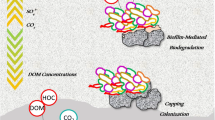
Abstract
Biochar is widely used for sediment remediation owing to its excellent adsorption properties and low carbon footprint. However, the impacts of biochar capping on phosphorus (P) bioavailability and mobility in the sediment are little known. In this study, the P mobilization processes in sediments capped with biochar were investigated by combining advanced high-resolution sampling techniques and microbiome analysis. The results showed that biochar is a double-edged sword for the sediment P release, depending on the application dosage and the capping time. In the short term (30 days), 2-cm biochar capping decreased the release flux of soluble reactive phosphorus (SRP) by 73.1%, whereas the 1-cm biochar capping significantly increased the release flux of SRP by 51.0%. After aging of biochar (80 days), the resupply capacity of sediment P was improved, resulting in increases of more than 33.7% and 121.5% in the release fluxes of SRP in the 1-cm and 2-cm capping groups, respectively, compared to the control group. Chemisorption played a pivotal role in regulating the levels of SRP, particularly during the short-term capping period. And more biochar can provide more adsorption sites on P. The P mobilization increase could be attributed to P desorption from biochar after biochar aging. Furthermore, biochar capping intensified the microbial-mediated iron reduction and organic matter decomposition, which enhanced P mobility. Our study highlights the importance of biochar application dosage and the capping time in sediment remediation, providing a scientific basis for the optimization of biochar capping techniques.
Graphical Abstract

Highlights
-
Biochar capping caused a sudden increase in organic matter and P concentration.
-
Aging biochar enhanced the bioavailability and release flux of P.
-
Biochar capping enhanced the P mobility by altering the microbial community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphorus (P) is an important environmental factor driving the steady-state transformation of aquatic ecosystems and is a significant limiting factor for water eutrophication (Wu et al. 2022). The primary sources of P in water encompass external input, internal release of sediment, and a small amount of atmospheric deposition. Some management and engineering measures have effectively controlled the input of external P. However, water eutrophication remains unresolved, which can mainly be caused by the internal P release (Li et al. 2023c). Studies have suggested that persistent P release from sediments can delay lake recovery by approximately 10 years (Søndergaard et al. 2012). Therefore, it makes sense to find a way to reduce internal P releases.
In situ capping is regarded as an effective remediation strategy to control water eutrophication due to its relatively modest cost and considerable remediation effectiveness (Pan et al. 2012; Sun et al. 2023). Biochar is produced through the thermal decomposition of biomass in the oxygen (O2)-restricted condition, and is regarded as an outstanding in situ capping material (Abel and Akkanen 2018; Zhu et al. 2019a). With its high porosity, numerous functional groups, and adsorption sites, biochar can immobilize contaminants via processes such as physical adsorption and redox reactions (Silvani et al. 2017; Zhao et al. 2022). A mount of research has found that biochar capping can effectively reduce the release of nitrogen from sediments using physical barriers, chemical adsorption and microbial transformation (Zhu et al. 2019a, b). Additionally, biochar has successfully remediated contaminated sediments by reducing the mobility of various pollutants (Yang et al. 2021b), such as organic compounds (Abel and Akkanen 2018), heavy metals and metalloids (Qian et al. 2022). Although biochar is often regarded as ‘black gold’ in ecological remediation engineering (Tan and Yu 2024), research on the use of biochar capping for sediment P remediation has only just been conducted (Gao et al. 2021). The effect of biochar capping on the bioavailability and mobility of P in sediments is not yet known.
Recently, studies have reported that biochar application can improve P availability in soils (Yang et al. 2021a). The water-soluble P content of most soils increased by 11–253% when biochar was added at 1% (w/w) (Parvage et al. 2012). Biochar has a high P content of 15–11,600 mg kg−1 due to the loss of volume of the biomass by 60–90% via the high-temperature carbonization process (Parvage et al. 2012; Sun et al. 2018). Therefore, it can act as a source of available P. In addition, biochar-derived dissolved organic matter (BDOM), a highly reactive product, plays an important role in the transport and transformation of P (Qi et al. 2023). BDOM may affect P mobilization in two ways. First, BDOM has a lot of reactive groups, such as ROH and RCOOH, which can replace adsorbed P on minerals (Schneider and Haderlein 2016). Second, BDOM can drive the reduction of Fe oxide as an electron shuttle, subsequently releasing P (Yang et al. 2021a). Consequently, we hypothesize that biochar addition may accelerate the P desorption from the sediment solid phase, which ultimately leads to internal P release, thus exacerbating water eutrophication. It is therefore crucial to accurately assess the impact of biochar capping on sediment P release.
Traditional chemical extraction procedures cannot provide comprehensive information about the P adsorption and desorption in sediments (Li et al. 2021, 2023b). Moreover, conventional sediment porewater sampling methods, sediment core slicing, centrifugation and subsequent analysis are not only generally limited in their spatial resolution, but also may change the analyte species in the porewater (Li et al. 2022b; Pedersen et al. 2015). Recently, multiple high-resolution sampling techniques have emerged that can compensate for these shortcomings, including diffusive gradients in thin films (DGT) (Davison and Zhang 1994), and high-resolution dialysis sampler (HR-Peeper) (Chen et al. 2022). The resupply kinetics of solutes can be characterized by combining these high-resolution sampling techniques (Zhang et al. 2020a). Therefore, fine-scale measurement of P mobilization processes can be achieved using multiple high-resolution sampling techniques.
Accordingly, this study aimed to assess how biochar remediation affects the bioavailability and mobility of P in sediments using high-resolution sampling techniques and microbiome analysis methods. Our findings provide a more comprehensive insight into the influence of biochar capping on the bioavailability and mobility of P in sediments, thus providing a scientific basis for the optimization of sediment restoration techniques.
