Abstract
Iron-carbon micro-electrolysis system is a promising method for promoting electron transfer in nitrate removal. However, many traditional approaches involving simple physical mixing inevitably suffered from the confined iron-carbon contact area and short validity period, leading to the overuse of iron. Here, a ceramsite-loaded microscale zero-valent iron (mZVI) and acidified carbon (AC) coupled-galvanic cell (CMC) was designed to support chemical, autotrophic and heterotrophic denitrification. Long-term experiments were conducted to monitor the nitrogen removal performance of denitrification reactors filled with CMC and thus optimized the denitrification performance by improving fabrication parameters and various operating conditions. The denitrification contributions test showed that the chemical denitrification pathway contributed most to nitrate removal (57.3%), followed by autotrophic (24.6%) and heterotrophic denitrification pathways (18.1%). The microbial analysis confirmed the significant aggregation of related denitrifying bacteria in the reactors, while AC promoted the expression of relevant nitrogen metabolism genes because of accelerated uptake and utilization of iron complexes. Meanwhile, the electrochemical analysis revealed a significantly improved electron transfer capacity of AC compared to pristine carbon. Overall, our study demonstrated the application of a novel mZVI-AC coupled material for effective nitrate removal and revealed the potential impact of CMC in the multipathway denitrification process.
Graphical Abstract

Highlights
-
Novel mZVI-AC coupled micro-electrolysis systems were established for denitrification
-
Chemical, autotrophic and heterotrophic denitrification pathways were observed in reactors
-
AC increased the activity of enzymes encoding denitrification and respiratory chains
-
The acidification brought greater capacitance and lower impedance to carbon to facilitate iron oxidation
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The discharge of high nitrate nitrogen (NO3−-N) loads from agricultural, domestic and industrial sources to surface water bodies is a growing concern in the environmental field (Rivett et al. 2008; Hosono et al. 2013). The high concentration of NO3−-N (> 0.4 mg L−1) is associated with significant environmental problems such as eutrophication of water bodies, which causes serious deterioration of local biodiversity and water quality (Auclair et al. 2010; Pennino et al. 2017). Since NO3−-N concentrations > 11.2 mg L−1 in drinking water have the potential to cause fatal health problems such as methemoglobinemia and cancer (Sadeq et al. 2008; Sadeq et al. 2008; Picetti et al. 2022), identifying possible mitigation practices and methods for NO3−-N removal in water sources is an international research hot spot.
Removal of excess nitrate from water bodies primarily involves chemical and microbial denitrification pathways, while microbial denitrification is currently the most widely applied technology in water treatment plants. Microbial denitrification has gained more potential because of its low cost, non-toxic residual effects, and wide range of applications (Liao et al. 2021; Zhou et al. 2022). However, the limitation of this technique is that the C/N ratios of most NO3−-N contaminated effluent and groundwater sources are relatively low and the carbon (C) source provided for denitrification reaction is insufficient (Pinney et al. 2000). On this basis, exogenous organic carbon sources such as methanol (Zhao et al. 2011) and acetic acid have been utilized in several studies to sustain a higher denitrification rate. However, the application of these organic sources resulted in secondary contamination by inorganic degradation products and organic residues. Therefore, multipathway denitrification without secondary pollution and the need for additional C sources is a more feasible and promising approach water treatment process.
Iron (Fe), as an abundant element in the natural world, profoundly affects the geochemical cycle of nitrogen (N) (Bibby et al. 2001). Owing to the strong reduction potential, Fe0 has been applied to iron-related NO3−-N removal through chemical and biological denitrification (Jamieson et al. 2018; Zhang et al. 2019). The denitrification process related to Fe0 includes the following pathways: (1) chemical denitrification via direct electron transfer from Fe0 to NO3−(Zhang et al. 2010, 2011; Guo et al. 2015); (2) promoted microbial hydrogen (H) autotrophic denitrification by H+ produced through the oxidation of Fe0; (3) supply of electron donor for NO3− reduction by the dissolved ferrous ions from Fe0 oxidation (Xing et al. 2016). Even though the application of Fe0 has shown good results, Fe0 with a smaller particle size was prone to form a layer of iron oxide on its surface, thus preventing the production of hydrogen (H2). Therefore, the use of microscale zero-valent iron (mZVI) and nanoscale zero-valent iron (nZVI) with a larger specific surface area and reactivity could effectively overcome the above drawback. Due to a larger specific surface area, the degradation rate of NO3−-N by nZVI is much greater than that of mZVI. The application of commercial mZVI had a nominal effect on the NO3−-N concentration within 2 h, while the application of nZVI made NO3−-N removed within half an hour(Wang et al. 2006). In engineering applications, continued oxidation of nZVI resulted in relatively short periods of effectiveness, thus requiring repeated replacement with new materials. In addition, the cost of nZVI is considerably higher than that of mZVI due to the difficulties of fabrication. To balance the oxidation rate and production cost, mZVI was chosen as the raw material in this study.
