalized MWCNTs into the recycled poly(ethylene terephthalate). Des Monomers Polym 19:283–289
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Wei Lu, Tour JM (2010) Improved Synthesis of Graphene Oxide. ACS Nano 4:4806–4814
Mbayachi VB, Ndayiragije E, Sammani T, Taj S, Mbuta ER, Ullah Khan A (2021) Graphene synthesis, characterization and its applications: A review. Results in Chemistry 3:100163
Mendoza-Carrasco R, Cuerda-Correa EM, Alexandre-Franco MF, Fernández-González C, Gómez-Serrano V (2016) Preparation of high-quality activated carbon from polyethyleneterephthalate (PET) bottle waste Its Use in the Removal of Pollutants in Aqueous Solution. J Environ Manag 181:522–535
Mishra S, Goje AS, Zope VS (2003) Chemical Recycling, Kinetics, and Thermodynamics of Poly (Ethylene Terephthalate) (PET) Waste Powder by Nitric Acid Hydrolysis. Polym React Eng 11:79–99
Mohan ANMB, B M, Panicker S (2019) Facile synthesis of graphene-tin oxide nanocomposite derived from agricultural waste for enhanced antibacterial activity against Pseudomonas aeruginosa. Sci Rep 9:4170
Nakagawa K, Namba A, Mukai SR, Tamon H, Ariyadejwanich P, Tanthapanichakoon W (2004) Adsorption of phenol and reactive dye from aqueous solution on activated carbons derived from solid wastes. Water Res 38:1791–1798
Nasir S, Hussein MZ, Yusof NA, Zainal Z (2017) Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterials 7:182
Neuberger N, Adidharma H, Fan M (2018) Graphene: A review of applications in the petroleum industry. J Petrol Sci Eng 167:152–159
Nováček M, Jankovský O, Luxa J, Sedmidubský D, Pumera M, Fila V, Lhotka M, Klímová K, Matějková S, Sofer Z (2017) Tuning of graphene oxide composition by multiple oxidations for carbon dioxide storage and capture of toxic metals. J Mater Chem A 5:2739–2748
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric Field Effect in Atomically Thin Carbon Films. Science 306:666–669
Padmajan Sasikala S, Lim J, Kim IH, Jung HJ, Yun T, Han TH, Kim SO (2018) Graphene oxide liquid crystals: A frontier 2D soft material for graphene-based functional materials. Chem Soc Rev 47:6013–6045
Pan Z, He Li, Qiu L, Korayem AH, Li G, Zhu JW, Collins F, Li D, Duan WH, Wang MC (2015) Mechanical properties and microstructure of a graphene oxide–cement composite. Cement Concr Compos 58:140–147
Park S, An J, Potts JR, Velamakanni A, Murali S, Ruoff RS (2011) Hydrazine-reduction of graphite- and graphene oxide. Carbon 49:3019–3023
Paulchamy B, Arthi G, Lignesh BD (2015) A simple approach to stepwise synthesis of graphene oxide nanomaterial. J Nanomed Nanotechnol 6:1
Quaschning, Volker (2016) Understanding renewable energy systems (Routledge). https://doi.org/10.4324/9781315769431
Ren PG, Yan DX, Ji X, Chen T, Li ZM (2011) Temperature dependence of graphene oxide reduced by hydrazine hydrate. Nanotechnology 22:055705
Rivero PJ, Garcia JA, Quintana I, Rodriguez R (2018) Design of Nanostructured Functional Coatings by Using Wet-Chemistry Methods. Coatings 8:76
Roy M, Kusurkar TS, Maurya SK, Kumar, Meena SK, Singh SK, Sethy N, Bhargava K, Sharma RK, Goswami D, Sarkar S, Das M (2014) Grapheneoxide from silk cocoon: a novel magnetic fluorophore for multi-photon imaging. 3 Biotech 4:67–75
Rümmeli MH, Rocha CG, Ortmann F, Ibrahim I, Sevincli H, Börrnert F, Kunstmann J, Bachmatiuk A, Pötschke M, Shiraishi M, Meyyappan M, Büchner B, Roche S, Cuniberti G (2011) Graphene: Piecing it together. Adv Mater 23:4471–4490
Safarpour, Mahdie, and Alireza Khataee (2019) 'Chapter 15 - Graphene-Based Materials for Water Purification.' in Sabu Thomas, Daniel Pasquini, Shao-Yuan Leu and Deepu A. Gopakumar (eds.), Nanoscale Materials in Water Purification (Elsevier). https://doi.org/10.1016/B978-0-12-813926-4.00021-5
Saha B, Ghoshal AK (2005) Thermal degradation kinetics of poly(ethylene terephthalate) from waste soft drinks bottles. Chem Eng J 111:39–43
Sahoo K, Shraban JK, Sahoo GK, Panigrahi DK, Pattanayak AS, Rout AS, Lenka A (2020) Preparation of graphene oxide from Bio-soot wastes: As an efficient adsorbent for highly noxious Congo red dye. FlatChem 24:100198