2 Materials and methods
2.1 Incubation experiment setup
The water and sediment were sampled from an area of eutrophication in Lake Taihu (Fig. S1, Tables S1 and S2) (Duan et al. 2023). The sediment was sifted through a 0.6 mm mesh to remove macrofauna and large particles, and subsequently mixed evenly. The water sample was filtered through a diaphragm vacuum pump (0.45 µm pore size). The homogenized sediments were transferred into 21 Plexiglas tubes (11 cm in diameter and 50 cm in height) and 3 transparent rhizoboxes (height × length × width: 30 cm × l10 cm × 50 cm) with detachable front windows (Fig. 1). The height of sediment was set at 25 cm in the Plexiglas tubes, with overlying water added to reach 20 cm above the interface between water and sediment (SWI). The sediment height in the rhizoboxes was 15 cm and overlying water was added at 10 cm SWI. Both the tubes and the rhizoboxes were precultured at 25 °C for 2 weeks before the addition of biochar (Fig. 1).
Biochar (Runkelvyuan Biological Engineering Co., Ltd, Shandong, China) produced from fruit tree branch was applied in this experiment, which was obtained at 420 °C for a duration of 1 h in a muffle furnace with limited oxygen conditions. Biochar was processed to pass through 80-mesh sieves before use. The detailed parameters of the biochar in this study are provided in Table S3. The sediment cores were treated in three ways: the control group (no biochar), 1-cm biochar capping group and 2-cm biochar capping group, respectively. Using the field capping restoration approach, pre-treated biochar was sprayed into the water column and settled into the sediment, forming a capping layer of approximately 1 or 2 cm (n = 9, respectively). All sediment tubes were cultured for 80 days at 25 °C and overlying water was also added continuously to maintain the 20 cm height during incubation. The sediment samples used to determine the P fractions and for DNA extraction were collected on day 80.
2.2 Measurement of the overlying water samples
A total of 17 days of overlying water physicochemical measurements, including SRP, UV254, dissolved oxygen (DO), and pH were conducted in Plexiglas tubes during the 80-day experiment. For the SRP and UV254, the water samples (approximately 1 mL) were obtained by the injector and subsequently filtered (0.45 μm pore size). The SRP and UV254 were determined immediately. SRP concentration was determined using molybdenum blue method by microplate spectrophotometer (Ren et al. 2020). UV254, as an alternative indicator for dissolved organic material (DOM), was determined using a quartz microtiter plate and a microplate spectrophotometer to estimate the DOM concentration (Zhang et al. 2018). The microplate spectrophotometer has been widely used for the detection of trace water samples. In addition, DO and pH in the water column were measured using a portable parameter meter (DPL-LS12) inserted directly into the water.
2.3 Acquisition of high-resolution data at the sediment–water interface
2.3.1 2D-distribution of O2 and pH in sediments
The distribution of pH and O2 in the SWI can be mapped using two-dimensional (2D) imaging techniques using pH and O2 planar optode, respectively (Li et al. 2019). Briefly, pH planar optodes (dimension: 10 cm × 5 cm) and O2 planar optodes (dimension: 10 cm × 5 cm) were affixed to the transparent detachable windows of rhizoboxes. pH and O2 imaging were performed before biochar capping, on the 1st, 2nd, 3rd, 15th, and 30th day after biochar capping. The imaging system PO2100 was provided by Easysensor Ltd (Nanjing, China). Considering the pH optodes lifetime (~ 7 days), the pH optodes were replaced on the 14th and 29th day. Fabrication and calibration information for pH and O2 planar optodes is provided in the Supplementary Materials.
2.3.2 1D-distribution of SRP/soluble Fe and labile P/Fe
The HR-Peeper sampler was used to collect the porewater for determining the SRP and soluble Fe on the 0th, 30th, and 80th days. Additionally, the labile P and Fe in the sediments were determined using ZrO-Chelex DGT on those same days. Both they were deoxygenated with nitrogen (N2) for 16 h before use. They were deployed in the sediment for 48 h and 24 h, respectively. Treatment of the HR-Peeper and Zr-oxide DGT probes was carried out according to the methods of Ma et al. (2020). Detailed operational processes can be found in the Supplementary Materials. Labile P was detected using the same method as that used for SRP. Fe was detected using the phenanthroline colorimetric method (Ren et al. 2020).
2.3.3 2D-distribution of soluble Fe(II) and labile S(−II) imaging
On day 30 and day 80, the 2D-distribution of soluble Fe(II) and labile S(−II) in sediments was determined using the combined DET/DGT probe according to the method of Robertson et al. (2008). To minimize solute diffusion in the gel, the DET/DGT probe was prepared by immobilizing the DET gel and DGT gel on both sides of the polyethylene terephthalate (PET) plate in a back-to-back manner. The DET/DGT probe was deployed in the sediments for 24 h. The DET staining gel was soaked in Ferrozine reagent for at least 12 h. After retrieval, the staining gel was placed on the DET gel, forming a stack of sheet/DET gel/staining gel/sheet. After 15 min coloration, the stack was scanned at 600 dpi resolution. The AgI DGT gel was briefly rinsed and directly scanned using a flat-bed scanner (Canon 5600F). The soluble Fe and labile S(−II) could be quantified based on the color change in the gel using computer-imaging densitometry techniques (Wang et al. 2019). More information on the DGT gel and DET gel fabrication and calibration processes is provided in Supplementary Materials.
2.4 Measurement of the sediment sample
DNA extraction, PCR amplification and sequence analysis were performed at Shanghai Personal Biotechnology Co., Ltd (Shanghai, China). DNA was extracted from freeze-dried homogenized sediment samples using the DNeasy PowerSoil Kit (QIAGEN, Inc., the Netherlands). The primers 515F and 907R (5ʹ-GTGCCAGCMGCCGCGGTAA-3ʹ and 5ʹ-CCGTCAATTCMTTTRAGTTT-3ʹ, respectively) were used for the PCR amplification of 16 S rRNA in the V3–V4 region. The analysis of amplicon sequences was conducted using Qiime2 (https://qiime2.org/). Specific details regarding the processing of microbial data can be found in Ma et al. (2023). The raw DNA sequencing data has been submitted to the NCBI SRA (PRJNA1047949).