Since the application of mZVI in NO3−-N degradation is restricted by the electron transfer rate, several researchers have attempted to enhance the denitrification rate by combining Fe0 with carbon to form galvanic cells (Oh et al. 2016; Zhang et al. 2022). For example, significant promotion in NO3−-N removal efficiency in the constructed wetland was achieved by mixing coke and iron scraps (Liu et al. 2019) and applying porous sponge iron containing carbon and iron (Si et al. 2020). However, the limitations of the above methods are also evident, including the limited contact area between iron and carbon, and the iron oxidation rate limited by the lower specific surface area. In this context, Zhang achieved complete NO3−-N removal of 100 mg L−1 within 28 h by employing biochar-supported nZVI (Zhang et al. 2020). Previous studies have shown that supporting nZVI on the nanographene surface improved the removal process by 125.1% compared with using nZVI alone (Salam et al. 2015). Unfortunately, there are some defects in the above materials including the low adhesion rate (< 10 wt%) of Fe0 on the substrate surface (Lee et al. 2016). Furthermore, the highly dispersed nZVI in the support materials was significantly susceptible to rapid oxidation to iron oxide, thus limiting its reducing ability. Besides, acid modification on carbon has been verified to improve the electron transfer capability, which could be used to facilitate the electron transfer rate in iron carbon microelectrolysis systems (Meng et al. 2021). Considering all above factors, this study provides an innovative form of iron-carbon galvanic cell, specifically by loading mixtures of acidified carbon (AC) and mZVI on ceramic particles for NO3− reduction. By using ceramic as carriers, the demand for mZVI and carbon was reduced during the manufacture, as well as the costs, while more galvanic cell structures for microelectrolysis were formed to boost the denitrification rate. The iron-carbon galvanic cell system was constructed in this form to meet the requirements of practical applications and purification efficiency.
In this study, a ceramsite-loaded mZVI and AC coupled-galvanic cell (CMC) was first applied to the multipathway denitrification reactor (MPDR). The main objectives of our work are to (1) optimize the performance of NO3− reduction under different mZVI to carbon ratios and different operating conditions, (2) determine the contribution of various denitrification pathways for NO3−-N reduction in MPDR, (3) evaluate the effect of mZVI to carbon ratios on the diversity of microbial community structure and functional genes, and (4) elucidate the influence of the acid-modification process on the denitrification capacity involving electron transfer.
2 Materials and methods
2.1 Synthesis of CMC under different manufacturing conditions
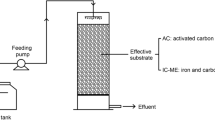
CMC, based on the mZVI–AC galvanic cell reaction, was developed for NO3−-N removal through multipathway denitrification. CMCs are spherical particles prepared with AC, mZVI (1.6–1.7 mm), ceramsite (15–20 mm) and adhesive (phenolic resin), and AC and mZVI were immobilized on the surface of ceramsite through the adhesive. AC was prepared by immersing carbon in a mixed solution of 12% H2O2 and 15% HNO3 and stirred using a magnetic stirring rod for 4 h (150 rpm, ambient temperature). Sludge carbon was used in this study and purchased from Qianjiang Water Sewage Treatment Factory (Qianjiang, China). The more detailed manufacturing process is shown in the (Additional file 1: Text S1). To explore the relationship between denitrification performance and the iron-carbon ratio in the CMC fabrication process, four CMCs were prepared with only carbon, mZVI-carbon ratios of 1:1 and 2:1, and only mZVI, labeled as C-MPDR, 1-MPDR, 2-MPDR, M-MPDR. Another material labeled as V-MPDR was prepared by replacing AC with virgin carbon under the same fabrication conditions as 2-MPDR to investigate the contribution of AC to electron transfer capability.
2.2 Establishment and continuous operation of MPDRs in long-term tests
Five denitrification reactors loaded with different CMCs (C-MPDR, 1-MPDR, 2-MPDR, M-MPDR and V-MPDR) were set up to investigate the removal efficiency under continuous operation experiments. The denitrification reactors are cylinders with a diameter of 22.5 cm (30 cm height difference between inlet and outlet, 25 cm effective reaction height). All reactors were initiated by inoculating with sludge obtained from a facultative secondary sedimentation tank of the LinAn sewage treatment plant (Hangzhou, China). Considering that the NO3−-N removal efficiency by Fe0 generally increased with rising reaction temperature (Ahn et al. 2008; Ji et al. 2011), the experiments were conducted in a plastic greenhouse with a general indoor temperature of 30–35 °C. Each column was inoculated with 500 ml of sludge suspension and maintained the nutrient by constant addition for 14 days. The continuous operation comprised of nine stabilization stages as well as adaptation periods, and the operation conditions for different stages are presented in Table 1. Since iron oxides on the interface of mZVI formed a physical barrier limiting the rate of chemical denitrification, 3 mg L−1 trichloroethylene (TCE) was introduced to eliminate surface oxidized material between each test stage (Ritter et al. 2002). The simulated municipal secondary effluent solution was generated by adding KNO3, NaHCO3 and trace element concentrates to tap water. The concentration of NaHCO3 in influent was controlled at 50 mg L−1 based on the conditions of the practical secondary effluent treatment. The microelement concentrated solution was prepared before the start of the experiment, and 1 mL of this concentrated solution was added to each litre of the simulated water body. Water samples obtained from the effluent of denitrification reactors were filtered through a membrane (0.45 μm pore size) before performing chemical analysis of NO3−-N, NO2−-N and NH4+-N, and subsequently determined via the enzyme-linked immunosorbent assay (Thermo Fisher Scientific, USA) according to the national standard method (APHA, 2005). The concentration of Fe ions was analyzed by inductively coupled plasma optical emission spectroscopy (ICP-OES) (ICP6000, Thermo Fisher Scientific, UK).