Saithanu T, Suksri A (2018) Coconut Shell Powder and Electrical Tree Inhibition of Solid Insulator. Key Eng Mater 775:89–93
Sajibul BM, Nizam AM, Maksudul UM, Islam FA, Bipasha A, Hossain SS (2016) “Synthesis of graphene”, International. Nano Lett 6:65–83
Sarhan H-A, El-Sakhawy M, Kamel S (2022) Development of magnetite/graphene oxide hydrogels from agricultural wastes for water treatment. J Renewable Materials 10:1–21
Shahriary L, Athawale AA (2014) Graphene oxide synthesized by using modified hummers approach. Int J Renew Energy Environ Eng 2:58–63
Sharma M, Behl K, Nigam S, Joshi M (2018) TiO2-GO nanocomposite for photocatalysis and environmental applications: A green synthesis approach. Vacuum 156:434–439
Shin DS, Kim HG, Ahn HS, Jeong HY, Kim Y-J, Dorj Odkhuu N, Tsogbadrakh H-B-R, Kim BH (2017) Distribution of oxygen functional groups of graphene oxide obtained from low-temperature atomic layer deposition of titanium oxide. RSC Adv 7:13979–13984
Siaw WC, Tsuji T, Abdul Manaf N, Abdul Patah MF, Mohamed B (2020) Synthesis of graphene oxide from industrial waste. IOP Conference Series: Materials Science and Engineering 778:012050
Sim LC, Leong KH, Ibrahim S, Saravanan P (2014) Graphene oxide and Ag engulfed TiO2 nanotube arrays for enhanced electron mobility and visible-light-driven photocatalytic performance. Journal of Materials Chemistry A 2:5315–5322
Singh SB, De M (2020) Thermally exfoliated graphene oxide for hydrogen storage. Mater Chem Phys 239:122102
Singhal K, Mehtab S, Pandey M, Zaidi MGH (2022) Sustainable development of graphene oxide from pine leaves for electrochemical energy storage and corrosion protection. Current Research in Green and Sustainable Chemistry 5:100266
Soltani T, Lee BK (2017) Low intensity-ultrasonic irradiation for highly efficient, eco-friendly and fast synthesis of graphene oxide. Ultrason Sonochem 38:693–703
Somanathan T, Prasad K, Ostrikov KK, Saravanan A, Krishna VM (2015) Graphene Oxide Synthesis from Agro Waste. Nanomaterials (basel) 5:826–834
Stanford MG, Zhang C, Fowlkes JD, Hoffman A, Ivanov IN, Rack PD, Tour JM (2020) ’High-Resolution Laser-Induced Graphene Flexible Electronics beyond the Visible Limit. ACS Appl Mater Interfaces 12:10902–10907
Staudenmaier L (1898) Verfahren zur Darstellung der Graphitsäure. Ber Dtsch Chem Ges 31:1481–1487
Sujiono, Eko Hadi, D Zabrian, MY Dahlan, BD Amin, J Agus (2020) Graphene oxide based coconut shell waste: synthesis by modified Hummers method and characterization, Heliyon, 6. https://doi.org/10.1016/j.heliyon.2020.e04568
Sun L (2019) Structure and synthesis of graphene oxide. Chin J Chem Eng 27:2251–2260
Sun X, Liu Z, Welsher K, Robinson JT, Goodwin A, Zaric S, Dai H (2008) Nano-graphene oxide for cellular imaging and drug delivery. Nano Res 1:203–212
Sun X, Wang W, Sun G, Jianqi Chen Lu, Wang L, Bi J (2022) Enhancement of the mechanical properties of nacre-like alumina ceramic by the synergism of graphene oxide and Si3N4 whisker. Ceram Int 48:941–946
Syed N, Sharma N, Kumar L (2017) Synthesis of Graphene Oxide (GO) by Modified Hummers Method and Its Thermal Reduction to Obtain Reduced Graphene Oxide (rGO) * Open Access. Graphene 6:1–18
Tamilselvi R, Ramesh M, Lekshmi GS, Bazaka O, Levchenko I, Bazaka K, Mandhakini M (2020) Graphene oxide – Based supercapacitors from agricultural wastes: A step to mass production of highly efficient electrodes for electrical transportation systems. Renewable Energy 151:731–739
Tatrari G, Tewari C, Pathak M, Karakoti M, Bohra BS, Pandey S, SanthiBhushan B, Srivastava A, Rana S, Sahoo NG (2022) Bulk production of zinc doped reduced graphene oxide from tire waste for supercapacitor application: Computation and experimental analysis. J Energy Storage 53:105098
Thangaraj B, Mumtaz F, Abbas Y, Anjum DH, Solomon PR, Hassan J (2023) Synthesis of Graphene Oxide from Sugarcane Dry Leaves by Two-Stage Pyrolysis. Molecules 28:3329
Tohamy H-A, Anis B, Youssef MA, Abdallah AE, El-Sakhawy M, Kamel S (2020a) Preparation of eco-friendly graphene oxide from agricultural wastes for water treatment. Desalin Water Treat 191:250–262