On day 80, the sediment cores were sectioned under nitrogen protection conditions, and freeze-dried for further analysis of the P fraction and alkaline phosphatase activities (ALP). The P fraction included exchangeable P (NH4Cl-P), Fe-adsorbed P (BD-P), Al-adsorbed P (NaOH-P), organic P (Org-P), Ca-bound P (HCl-P), and residual P (Res-P). Detailed extraction methods for inorganic and organic P can be found in Janssen et al. (2021). The ALP activities in the sediments were measured using an S-AKP kit (Boxbio, China).
2.5 Data analysis
The pH and DO optodes were calculated according to color ratiometric principles (Li et al. 2022a). The labile P and Fe concentrations were calculated according to (Chen et al. 2018). The net diffusion fluxes of soluble and labile P within 20 mm at SWI were calculated based on Fick's diffusion law (Wang et al. 2024). The grayscale of the DET gel images and DGT gel images were applied to determine the soluble Fe(II) concentration and labile S(−II) accumulation quantity (Li et al. 2022b). Details of the calculations are provided in the Supplementary Materials. The parameter R, which indicated the P resupply capacities from the solid phase to the porewater, was calculated from the ratio of the labile P and the SRP concentration (Wang et al. 2018).
The diffusion kinetics and resupply of P in sediments were revealed by the model of DGT induced fluxes in sediments and soils (DIFS) (Wu et al. 2018). The main output parameters of the model include K1 and K−1, representing the adsorption rate constant, and desorption rate constant, respectively (Menezes-Blackburn et al. 2016). The desorption rate was calculated according to Chen et al. (2022). Details of the calculations are provided in the Supplementary Materials (Table S4).
The alpha diversity indexes and principal coordinates analysis (PCoA) were determined using the “Vegan” package. In addition, the potential effects of environmental variables on the microbial community were determined using canonical correlation analysis (CCA). PICRUSt2 was used to predict the P-cycle function. According to KEGG orthologs, the P-cycle functional genes were classified into four types, namely inorganic P solubilization, organic P mineralization, P transportation and P regulation (Hu et al. 2023). All graphs were produced using Origin 2018, R v.4.1.2. and Surfer 13. Pearson correlation analysis and t-test were conducted in SPSS 25 to evaluate the association between parameters.
3 Results and discussion
3.1 Changes in pH, DO, UV254 and SRP in the incubation experiment
After biochar capping, the pH and redox conditions in the incubation system were significantly changed (Fig. 2 and Fig. S2). The average pH of the three different treatment groups was maintained at around 8.46, 8.53 and 8.65 for the control, 1-cm and 2-cm biochar capping groups, respectively. The water pH after biochar capping was significantly higher than the control group (p < 0.05), and it increased with the amount of biochar added (Fig. 2a). The increase in pH was more evident at the sediment-biochar interface, and the pH gradually decreased with depth (Fig. 2b). The 2-cm biochar capping led to a greater area of pH increase than 1-cm capping in the sediment. This phenomenon is consistent with previous findings at the soil-biochar interface (Buss et al. 2018). A large amount of inorganic ash and negatively charged functional groups in the biochar bound tightly to the H+ ions, leading to an increase in pH (Chintala et al. 2013). The DO in the overlying water decreased by approximately 70% after biochar capping (Fig. S2). It was initially decreased in the aqueous phase specifically at 0.5 cm above sediment and then increased to its original level on day 30. The concentration of organic matter was increased in the water column after biochar capping (Fig. 2a). The decomposition of organic matter consumed oxygen, which reduced the amount of DO in the water column (Cui et al. 2022). Additionally, the aerobic layer at the SWI decreased following the biochar capping.
Biochar capping caused a sharp increase in organic matter (UV254) and SRP concentration in the overlying water, which was associated with the amount of biochar capping (Fig. 2a). The UV254 increased from 0.0247 in control to 0.0494 and 0.0701 in the 1-cm and 2-cm capping groups, respectively. During the sediment incubation, the capping groups consistently maintained higher UV254 levels compared with the control group. As a carbon-rich solid material, biochar contains a significant amount of organic matter that can be dissolved in the water, resulting in higher dissolved organic carbon (DOC) concentration (Azeem et al. 2023). The SRP concentration changes in the water column were similar to those for UV254, with a sharp increase and a maximum value on the first day of biochar capping. The highest SRP concentrations were 1.11 mg L−1 and 2.10 mg L−1 in the 1-cm and 2-cm capping groups, respectively. The solubilization of dissolved P in biochar likely contributed to the elevated SRP concentrations in the water column (Padilla et al. 2023). Subsequently, the SRP concentration gradually decreased and returned to its original level after 20 days. This study revealed that biochar capping led to a sudden increase in organic matter and P concentration, which may adversely affect the water quality. Furthermore, biochar capping-induced changes in pH, redox conditions and UV254 at the sediment interface could affect the P transport and transformation processes in sediments.