2.3 Characterization, microbial community analysis and electrochemical analysis
The crystalline structures of biochar were measured using an X-ray diffractometer (XRD) (Bruker D8 Advance) with a Cu-K target (λ = 1.54056 Å) and ceramic X-ray tube, in the angular range of 10° < 2θ < 80° at a scan rate of 1.00° min−1. Scanning electron microscopy (SEM, Gemini SEM 300, Germany) was employed in conjunction with an energy-dispersive X-ray analyzer (EDS, Bruker Nano Berlin, Germany) to visualize the microsurface characteristics and surface chemical composition of the prepared materials. Thermo Scientific ESCALAB 250Xi was used for X-ray photoelectron spectroscopy (XPS) analysis of AC and carbon with Al Kα X-ray radiation (1486.6 eV), and the C peak standard was 284.8 eV. At the end of the experiment, microbial samples were collected from the surface of CMC in five denitrification reactors using 0.1 M PBS (Phosphate buffer saline). Sampling methods for microorganisms are the elution method and described in Additional file 1: Text S2. The 1470E electrochemical workstation (Solartron Technology Co., Ltd) was used to analyze Nyquist impedance and cyclic voltammogram curves with a three-electrode system. The specific test steps and parameters are described in the Additional file 1: Text S3.
2.4 Denitrification contribution ratios test for 2-MPDR
The denitrification contribution ratios test was designed to determine the contribution of chemical, autotrophic and heterotrophic denitrification to the NO3−-N removal from 2-MPDR. Three groups of reaction systems labeled as chemical denitrification (CD), autotrophic denitrification (AD) and heterotrophic denitrification (HD) were established. Each group contained 6 Schott bottles, and the Schott bottles in AD and HD groups were each added with the same amounts of CMCs mass, while the Schott bottles in CD group contained CMCs with different quality gradients. In CD, different amounts of CMC additives were set to determine the relationship between CMC addition and denitrification rate, and then the chemical denitrification rates in AD and HD were calculated. Schott bottles were labeled as 1-CD, 2-CD, 3-CD, 4-CD, 5-CD and 6-CD according to the amount of CMC additives in ascending order. Twelve CMCs with attached biofilms were gathered from the upper, middle and bottom layers of 2-MPDR by the end of stage 3–3. The biofilms were then eluted into 200 mL of the identical aqueous solution as the stage 3 influent and preincubated under the same operating conditions for 24 h. The biofilm solution was evenly allocated into groups AD and HD. AD and HD were subsequently added with equal quality of CMCs, and then fed with sterilized influent used in stage 9 through a 1:4 effective volume of CMC and water volume. All Schott bottles were set in a shaking incubator at 100 rpm and 30 °C. In addition, AD was injected with 50 mg L−1 NaHCO3 to determine the autotrophic denitrification rate, whereas HD was fed with an identical influent using methanol as the organic carbon source (COD = 28.58 mg L−1) to determine the gross denitrification rate. The influents of AD and HD shared the same carbon mass fraction, and all Schott bottles were sealed with rubber stoppers. At 0, 10, 20, 40, 60, 90, 120, 180 and 240 min, 2 mL samples were aspirated from each Schott bottle with a syringe, and processed in triplicate each time. The Schott bottles remained sealed throughout the sampling process. Denitrification rates of different pathways were calculated by the following equations:
where, S1, S2 and S3 (g N (m3 d)−1) are the rates of chemical denitrification, autotrophic denitrification and heterotrophic denitrification, respectively; C1 and C2 are the final NO3−-N concentrations in AD and HD (mg L−1); Cx and Cy are the initial NO3−- N concentrations in AD and HD (mg L−1); M is the total volume of the reaction system (m3); V is the total volume of the reaction solution (L); T is the reaction time up to the time of sampling (d).
3 Results and discussion
3.1 Performance of MPDRs for nitrate-containing water treatment
Five reactors were continuously operated for 137 days to investigate the interaction between different mZVI-carbon ratios and denitrification performance (Fig. 1a), and the operational phase was divided into three stages with HRT, influent NO3− concentration and additional carbon source as variables (Table 1). In each stage, the denitrification reactors demonstrated a certain potential for NO3−-N removal. In general, 2-MPDR showed a consistent and effective performance in denitrification capacity under various operating conditions. During the whole experimental period, 2-MPDR achieved a maximum NO3−-N removal efficiency of 97.6 ± 1.3% and a maximum denitrification load of 79.68 ± 1.92 g N (m3 d)−1, achieving a basic removal of NO3−. The NO3−-N removal efficiency in four reactors ( not including C-MPDR) decreased with the shortening of hydraulic retention time (HRT) in stage 1, but the denitrification load continued to increase as a response to more frequent interactions. In stage 2 (from day 48–89), the differences in denitrification performance between individual denitrification reactors increased as the influent NO3− concentration increased. C-MPDR presented an enhanced denitrification potential under high NO3−-N concentration influent conditions, and this effect can be attributed to the absorption capacity of AC for NO3−. Subsequently, COD at 10, 20 and 40 mg L−1 was introduced into the denitrification reactors in stage 3 (from day 96–137) to investigate the potential impact of carbon sources on the heterotrophic denitrification pathway. The introduction of methanol assisted each denitrification reactor in reducing NO3−-N, demonstrating the potential for heterotrophic denitrification in each denitrification reactor. Unexpectedly, the effluent NO3− of M-MPDR was not significantly reduced in response to the addition of methanol. In any case, the denitrification capacity of M-MPDR was lower than that of other reactors except C-MPDR in all reaction stages.