Tohamy H-AS, Kamel S, El-Sakhawy M, Youssef MA, Abdallah AE, Anis B (2020b) Thermal properties of graphene oxide prepared from different agricultural wastes. Egyptian J Chem 63(10):3619–3629. https://doi.org/10.21608/ejchem.2020.22915.2375
Tohamy H-AS, El-Sakhawy M, Kamel S (2022) Development of magnetite/graphene oxide hydrogels from agricultural wastes for water treatment. J Renewable Mater 10:1–21
Torres FG, Troncoso OP, Rodriguez L, De-la-Torre GE (2021) Sustainable synthesis, reduction and applications of graphene obtained from renewable resources. Sustain Mater Technol 29:e00310
Tour JM (2014) Top-Down versus Bottom-Up Fabrication of Graphene-Based Electronics. Chem Mater 26:163–171
Wachid FM, Perkasa AY, Prasetya FA, Rosyidah N, Darminto (2014) Synthesis and characterization of nanocrystalline graphite from coconut shell with heating process. AIP Conf Proc 1586:202–206
Wang Bo, Ruan T, Chen Y, Jin F, Li Peng Yu, Zhou DW, Dou S (2020) Graphene-based composites for electrochemical energy storage. Energy Storage Materials 24:22–51
Wu Z-S, Zhou G, Yin L-C, Ren W, Li F, Cheng H-M (2012) Graphene/metal oxide composite electrode materials for energy storage. Nano Energy 1:107–131
Yang D, Velamakanni A, Bozoklu G, Park S, Stoller M, Piner RD, Stankovich S, Jung I, Field DA, Ventrice CA, Ruoff RS (2009) Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and Micro-Raman spectroscopy. Carbon 47:145–152
Yang Q, Geng Y, Dong H, Zhang J, Xiaoman Yu, Sun Lu, Xiaorong Lu, Chen Y (2017) Effect of environmental regulations on China’s graphite export. J Clean Prod 161:327–334
Yang H, Yeow BS, Chang T-H, Li K, Fanfan Fu, Ren H, Chen P-Y (2019) Graphene Oxide-Enabled Synthesis of Metal Oxide Origamis for Soft Robotics. ACS Nano 13:5410–5420
Yap YW, Mahmed N, Norizan MN, Rahim SZA, Salimi MNA, Razak KA, Mohamad IS, Abdullah M-B, Yunus MYM (2023) Recent Advances in Synthesis of Graphite from Agricultural Bio-Waste Material: A Review. Materials 16:3601
Yu H, Zhang B, Bulin C, Li R, Xing R (2016) High-efficient synthesis of graphene oxide based on improved hummers method. Sci Rep 6:36143
Zaaba NI, Foo KL, Hashim U, Tan SJ, Liu W-W, Voon CH (2017) Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Engineering 184:469–477
Zaid O, Hashmi SRZ, Aslam F, Abedin ZU, Ullah A (2022) Experimental study on the properties improvement of hybrid graphene oxide fiber-reinforced composite concrete. Diam Relat Mater 124:108883
Zhang Z, Schniepp HC, Adamson DH (2019) Characterization of graphene oxide: Variations in reported approaches. Carbon 154:510–521
Zheng Q, Li Z, Yang J, Kim J-K (2014) Graphene oxide-based transparent conductive films. Prog Mater Sci 64:200–247
Zheng X, Zhai R, Zhang Z, Zhang B, Liu J, Razaq A, Ahmad MA, Raza R, Saleem M, Rizwan S, Jafri SHM, Li H, Papadakis R (2022) Graphene-oxide-based fluoro- and chromo-genic materials and their applications. Molecules 27(6):2018
Acknowledgements
This research received support from James Cook University Postgraduate Research Scholarship (JCUPRS).
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions Open access funding provided by James Cook University, Townsville, Australia.
Author information
Authors and Affiliations
Contributions
WCHS: investigation, conceptualized, designed, and performed the study, writing—original draft preparation. RT and MVJ: work supervision, conceptualized, the guidance of paper framework, data validation, writing—review, and editing. MAZ and SA: data validation, writing- review, and editing. All authors read, edited, and approved the final draft.
Corresponding author
Ethics declarations
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Silva, W.C.H., Zafar, M.A., Allende, S. et al. Sustainable Synthesis of Graphene Oxide from Waste Sources: A Comprehensive Review of Methods and Applications. Mater Circ Econ 6, 23 (2024). https://doi.org/10.1007/s42824-024-00117-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42824-024-00117-w