3.2 Effects of biochar capping on P mobilization in SWI
The spatial distribution of soluble and labile P in the sediment profiles showed that the mobility and bioavailability of P can be altered by biochar capping (Fig. 3). On day 30, the profile-averaged SRP concentration of 1-cm capping group (0.87 mg L−1) was significantly higher than that of the control group (0.68 mg L−1) (p < 0.001), while there was no significant difference in the profile-averaged SRP concentration of 2-cm capping group (0.67 mg L−1) compared to the control group (p > 0.05) (Fig. 3a and Fig. S3). The changes in labile P were consistent with the SRP in the sediment profiles (Fig. 3b and Fig. S3). The 2-cm biochar capping can effectively control porewater SRP concentration at the surface, in particular in the surface sediment (0–50 mm), and the SRP concentration was significantly lower than that of the control and 1-cm capping groups (p < 0.05). After 30 days of the biochar capping, the K−1 value in the treatment groups increased significantly, while K1/K−1 values of the 1-cm capping group decreased and those of the 2-cm capping group increased (Table S5). The P desorption from solid was dominated in the 1-cm capping group, and the P adsorption from solution was dominated in the 2-cm capping group. The adsorption of phosphorus by biochar involves a rapid chemisorption phase, followed by a surface diffusion-controlled phase. Therefore, 2-cm biochar capping provided enough adsorption sites for SRP, leading to a decline in SRP concentration in the porewater in the short term. For 1-cm biochar capping, however, the surface adsorption sites of biochar were saturated and subsequent adsorption of P via intraparticle surface diffusion was also limited, which led to a higher desorption than adsorption of phosphorus by the biochar (Table S5). In addition, it was found that soluble and labile Fe decreased in the surface sediments (Fig. 3), which could be attributed to enhanced Fe adsorption by the biochar (Chen et al. 2022). Also, the significant positive correlation (p < 0.01) between Fe and UV254 indicated that organic matter released from the biochar may bind with Fe oxides, contributing to a decline in the adsorption of Fe oxides on P. Consequently, in the early stages of biochar capping, the adsorption–desorption processes of biochar on P are the key factors controlling the P mobilization.
The effects of biochar aging on P mobilization were further investigated. On day 80, both the SRP and labile P concentration in the sediments significantly increased in the capping groups compared with the control group (p < 0.001) (Fig. 3a, b, and Fig. S3). The SRP concentration increased by 35.50% and 23.84% in the 1-cm and 2-cm biochar capping groups, respectively. Furthermore, the labile P concentration increased by 36.56% and 23.06%, respectively. The P desorption rate in the treatment groups increased significantly, especially in the 2-cm capping group (Table S5). The increases in P mobility and bioavailability could be attributed to the competitive organic matter adsorption and biochar aging. As biochar ages, more BDOM is released, which could facilitate P desorption through surface adsorption of Fe oxides (Xu et al. 2014). In addition, the surface of aged biochar is rich in oxygen-containing functional groups, which increases the negative charge density of the biochar (Soinne et al. 2014; Yang et al. 2021a). The resultant electrostatic repulsion can lead to the desorption of sorbed P on the biochar, thus increasing P mobility and bioavailability. Biochar capping also increased the anaerobic conditions in the deep sediments. The reduction of SO42− and the formation of FeS can also promote the release of sediment P (Chen et al. 2016). In this study, it was found that both soluble Fe(II) and labile S(−II) decreased as the capping time of the biochar increased (Fig. 4), resulting from the formation of FeS. Consequently, the upward flux of reduced Fe decreased, resulting in a reduced availability of Fe(II) for re-oxidation and P sequestration in the surface sediment, thereby reducing the P retention capacity.
Furthermore, it was found that 2-cm biochar capping decreased the release flux of SRP by 73.1%, while the 1-cm biochar capping significantly increased the release flux of SRP by 51.0%, on day 30 (Fig. 3). However, the resupply ability (R) of P in the 1-cm biochar capping group was significantly lower compared to the control and 2-cm biochar capping group (p < 0.001; Fig. 5 and Fig. S3). The rising changes in SRP and labile P concentrations caused by biochar capping were disproportionate over the short-term period of biochar capping, resulting in differences in P resupply ability. After aging of biochar (80 days), the resupply capacity of sediment P improved, resulting in increases of more than 121.5% and 33.7% in the release fluxes of SRP in 1-cm and 2-cm capping groups compared to the control group, respectively (Fig. 5 and Fig. S3). The P fraction analysis also further confirmed a rise in the sediment P resupply. The solid labile P and Ca-P contents were lower than those for the control group (Table 1). Labile P and Ca-P are recognized as one of the main P fractions adsorbed by biochar (Jung et al. 2015; Zhang et al. 2020b). In summary, in the short term, a large amount of biochar capping can reduce SRP concentrations and release flux by chemisorption of P, whereas with biochar aging, the remediation effects of biochar capping decrease, leading to a rise in the resupply capacity and release flux of sediment P.
3.3 Response of the microbial community composition to biochar capping
To further investigate the impact of biochar capping on P mobilization processes, the response of microbial community composition to biochar capping was analyzed. After DNA metabarcoding and bioinformatic analysis, a total of 21,224 bacterial amplicon sequence variants (ASVs) were identified in sediments. Proteobacteria accounted for 39.7–43.7% of the total bacterial sequences in sediments, which was the dominant phylum (Fig. 6a), followed by Acidobacteria, Chloroflexi, and Nitrospirae. The relative abundance of Proteobacteria and Bacteroidetes in sediments was higher after biochar capping, whereas some microorganisms, such as Acidobacteria and Chloroflexi, showed opposite changes (Fig. 6a). In addition, PCoA showed that microbial communities were significantly separated in sediments after biochar capping (Fig. 6b). Changes in bacterial community composition after the addition of biochar may be attributed primarily to the alkalinity of the biochar (Fig. S4). Acidobacteria is thought to be the phylum most sensitive to biochar and is dominant in low pH soils (Xu et al. 2016). In this study, it was found that Acidobacteria were significantly reduced in biochar-capped sediments, which is consistent with previous studies (Sheng and Zhu 2018). Benthic microorganisms are mostly responsible for the material cycling process in sediments (Ma et al. 2023). Biochar capping may alter the specific microbial activity and thus affect the process of material transport and transformation (Li et al. 2022c). On one hand, the nutrients adsorbed by biochar could provide some nutrients to benthic microorganisms and increase microbial richness (Fig. 6c) (Xu et al. 2019). On the other hand, biochar can provide a better environment for microorganisms to survive and reproduce due to its porosity and high specific surface area. These pore structures can allow microorganisms to hide to avoid predation, thus enhancing the abundance of microorganisms in the soil (Ameloot et al. 2013; Liang et al. 2010).