Performance of denitrification reactors during the continuous operation (stages 1–3). a The influent NO3−-N; the effluent NO3−-N of C-MPDR, 1-MPDR, 2-MPDR, M-MPDR and V-MPDR; b NH4+-N concentration, NO2−-N concentration, NO3−-N removal loads, NO3−-N removal efficiency and NH4+ generation rate in 2-MPDR in entire test stage (0–137 d)
In addition, CMC produced by AC enabled 2-MPDR in enhancing the average denitrification rate by 8.4% relative to V-MPDR (P = 0.057). The enhanced denitrification performance of 2-MPDR could be linked to the acid modification, which is likely to facilitate the electron transfer process between NO3− and mZVI. And the oxidation rate of mZVI has been established as the rate-limiting factor for the chemical denitrification pathway. 2-MPDR demonstrated a greater NO3−-N removal capacity than 1-MPDR in both stages 1 and 2, which provides strong evidence that CMC performed the most effective NO3−-N removal at a 2:1 ratio of mZVI to carbon. Without mZVI, C-MPDR demonstrated a nominal denitrification capacity in stages 1 and 2, and this further suggests that multipathway denitrification primarily occurred in the presence of mZVI.
With the observation of nitrogen form transformation in 2-MPDR (Fig. 1b), we found the hybrid denitrification process combined chemical and biological denitrification in the denitrification reactors. In stage 1, NH4+-N concentration decreased from the highest 2.53 ± 0.26 mg L−1 to 0.71 ± 0.09 mg L−1 as HRT dropped from 24 to 12 h and 6 h. Simultaneously, higher rates of NH4+ production were observed at longer HRT, implying that the chemical denitrification reaction requires at least 12 h for sufficient interaction. Moreover, a significant increase in effluent NH4+-N concentration was attributed to the increase in influent NO3−-N concentration in stage 2. This result was assigned to the promotion of reaction ③ and ⑤ (Additional file 1: Text S4), as the increase in NO3− concentration enhanced the frequency of collisions between mZVI and NO3− ions. We found that the concentration of NO2−-N in M-MPDR was much higher than in other reactors throughout the whole stages (Additional file 1: Fig. S1). This result indicated that the pronounced reaction occurring in M-MPDR was chemical denitrification involving reaction ④ (Additional file 1: Text S4). Interestingly, we observed a lower effluent concentration of NO2−-N in 2-MPDR than in M-MPDR, while 2-MPDR exhibited higher NO3−-N removal, suggesting the presence of the microbial-induced biological denitrification pathway. Since M-MPDR effluent had the highest concentrations of NO2−-N, it is puzzling that M-MPDR employing only mZVI resulted in such a low NO3−-N removal rate. At the start of the experiment, we anticipated that AC only performed an auxiliary role, while mZVI was the predominant reactant for multipathway denitrification. It is speculated that AC could provide an abundant pore structure for microbial growth and metabolism while promoting the rate of mZVI oxidation, and the abundant elements on the surface of AC also supported the growth of related denitrifying bacteria. In the SEM–EDS assay, a variety of elements such as phosphorus (P) and nitrogen (N) were detected on the surface of AC (Additional file 1: Fig. S2).
3.2 Observation on the surface of CMC in different MPDRs
SEM analysis was performed after stage 3–3 to visualize the microstructures of CMC, and the bacteria occurrence in the biofilms. Figure 2 illustrates the adhesive's (phenolic resin) porous microstructure of CMC. Given that both iron and carbon materials have smooth surfaces, the porous microstructure of CMC could extend to a larger area based on iron and carbon, which could be conducive to providing more sites for the proliferation of denitrifying bacteria. The microstructure of mZVI surface was rough and strongly weathered, and microbes in the biofilm were observed to colonize the mZVI surface (red dashed line) (Fig. 2B). The irregular structure could effectively alleviate the passivation of Fe by reducing the exposed area, thus allowing the continuous oxidation of mZVI and the transfer of electrons. Additionly, the relative content of oxygen (O) element on the CMC surface in 2-MPDR was significantly higher than that in V-MPDR, while the amounts of Fe and C were relatively reduced (Additional file 1: Fig. S2).
Although it was observed that Fe transferred electrons to NO3− in the reactors and promoted inefficient denitrification by AC, the mineral species formed by the oxidation of mZVI on the CMC surface in different reactors was unclear at the end of the experiment. XRD patterns confirmed that the presence of AC promoted the generation of goethite on the CMC surface in 2-MPDR. The diffraction peaks at 2θ values of 26.2° (002) and 42.8° (100) revealed the graphitized properties of carbon after denitrification in C-MPDR (Mitravinda et al. 2021). For M-MPDR, three diffraction peaks occurred at around 44.7° (110), 65.0° (200), and 82.3° (211) corresponding to the α-Fe crystalline phase. The diffraction peaks of Fe were still detectable throughout the experiment. This suggests the incomplete reaction of mZVI in M-MPDR within the experimental period. In addition, diffraction peaks representing carbon at 2θ values of 26.6° and goethite (α-FeOOH) at 2θ values of 21.2° (101) and 36.6° (021) were detected on the surface of 2-MPDR. Goethite was the primary product of the multipathway denitrification, deposited on the surface of mZVI, and was observed in Fe0 corrosion progression elsewhere (Yang et al. 2012; Li et al. 2015). Fe3O4 as an iron mineral with excellent electron transfer capacity was not observed in denitrification reactors, which was attributed to the specific environmental elements (high pH and low ORP) necessary for Fe3O4 generation (Chen et al. 2004), while the pH in 2-MPDR remained around 7.8 throughout the experiment. It is known that the precipitation of goethite on the CMC surface can potentially inhibit the continued oxidation of mZVI. Furthermore, the oxidation process of carbon and mineral intercalation could form a biochar-mineral composite layer on the surface, and this effect could result in greater resistance to chemical oxidation (Wang et al. 2021a). The obvious Fe-carbon composite layer was not observed in this study, thus indicating that the rate of mZVI oxidation was not limited in 2-MPDR.