a The dominant bacterial communities at the phylum level in the control, 1-cm and 2-cm biochar capping sediments. b The composition and structure differences of microbial communities at the ASV level. c The richness, relative abundance of Pseudomonadaceae and Geobacteraceae and the ALP activity for the control, 1-cm and 2-cm biochar capping sediments
Functional P-cycling genes were analyzed to understand how biochar affects microbial-controlled P-cycling processes in sediments. There was no significant effect on the overall abundance of P-cycling genes associated with inorganic P solubilization, organic P mineralization, P transportation, and P regulation. However, the relative abundance of individual genes, such as ppa and phnO was significantly affected by biochar (Fig. S5). The biochar capping increased the relative abundance of ppa-involved inorganic P solubilization and decreased the relative abundance of phnO-involved organic P mineralization. In addition, the abundance of phosphate-solubilizing bacteria, particularly Pseudomonadaceae (the dominant genus), was influenced by biochar (Fig. 6c). The relative abundance of Pseudomonadaceae in the treatment group was lower compared to the control group (Fig. 6c). ALP activity also showed a decreasing trend with the increase in biochar, decreasing from 1714.3 U g−1 in the control group to 1048.8 U g−1 and 526.7 U g−1 in the 1-cm and 2-cm capping groups, respectively (Fig. 6c). Previous studies found increases in phosphate-solubilizing bacteria and ALP activity in flooded rice rhizospheres following biochar addition (Chen et al. 2022). However, our findings suggest that the presence of excess labile P in sediments may contribute to the decrease in ALP activity. Long-term capping of biochar promoted P desorption. High concentrations of dissolved inorganic P can have a negative effect on organic P mineralization processes in sediments (Zhai et al. 2014).
Phosphorus cycling in sediments has also been reported to be strongly linked to Fe redox conditions (Liang et al. 2022). The biochar capping group had a higher abundance of Geobacteraceae, which involved in the reduction of Fe(III), compared to the control group (Fig. 6c). Fe(III) reduction bacteria-mediated reductive dissolution of Fe oxides enhances P release from sediments (Li et al. 2023a). The capping of biochar provided suitable conditions for the growth of Fe(III) reduction bacteria, which increased the biotic reduction of Fe oxides. Also, the capping of biochar also increased BDOM concentration and promoted the desorption of P (Fig. 3). In this study, the Fe(II) reduced by microorganisms was bound by biochar and S(−II), and the adsorption rate of Fe(II) was higher than the reduction rate of Fe oxides, resulting in lower concentrations of soluble and labile Fe(II) in the capping group compared to the control group (Fig. 3). The decrease in the amount of Fe(II) that can be oxidized on the sediment surface caused by biochar capping reduces the sediment's capacity to retain P and increases P release from the sediment. Overall, biochar capping can alter the sediment microbial community structure and function, and, in turn, lead to the P release from sediments.
3.4 Implications of biochar capping on sediment restoration
Biochar has been extensively used for the remediation of contaminated sediments; however, the accompanying adverse effects of biochar capping on the aquatic environment, especially on sediment P release, have not been fully understood. In this study, we examined the mobility, bioavailability and release risk of P under different biochar additions and treatment times using microcosm experiments (Fig. 7). The results showed that both the capping amount and the treatment time affected the mobility and P release in sediments. In the short term, biochar capping can effectively control SRP concentrations in porewater in surface sediments by rapid chemisorption. After a longer incubation period (80 days), the sediment P resupply capacity increased, resulting in higher P release from sediments in the biochar capping group compared to the control group (Fig. 5). The changes in biochar composition and surface chemistry, as well as sediment microbial community structure after biochar capping over time, reduce the effectiveness of biochar-based sediment remediation (Yang et al. 2021b). Furthermore, the sharply increased process of SRP concentration in the overlying water due to biochar capping during the initial phase should be of wide concern. Thus, modifying biochar in a way that sequesters phosphorus release and slows aging is critical for biochar remediation of water bodies and sediments.
In summary, biochar capping has adverse effects on sediment P release, although it is effective for controlling P release in short-term applications by using a large amount of biochar. Therefore, the effectiveness of biochar in the long term is an essential foundation for exploring the potential environmental risks of biochar. Additionally, a systematic assessment of the effects of biochar capping on aquatic ecosystems by combining aquatic organisms, water environment and other elements needs to be further explored.
4 Conclusions
Using advanced fine-scale measurement methods, this study demonstrated the effects of biochar capping on the P mobilization processes in sediments. The application dosage and capping time of the biochar were two important factors that influenced sediment P release. Adequate biochar capping can act as a barrier to sediment P release in the short term, while the sharp increase in SRP and organic matter in the overlying water should be noted. After the aging of biochar (80 days), the bioavailability and P release flux were increased, leading to the P desorption from the aging biochar. Furthermore, biochar capping intensified the microbial-mediated Fe reduction, which was subsequently sequestrated by biochar and S(−II), reducing the sediments retention capacity for P and thereby enhancing P mobility. The novel P mobilization processes in sediment from biochar capping determined in this study will contribute to the assessment of the ecological risks of biochar remediation for ecosystem function and service.
Data availability
Data will be made available on reasonable request.
References
Abel S, Akkanen J (2018) A combined field and laboratory study on activated carbon-based thin layer capping in a PCB-contaminated boreal lake. Environ Sci Technol 52(8):4702–4710. https://doi.org/10.1021/acs.est.7b05114
Ameloot N, Graber ER, Verheijen FGA, De Neve S (2013) Interactions between biochar stability and soil organisms: review and research needs. Eur J Soil Sci 64(4):379–390. https://doi.org/10.1111/ejss.12064
Azeem M, Sun T-R, Jeyasundar PGSA, Han R-X, Li H, Abdelrahman H, Shaheen SM, Zhu Y-G, Li G (2023) Biochar-derived dissolved organic matter (BDOM) and its influence on soil microbial community composition, function, and activity: a review. Crit Rev Environ Sci Technol 53(21):1912–1934. https://doi.org/10.1080/10643389.2023.2190333
Buss W, Shepherd JG, Heal KV, Mašek O (2018) Spatial and temporal microscale pH change at the soil-biochar interface. Geoderma 331:50–52. https://doi.org/10.1016/j.geoderma.2018.06.016
Chen M, Li XH, He YH, Song N, Cai HY, Wang C, Li YT, Chu HY, Krumholz LR, Jiang HL (2016) Increasing sulfate concentrations result in higher sulfide production and phosphorous mobilization in a shallow eutrophic freshwater lake. Water Res 96:94–104. https://doi.org/10.1016/j.watres.2016.03.030
Chen M, Ding S, Chen X, Sun Q, Fan X, Lin J, Ren M, Yang L, Zhang C (2018) Mechanisms driving phosphorus release during algal blooms based on hourly changes in iron and phosphorus concentrations in sediments. Water Res 133:153–164. https://doi.org/10.1016/j.watres.2018.01.040
Chen H, Yuan J, Chen G, Zhao X, Wang S, Wang D, Wang L, Wang Y, Wang Y (2022) Long-term biochar addition significantly decreases rice rhizosphere available phosphorus and its release risk to the environment. Biochar. https://doi.org/10.1007/s42773-022-00178-7
Chintala R, Schumacher TE, McDonald LM, Clay DE, Malo DD, Papiernik SK, Clay SA, Julson JL (2013) Phosphorus sorption and availability from biochars and soil/biochar mixtures. Clean: Soil, Air, Water 42(5):626–634. https://doi.org/10.1002/clen.201300089
Cui Y, Wu J, Tan E, Kao SJ (2022) Role of particle resuspension in maintaining hypoxic level in the pearl river estuary. J Geophys Res Oceans. https://doi.org/10.1029/2021jc018166
Davison W, Zhang H (1994) In situ speciation measurements of trace components in natural waters using thin-film gels. Nature 367:546–548. https://doi.org/10.1038/367546a0
Duan Z, Tan X, Shi L, Zeng Q, Ali I, Zhu R, Chen H, Parajuli K (2023) Phosphorus accumulation in extracellular polymeric substances (EPS) of colony-forming cyanobacteria challenges imbalanced nutrient reduction strategies in eutrophic lakes. Environ Sci Technol 57(4):1600–1612. https://doi.org/10.1021/acs.est.2c04398
Gao C, Fan J, Zhang X, Gong Z, Tan Z (2021) Sediment metals adhering to biochar enhanced phosphorus adsorption in sediment capping. Water Sci Technol 84(8):2057–2067. https://doi.org/10.2166/wst.2021.411
Hu M, Le Y, Sardans J, Yan R, Zhong Y, Sun D, Tong C, Penuelas J (2023) Moderate salinity improves the availability of soil P by regulating P-cycling microbial communities in coastal wetlands. Glob Chang Biol 29(1):276–288. https://doi.org/10.1111/gcb.16465
Janssen ABG, Droppers B, Kong X, Teurlincx S, Tong Y, Kroeze C (2021) Characterizing 19 thousand Chinese lakes, ponds and reservoirs by morphometric, climate and sediment characteristics. Water Res 202:117427. https://doi.org/10.1016/j.watres.2021.117427
Jung KW, Hwang MJ, Ahn KH, Ok YS (2015) Kinetic study on phosphate removal from aqueous solution by biochar derived from peanut shell as renewable adsorptive media. Int J Environ Sci Te 12(10):3363–3372. https://doi.org/10.1007/s13762-015-0766-5
Li C, Ding S, Yang L, Zhu Q, Chen M, Tsang DCW, Cai G, Feng C, Wang Y, Zhang C (2019) Planar optode: a two-dimensional imaging technique for studying spatial-temporal dynamics of solutes in sediment and soil. Earth Sci Rev 197:102916. https://doi.org/10.1016/j.earscirev.2019.102916
Li C, Ding S, Ma X, Chen M, Zhong Z, Zhang Y, Ren M, Zhang M, Yang L, Rong N, Wang Y (2021) O2 distribution and dynamics in the rhizosphere of Phragmites australis, and implications for nutrient removal in sediments. Environ Pollut 287:117193. https://doi.org/10.1016/j.envpol.2021.117193
Li C, Ding S, Chen M, Sun Q, Zhang Y, Ma X, Zhong Z, Tsang DCW, Wang Y (2022a) Mechanistic insights into trace metal mobilization at the micro-scale in the rhizosphere of Vallisneria spiralis. Sci Total Environ 806:150735. https://doi.org/10.1016/j.scitotenv.2021.150735
Li C, Ding S, Chen M, Zhong Z, Sun Q, Wang Y (2022b) Visualizing biogeochemical heterogeneity in soils and sediments: a review of advanced micro-scale sampling and imaging methods. Crit Rev Environ Sci Technol 53(12):1229–1253. https://doi.org/10.1080/10643389.2022.2128239
Li Y, Xiong X, Zhang C, Liu A (2022c) Sustainable restoration of anoxic freshwater using environmentally-compatible oxygen-carrying biochar: performance and mechanisms. Water Res 214:118204. https://doi.org/10.1016/j.watres.2022.118204
Li R, Wang W, Zhao R, Zhang J, Sun L, Li X, Li B (2023a) New insights into the microbial-driven metal reductive dissolution for enhanced phosphorus release from iron-rich sludge. J Clean Prod 392:136290. https://doi.org/10.1016/j.jclepro.2023.136290