3.3 Contribution ratios of denitrification in different pathways in 2-MPDR
Analysis of long-term experiments confirmed the pronounced chemical denitrification process in MPDRs, while the bio-denitrification pathway could also be involved in the NO3− removal process, but the contribution of individual pathway remains unclear. The denitrification contribution ratios test was performed to determine the proportion of different denitrification pathways in NO3−-N removal in 2-MPDR. AD and HD (exogenous NaHCO3, methanol) differed in the initial NO3−-N concentration since the added microbial inoculum contained a small amount of nitrogen. The result in CD group indicated a significant correlation between the NO3−-N removal rate and the mass of CMC addition (P < 0.01). CD-6 exhibited a greater NO3−-N removal efficiency of 81.9% with maximum CMC addition, but it was slightly lower than the 91.5% removal efficiency in HD but higher than the 76.7% removal efficiency in AD (Fig. 3a). The results indicated that the chemical denitrification was more pronounced than biological denitrification in the initial experimental period. Low concentrations of NH4+-N and NO2−-N accumulated in HD and AD before the test, with the NO2−-N concentration decreasing slowly throughout the test (Fig. 3b). NH4+-N accumulated in the first 10 min, but decreased in the subsequent 230 min. This trend further proved the dominant role of chemical denitrification in the early stage of the reaction. The addition of microorganisms increased the average concentrations of Fe (Fe2+ and Fe3+) in reaction systems (Additional file 1: Fig. S3a), which could be attributed to the facilitated dissolution of mZVI by the secretion of denitrifying microorganisms.
The addition of CMC in CD was fitted to the NO3−-N removal rate as a linear fitting equation for calculating the contributions of chemical denitrification reactions in AD and HD (Additional file 1: Fig. S3b). NO3−-N removal was calculated based on the average NO3−-N concentration at 240 min HRT (7.01 mg L−1 in AD and 2.46 mg L−1 in HD). The average removal of NO3−-N in AD and HD was calculated to be 48.47 and 45.64 g N (m3 d)−1, respectively. Based on the linear fitting equation of the chemical denitrification rate in CD, the contributions of autotrophic denitrification pathway in AD and heterotrophic denitrification pathway in HD were calculated to be 19. 58 and 14.45 g N (m3 d)−1. The result showed that chemical, autotrophic and heterotrophic denitrification accounted for 57.3%, 24.6% and 18.1% of the total denitrification, respectively. However, autotrophic denitrification (84.1%) occupied a greater share compared to heterotrophic denitrification (18.9%) in similar reported iron-carbon reactors (Deng et al. 2016). Although heterotrophic denitrification was thermodynamically superior to autotrophic denitrification (Rezania et al. 2005), autotrophic denitrification was observed as the more dominant bio-denitrification pathway in this study.
3.4 Determination and variance analysis of microbial communities in MPDRs
Limited by the reaction time and hydraulic conditions in denitrification reactors, the acidification of biochar had no significant contribution to the denitrification capacity of denitrification reactors, while increasing the ratio of mZVI to carbon in CMC remarkably reduced the NO3−-N effluent concentration in these long-term trials. Therefore, it is necessary to ascertain the impact of AC and ratios of mZVI to carbon on community structures and the variations of autotrophic and heterotrophic denitrification populations in each reactor through microbial and enzyme activity analysis. The dominant species of the genus in denitrification reactors were successively Acidovorax, Azoarcus and Limnobacter, all of which belong to the phylum Proteobacteria (Fig. 4). Proteobacteria were the predominant microbial phylum in denitrification reactors and were also identified as the phylum of most denitrifying bacteria widely existing in sewage treatment plants (Additional file 1: Fig. S4) (Chen et al. 2018). Acidovorax, which belongs to the phylum of Proteobacteria, is widely recognized as dominant mixotrophic denitrifying bacteria (Shen et al. 2013; Jiang et al. 2020). The majority of species in the genus Azoarcus could reduce NO3− and NO2− (Myung et al. 2018). It is noteworthy that this genus was found mainly in numerous bio-electrochemical studies (Shehab et al. 2013; Mei et al. 2015), while the application of electrodes to enhance electron transfer shares a certain degree of similarity with mZVI-carbon micro-electrolytic galvanic cells. Previous studies have demonstrated that Limnobacter was the heterotrophic denitrifying bacteria with the ability to reduce NO3− and NO2−(Wang et al. 2021b). Additionally, Denitratisoma and Thauera are both well-known relevant denitrifying bacteria, both of which were significantly dispersed in 2-MPDR (10.22% and 5.07%, respectively) and V-MPDR (6.96% and 1.46%, respectively) (Wang et al. 2021b). By accounting for the relative abundance of autotrophic and denitrification-related genera, it was revealed that the average percentage of autotrophic denitrifying bacteria increased from 1.2% to 5.6% with the application of AC than virgin carbon. The above results suggest that AC could further promote the growth of autotrophic denitrifying bacteria and assist the expansion of the dominance ascribed to autotrophic denitrifying bacteria. This effect may originate from the acceleration of electron transfer related to mZVI. It is worth noting that according to α-diversity analysis (Shannon and Simpson index), microorganisms in M-MPDR manifested the greatest diversity and absolute abundance, while the lowest microbial abundance was found in 1-MPDR and 2-MPDR utilizing AC (Fig. 4a). Samples from M-MPDR did not cluster significantly in the principal component analysis (PCA), revealing a greater bacterial species abundance (Additional file 1: Fig. S5). It was consistent with the α-diversity analysis (Fig. 4a) and disclosed the most significant differences in microbial community composition between M-MPDR and other denitrification reactors. Simultaneously, the samples from 2-MPDR and V-MPDR were clustered closely along PCA 2 axis, indicating a shared majority microbial community composition across both systems.