Li X, Huang L, Reible D, Zeng X, Liu S, Fu J, Wang K, Fang H (2023b) Inhibition of sediment erosion and phosphorus release by remediation strategy of contaminated sediment backfilling. Water Res 239:120055. https://doi.org/10.1016/j.watres.2023.120055
Li Y, Liu Y, Wang H, Zuo Z, Yan Z, Wang L, Wang D, Liu C, Yu D (2023c) In situ remediation mechanism of internal nitrogen and phosphorus regeneration and release in shallow eutrophic lakes by combining multiple remediation techniques. Water Res 229:119394. https://doi.org/10.1016/j.watres.2022.119394
Liang B, Lehmann J, Sohi SP, Thies JE, O’Neill B, Trujillo L, Gaunt J, Solomon D, Grossman J, Neves EG, Luizão FJ (2010) Black carbon affects the cycling of non-black carbon in soil. Org Geochem 41(2):206–213. https://doi.org/10.1016/j.orggeochem.2009.09.007
Liang Z, Letscher RT, Knapp AN (2022) Dissolved organic phosphorus concentrations in the surface ocean controlled by both phosphate and iron stress. Nat Geosci 15:651–657. https://doi.org/10.1038/s41561-022-00988-1
Ma X, Li C, Yang L, Ding S, Zhang M, Zhang Y, Zhao T (2020) Evaluating the mobility and labile of As and Sb using diffusive gradients in thin-films (DGT) in the sediments of Nansi Lake. China Sci Total Environ 713:136569. https://doi.org/10.1016/j.scitotenv.2020.136569
Ma X, Li Y, Niu L, Shang J, Yang N (2023) Microbial community structure and denitrification responses to cascade low-head dams and their contribution to eutrophication in urban rivers. Environ Res 221:115242. https://doi.org/10.1016/j.envres.2023.115242
Menezes-Blackburn D, Zhang H, Stutter M, Giles CD, Darch T, George TS, Shand C, Lumsdon D, Blackwell M, Wearing C, Cooper P, Wendler R, Brown L, Haygarth PM (2016) A holistic approach to understanding the desorption of phosphorus in soils. Environ Sci Technol 50(7):3371–3381. https://doi.org/10.1021/acs.est.5b05395
Padilla JT, Watts DW, Novak JM, Cerven V, Ippolito JA, Szogi AA, Johnson MG (2023) Magnesium activation affects the properties and phosphate sorption capacity of poultry litter biochar. Biochar. https://doi.org/10.1007/s42773-023-00263-5
Pan G, Dai L, Li L, He L, Li H, Bi L, Gulati RD (2012) Reducing the recruitment of sedimented algae and nutrient release into the overlying water using modified soil/sand flocculation-capping in eutrophic lakes. Environ Sci Technol 46(9):5077–5084. https://doi.org/10.1021/es3000307
Parvage MM, Ulén B, Eriksson J, Strock J, Kirchmann H (2012) Phosphorus availability in soils amended with wheat residue char. Biol Fertil Soils 49(2):245–250. https://doi.org/10.1007/s00374-012-0746-6
Pedersen LL, Smets BF, Dechesne A (2015) Measuring biogeochemical heterogeneity at the micro scale in soils and sediments. Soil Biol Biochem 90:122–138. https://doi.org/10.1016/j.soilbio.2015.08.003
Qi Y, Yang F, Gao Y, Zhu Q, Tang X, Wang C, Sun H (2023) Role of biochar-derived DOM compositions in enhanced biodegradation of sulfamethoxazole and chloramphenicol. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2023.131979
Qian W, Zhang S, Tong C, Sardans J, Peñuelas J, Li X (2022) Long-term patterns of dissolved oxygen dynamics in the pearl river estuary. J Geophys Res Biogeosci. https://doi.org/10.1029/2022jg006967
Ren M, Ding S, Shi D, Zhong Z, Cao J, Yang L, Tsang DCW, Wang D, Zhao D, Wang Y (2020) A new DGT technique comprised in a hybrid sensor for the simultaneous measurement of ammonium, nitrate, phosphorus and dissolved oxygen. Sci Total Environ 725:138447. https://doi.org/10.1016/j.scitotenv.2020.138447
Robertson D, Teasdale PR, Welsh DT (2008) A novel gel-based technique for the high resolution, two-dimensional determination of iron (II) and sulfide in sediment. Limnol Oceanog-Meth 6(10):502–512. https://doi.org/10.4319/lom.2008.6.502
Schneider F, Haderlein SB (2016) Potential effects of biochar on the availability of phosphorus—mechanistic insights. Geoderma 277:83–90. https://doi.org/10.1016/j.geoderma.2016.05.007
Sheng Y, Zhu L (2018) Biochar alters microbial community and carbon sequestration potential across different soil pH. Sci Total Environ 622–623:1391–1399. https://doi.org/10.1016/j.scitotenv.2017.11.337
Silvani L, Di Palma PR, Riccardi C, Eek E, Hale SE, Viotti P, Petrangeli Papini M (2017) Use of biochar as alternative sorbent for the active capping of oil contaminated sediments. J Environ Chem Eng 5(5):5241–5249. https://doi.org/10.1016/j.jece.2017.10.004
Soinne H, Hovi J, Tammeorg P, Turtola E (2014) Effect of biochar on phosphorus sorption and clay soil aggregate stability. Geoderma 219–220:162–167. https://doi.org/10.1016/j.geoderma.2013.12.022
Søndergaard M, Bjerring R, Jeppesen E (2012) Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia 710(1):95–107. https://doi.org/10.1007/s10750-012-1091-3
Sun K, Qiu M, Han L, Jin J, Wang Z, Pan Z, Xing B (2018) Speciation of phosphorus in plant- and manure-derived biochars and its dissolution under various aqueous conditions. Sci Total Environ 634:1300–1307. https://doi.org/10.1016/j.scitotenv.2018.04.099
Sun C, Zhong J, Pan G, Mortimer RJG, Yu J, Wen S, Zhang L, Yin H, Fan C (2023) Controlling internal nitrogen and phosphorus loading using Ca-poor soil capping in shallow eutrophic lakes: long-term effects and mechanisms. Water Res 233:119797. https://doi.org/10.1016/j.watres.2023.119797