a Relative abundance of microbial composition at the genus level in denitrification reactors; b Relative abundance of functional genes encoding for NR, NAR, NIR, NCR and NOR in 2-MPDR and V-MPDR; c Relative abundance of functional genes encoding for NAD(P)H dehydrogenase, ubiquinone, riboflavin and iron acceptor protein in reactors
Moreover, it was observed that the relative abundance of Thermomonas and Thauera was higher in 2-MPDR (0.23% and 5.07%) compared to V-MPDR (0.06% and 1.46%). In previous studies, Thermomonas and Thauera were identified to be efficient in removing NO3− using hydrogen as an electron donor (Wang et al. 2022; Shi et al. 2022). The NO3−-dependent genera Azospira (1.94%) and Pseudomonas (1.47%), known as denitrifying bacteria, were also detected exclusively in the biofilm of 2-MPDR (Zhang et al. 2021). Notably, Comamonadaceae, identified to use NO2− for anaerobic respiration (Yang et al. 2020), was observed with the highest relative abundance (3.30%) in V-MPDR. In contrast, this dominant bacterium was not detected in M-MPDR, which also had NO2−-N accumulation in long-term trials. This result was attributed to the absence of NO2− conversion pathway in M-MPDR, which took chemical denitrification as the main denitrification reaction.
Based on the above analysis, the relative abundance of denitrification functional genes was associated with the denitrification capacity of denitrification reactors by encoding denitrification functional enzymes. The relative abundance of denitrification functional genes encoding NO3− and NO2− reductase (NR), nitrate reductase (NAR), nitrite reductase (NIR), nitric oxide reductase (NCR), and nitrous oxide reductase (NOR) were observed to be highest in 2-MPDR (Fig. 4b). All of these functional genes showed a synergistic effect in catalyzing the reduction of NO3− to N2(Chen et al. 2018), thereby contributing to the denitrification process. Additional file 1: Fig. S4 illustrates that the relative abundance of relevant denitrification functional genes in different reactors was ranked as 2-MPDR > V-MPDR > 1-MPDR > M-MPDR > C-MPDR. Thus, in the presence of mZVI, carbon has been shown to affect the bio-denitrification system, while AC could further promote microbial activity in bio-denitrification. It is worth noting that the gene encoding iron complex receptor protein was significantly expressed in 2-MPDR (Fig. 4c). Iron complex receptor proteins are closely associated with iron transporters, which serve as vectors for the microbial acquisition of iron-containing oxides and hydroxides (Zhou et al. 2021). These results suggested that AC could generate more iron oxides by promoting iron electron transfer, thus accelerating the uptake and utilization of iron complexes by microorganisms. Previous studies have confirmed that the uptake of iron by bacteria could promote the activity of nitrate and nitrite reductase (Pintathong et al. 2009; Zou et al. 2014). Thus, the presence of AC could assist in the enhancement of the inherent denitrification capacity of related bacteria strains by mZVI.
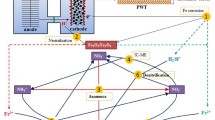
Nitrate reductase of anaerobic denitrifying bacteria is normally integrated with the cell membrane, and NADH dehydrogenase employs a redox medium as the electron shuttle under anaerobic conditions (Sarkar et al. 2017). The redox medium in this study was primarily the substrate used by denitrification microorganisms, such as scattered ferric ions and their compounds in solution. Besides, genes encoding for NAD(P)H dehydrogenase (K03885), ubiquinone (K06134) and riboflavin (K00793, K11753) biosynthesis were identified with higher relative abundance in reactor 2-MPDR (Additional file 1: Fig. S6), and these substances combined to promote the electron transfer process reagarding NO3− reduction. Based on the above results, the nitrogen metabolism and electron transfer pathways during denitrification were configured and displayed (Fig. 5). Overall, CMC increased the relative abundance of denitrification functional genes in 2-MPDR, demonstrating the enhanced bio-denitrification capacity in denitrification reactors, while AC further accelerated the electron transfer and nitrogen metabolism processes by promoting iron oxidation and hydrogen production.
3.5 Acidification enhanced the electron transfer capacity of AC in CMC-based multipathway denitrification
Although it is known that AC promoted the NO3−-N removal performance in 2-MPDR through the denitrification process and increased the expression of enzymes related to nitrogen metabolism, the potential impact of the acidification process on AC has not been confirmed. Results of electrochemical tests performed on AC indicate that the acidification process appeared to confer a higher electron transfer capacity to AC. Four different electrodes were fabricated with virgin carbon (C), HNO3 acidified carbon (C-N), H2O2 acidified carbon (C-H), and HNO3-H2O2 mixed acidified carbon (AC). As shown in Fig. 6a, the peak current varied between electrodes with different carbons applied. It was observed that AC exhibited the highest current of 1.78 mA, while the others exhibited currents of 1.32 mA (C-N), 0.79 mA (C-H) and 0.35 mA (C) around 0.6 V, respectively. The enhancement of peak current indicated a significant elevation of electron transfer capacity at the electrodes using AC. Theoretically, the area of the enclosed area represents the number of electrons transferred from the electrode and consequently reflects the capacitance of the electrode (Cakici et al. 2017; Zhong et al. 2018). The largest closed area was reached in the CV curve of AC, indicating that the mixed HNO3-H2O2acidification process assigned greater electrochemical activity and capacitance to AC compared to other carbon-based electrodes (Wu et al. 2019). Consequently, AC with a faster electron transfer rate can facilitate the electron transfer in the oxidation of Fe0 and NO3− reduction process in denitrification reactors.
As an indication of electrode potential loss, EIS analysis was performed to determine the Nyquist curves for the different carbon electrodes. The ohmic resistance obtained from the Nyquist curve is the electron transfer barrier induced by the electrical circuit structure and electrodes. Smaller diameter of the circle in the low-frequency region represents smaller resistance. Our results showed that AC demonstrated the smallest impedance arc diameter, followed by C-N, C-H and C (Fig. 6b). The unique half-circle in the high-frequency region illustrated the absence of diffusion effects in the circuit. Thus, AC could obtain higher currents, larger capacitance and lower apparent resistance, which indicated the expected potential to increase the electron transfer rate.
Further, XPS analysis on the carbon surface was performed to ascertain the mechanism of the enhanced electron transfer rate of AC. The results showed that the acidification process significantly increased the relative oxygen content of the carbon surface (Fig. 6c). The relative area of the O1s peak containing OH− and H2O increased by 77.4% after acid modification. Among them, the peak corresponding to OH− showed a significant increase in area, while the peak corresponding to lattice oxygen (H2O) remained unchanged (Goh et al. 2010). The addition of numerous oxygen-containing functional groups (hydroxyl and carboxyl groups) to the surface of carbon during acid modification process endowed it with unique redox properties, which could be responsible for the enhanced electron transfer rate. As previously mentioned, AC with higher capacitance and electron transfer rates exhibited considerable potential in promoting nitrogen metabolism by indirectly enhancing hydrogen production and iron oxidation.
3.6 Potential for advanced nitrate removal with 2-MPDR
To date, chemical and mixotrophic denitrification using the iron-carbon micro-electrolysis system is an effective solution for NO3−-N removal in secondary effluent and wastewater. Thus, carbon plays an important role in facilitating electron transfer and improving the microstructure. However, the iron-carbon micro electrolysis system still faces challenges. The low loading of Fe0 on carbon, susceptible oxidation, and paucity in nitrogen selectivity of conventional iron-carbon composites seemed to block further practical application. Limited contact area, poor electron transfer efficiency, and excessive iron usage also restrict the widespread application of simple iron-carbon compounds. CMC in this study could effectively help to improve the mentioned downsides and reduce iron consumption while ensuring an advanced NO3− removal rate through the denitrification process. Through the removal effect of trichloroethylene to the iron oxide layer on the CMC surface, the original denitrification performance of CMC can be regained after regeneration (Ritter et al. 2002). In addition, the lower manufacturing cost of CMC and the operating budget of 2-MPDR alleviate the concerns of practical applications. Combined with the contribution ratios of chemical, autotrophic and heterotrophic denitrification pathways mentioned above in this study and the equation concerning heterotrophic denitrification (Additional file 1: Text S5) (Mccarty 1969), the amount of additional carbon source required for complete denitrification was calculated. With an average influent concentration of 40 mg L−1 NO3−-N in 2-MPDR, an additional 7.82 mg L−1 methanol was required by calculation. This calculation was performed under the influent containing NO3−-N only, while the actual methanol demand will become greater in response to the consumption of NO2− and NH4+. Therefore, reducing additional carbon sources while maintaining the denitrification performance of CMC in low C/N ratio wastewater is of great practical importance for the efficient utilization of resources. Future research should target the operation mode of 2-MPDR to develop alternative removal strategies for water columns with various C/N ratios and mitigate additional operating costs.
4 Conclusion
In this study, a novel micro-electrolysis system coupled with mZVI and AC was used to construct denitrification reactors, with potential applications in large-scale engineering projects. The efficient and stable denitrification capacity of the reactors was verified in long-term tests, with reactor 2-MPDR achieving a maximum of 97.6% nitrate removal, while multiple pathways of denitrification reactions were observed. The denitrification contribution ratios test was conducted to further determine the contribution of chemical, autotrophic and heterotrophic denitrification in 2-MPDR. AC further improved the activity of enzymes encoding denitrification and the respiratory chain, thus increasing the rate of bio-denitrification. Interestingly the significant expression of iron complex receptor proteins increased the uptake of iron complexes in the medium, thus promoting the synthesis of iron sulfide protein in related denitrification enzymes. The improved denitrification capacity is ascribed to the elevated electrochemical activity of carbon by acidification process, which accelerated a series of reactions associated with iron corrosion. Overall, the iron-carbon coupling micro-electrolysis system with economic feasibility has promising applications in wastewater denitrification.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Ahn SC, Oh S-Y, Cha DK (2008) Enhanced reduction of nitrate by zero-valent iron at elevated temperatures. J Hazard Mater 156(1):17–22. https://doi.org/10.1016/j.jhazmat.2007.11.104