Tan G, Yu H-Q (2024) Rethinking biochar: black gold or not? Nat Rev Mater 9:4–5. https://doi.org/10.1038/s41578-023-00634-1
Wang S, Wu Z, Luo J (2018) Transfer mechanism, uptake kinetic process, and bioavailability of P, Cu, Cd, Pb, and Zn in macrophyte rhizosphere using diffusive gradients in thin films. Environ Sci Technol 52(3):1096–1108. https://doi.org/10.1021/acs.est.7b01578
Wang Y, Ding S, Ren M, Li C, Xu S, Sun Q, Xu L (2019) Enhanced DGT capability for measurements of multiple types of analytes using synergistic effects among different binding agents. Sci Total Environ 657:446–456. https://doi.org/10.1016/j.scitotenv.2018.12.016
Wang S, Ding S, Zhao H, Chen M, Yang D, Li C (2024) Seasonal variations in spatial distribution, mobilization kinetic and toxicity risk of arsenic in sediments of Lake Taihu. China J Hazard Mater 463:132852. https://doi.org/10.1016/j.jhazmat.2023.132852
Wu Z, Wang S, Luo J (2018) Transfer kinetics of phosphorus (P) in macrophyte rhizosphere and phytoremoval performance for lake sediments using DGT technique. J Hazard Mater 350:189–200. https://doi.org/10.1016/j.jhazmat.2018.02.005
Wu Z, Li J, Sun Y, Peñuelas J, Huang J, Sardans J, Jiang Q, Finlay JC, Britten GL, Follows MJ, Gao W, Qin B, Ni J, Huo S, Liu Y (2022) Imbalance of global nutrient cycles exacerbated by the greater retention of phosphorus over nitrogen in lakes. Nat Geosci 15(6):464–468. https://doi.org/10.1038/s41561-022-00958-7
Xu G, Sun J, Shao H, Chang SX (2014) Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol Eng 62:54–60. https://doi.org/10.1016/j.ecoleng.2013.10.027
Xu N, Tan G, Wang H, Gai X (2016) Effect of biochar additions to soil on nitrogen leaching, microbial biomass and bacterial community structure. Eur J Soil Biol 74:1–8. https://doi.org/10.1016/j.ejsobi.2016.02.004
Xu M, Gao P, Yang Z, Su L, Wu J, Yang G, Zhang X, Ma J, Peng H, Xiao Y (2019) Biochar impacts on phosphorus cycling in rice ecosystem. Chemosphere 225:311–319. https://doi.org/10.1016/j.chemosphere.2019.03.069
Yang L, Wu Y, Wang Y, An W, Jin J, Sun K, Wang X (2021a) Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci Total Environ 758:143657. https://doi.org/10.1016/j.scitotenv.2020.143657
Yang Q, Wang Y, Zhong H (2021b) Remediation of mercury-contaminated soils and sediments using biochar: a critical review. Biochar 3(1):23–35. https://doi.org/10.1007/s42773-021-00087-1
Zhai L, CaiJi Z, Liu J, Wang H, Ren T, Gai X, Xi B, Liu H (2014) Short-term effects of maize residue biochar on phosphorus availability in two soils with different phosphorus sorption capacities. Biol Fertil Soils 51(1):113–122. https://doi.org/10.1007/s00374-014-0954-3
Zhang Y, Zhou Y, Shi K, Qin B, Yao X, Zhang Y (2018) Optical properties and composition changes in chromophoric dissolved organic matter along trophic gradients: Implications for monitoring and assessing lake eutrophication. Water Res 131:255–263. https://doi.org/10.1016/j.watres.2017.12.051
Zhang M, Li C, Yang L, Ding S, Ma X, Zhang Y, Zhao T (2020a) Application of DGT/DIFS combined with BCR to assess the mobility and release risk of heavy metals in the sediments of Nansi Lake. China Environ Geochem Health 42(11):3765–3778. https://doi.org/10.1007/s10653-020-00638-8
Zhang M, Song G, Gelardi DL, Huang L, Khan E, Masek O, Parikh SJ, Ok YS (2020b) Evaluating biochar and its modifications for the removal of ammonium, nitrate, and phosphate in water. Water Res 186:116303. https://doi.org/10.1016/j.watres.2020.116303
Zhao M, Ma X, Liao X, Cheng S, Liu Q, Wang H, Zheng H, Li X, Luo X, Zhao J, Li F, Xing B (2022) Characteristics of algae-derived biochars and their sorption and remediation performance for sulfamethoxazole in marine environment. Chem Eng J. https://doi.org/10.1016/j.cej.2021.133092
Zhu Y, Shan B, Huang J, Teasdale PR, Tang W (2019a) In situ biochar capping is feasible to control ammonia nitrogen release from sediments evaluated by DGT. Chem Eng J 374:811–821. https://doi.org/10.1016/j.cej.2019.06.007
Zhu Y, Tang W, Jin X, Shan B (2019b) Using biochar capping to reduce nitrogen release from sediments in eutrophic lakes. Sci Total Environ 646:93–104. https://doi.org/10.1016/j.scitotenv.2018.07.277
Acknowledgements
Not applicable.
Funding
This research was funded by the National Key Research and Development Program of China (2018YFA0903000), the National Natural Science Foundation of China (U2102210, 41877492, and 42277393), the Postdoctoral Fellowship Program of CPSF (GZB20230782).
Author information
Authors and Affiliations
Contributions
Xin Ma contributed to methodology, data collection, and writing-original draft. Yijun Song contributed to data analysis and validation. Yilun Shen contributed to data collection. Liyuan Yang contributed to the methodology. Shiming Ding contributed to resources and editing. Cai Li contributed to the data analysis, review and editing. Qin Sun contributed to project administration, resources, and editing. The author(s) read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Jun Meng
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ma, X., Song, Y., Shen, Y. et al. Fine-scale measurements unravel the side effects of biochar capping on the bioavailability and mobility of phosphorus in sediments. Biochar 6, 49 (2024). https://doi.org/10.1007/s42773-024-00343-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-024-00343-0