Auclair J, Lépine F, Parent S, Villemur R (2010) Dissimilatory reduction of nitrate in seawater by a Methylophaga strain containing two highly divergent narG sequences. ISME J 4(10):1302–1313. https://doi.org/10.1038/ismej.2010.47
Bibby TS, Nield J, Barber J (2001) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412(6848):743–745. https://doi.org/10.1038/35089098
Cakici M, Kakarla RR, Alonso-Marroquin F (2017) Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem Eng J 309:151–158. https://doi.org/10.1016/j.cej.2016.10.012
Chen S-S, Hsu H-D, Li C-W (2004) A new method to produce nanoscale iron for nitrate removal. J Nanopart Res 6(6):639–647. https://doi.org/10.1007/s11051-004-6672-2
Chen H, Zhao X, Cheng Y, Jiang M, Li X, Xue G (2018) Iron Robustly Stimulates Simultaneous Nitrification and Denitrification Under Aerobic Conditions. Environ Sci Technol 52(3):1404–1412. https://doi.org/10.1021/acs.est.7b04751
Deng S, Li D, Yang X, Xing W, Li J, Zhang Q (2016) Biological denitrification process based on the Fe(0)–carbon micro-electrolysis for simultaneous ammonia and nitrate removal from low organic carbon water under a microaerobic condition. Biores Technol 219:677–686. https://doi.org/10.1016/j.biortech.2016.08.014
Goh K-H, Lim T-T, Banas A, Dong Z (2010) Sorption characteristics and mechanisms of oxyanions and oxyhalides having different molecular properties on Mg/Al layered double hydroxide nanoparticles. J Hazard Mater 179(1):818–827. https://doi.org/10.1016/j.jhazmat.2010.03.077
Guo X, Yang Z, Liu H, Lv X, Tu Q, Ren Q, Xia X, Jing C (2015) Common oxidants activate the reactivity of zero-valent iron (ZVI) and hence remarkably enhance nitrate reduction from water. Sep Purif Technol 146:227–234. https://doi.org/10.1016/j.seppur.2015.03.059
Hosono T, Tokunaga T, Kagabu M, Nakata H, Orishikida T, Lin I-T, Shimada J (2013) The use of δ15N and δ18O tracers with an understanding of groundwater flow dynamics for evaluating the origins and attenuation mechanisms of nitrate pollution. Water Res 47(8):2661–2675. https://doi.org/10.1016/j.watres.2013.02.020
Jamieson J, Prommer H, Kaksonen AH, Sun J, Siade AJ, Yusov A, Bostick B (2018) Identifying and quantifying the intermediate processes during nitrate-dependent iron(II) oxidation. Environ Sci Technol 52(10):5771–5781. https://doi.org/10.1021/acs.est.8b01122
Ji M-K, Ahn Y-T, Ali Khan M, Abou-Shanab RAI, Cho Y, Choi J-Y, Je Kim Y, Song H, Jeon B-H (2011) Removal of nitrate and ammonium ions from livestock wastewater by hybrid systems composed of zero-valent iron and adsorbents. Environ Technol 32(16):1851–1857. https://doi.org/10.1080/09593330.2011.565079
Jiang L, Wu A, Fang D, Zhang Y, Shen Q, Xu X, Ji F (2020) Denitrification performance and microbial diversity using starch-polycaprolactone blends as external solid carbon source and biofilm carriers for advanced treatment. Chemosphere 255:126901. https://doi.org/10.1016/j.chemosphere.2020.126901
Lee C-S, Gong J, Huong CV, Oh D-S, Chang Y-S (2016) Macroporous alginate substrate-bound growth of Fe0 nanoparticles with high redox activities for nitrate removal from aqueous solutions. Chem Eng J 298:206–213. https://doi.org/10.1016/j.cej.2016.03.113
Li X, Wang H, Hu C, Yang M, Hu H, Niu J (2015) Characteristics of biofilms and iron corrosion scales with ground and surface waters in drinking water distribution systems. Corros Sci 90:331–339. https://doi.org/10.1016/j.corsci.2014.10.028
Liao Y, Li S, Zhu X, Dang Z, Tang S, Ji G (2021) The promotion and inhibition effect of graphene oxide on the process of microbial denitrification at low temperature. Biores Technol 340:125636. https://doi.org/10.1016/j.biortech.2021.125636
Liu X, Xu J, Huang J, Huang M, Wang T, Bao S, Tang W, Fang T (2019) Bacteria-supported iron scraps for the removal of nitrate from low carbon-to-nitrogen ratio wastewater. RSC Adv 9(6):3285–3293. https://doi.org/10.1039/C8RA09091B
Mccarty P (1969) Biological denitrification of wastewaters by addition of organic materials. In: Proc Industrial Waste Conference
Mei X, Guo C, Liu B, Tang Y, Xing D (2015) Shaping of bacterial community structure in microbial fuel cells by different inocula. RSC Adv 5(95):78136–78141. https://doi.org/10.1039/C5RA16382J
Meng F, Li Z, Lei C, Yang K, Lin D (2021) Removal of trichloroethene by iron-based biochar from anaerobic water: Key roles of Fe/C ratio and iron carbides. Chem Eng J 413:127391. https://doi.org/10.1016/j.cej.2020.127391
Mitravinda T, Anandan S, Sharma CS, Rao TN (2021) Design and development of honeycomb structured nitrogen-rich cork derived nanoporous activated carbon for high-performance supercapacitors. Journal of Energy Storage 34:102017. https://doi.org/10.1016/j.est.2020.102017
Myung J, Yang W, Saikaly PE, Logan BE (2018) Copper current collectors reduce long-term fouling of air cathodes in microbial fuel cells. Environ Sci: Water Res Technol 4(4):513–519. https://doi.org/10.1039/C7EW00518K
Oh S-Y, Seo Y-D, Kim B, Kim IY, Cha DK (2016) Microbial reduction of nitrate in the presence of zero-valent iron and biochar. Biores Technol 200:891–896. https://doi.org/10.1016/j.biortech.2015.11.021
Pennino MJ, Compton JE, Leibowitz SG (2017) Trends in drinking water nitrate violations across the United States. Environ Sci Technol 51(22):13450–13460. https://doi.org/10.1021/acs.est.7b04269
Picetti R, Deeney M, Pastorino S, Miller MR, Shah A, Leon DA, Dangour AD, Green R (2022) Nitrate and nitrite contamination in drinking water and cancer risk: A systematic review with meta-analysis. Environ Res 210:112988. https://doi.org/10.1016/j.envres.2022.112988
Pinney ML, Westerhoff PK, Baker L (2000) Transformations in dissolved organic carbon through constructed wetlands. Water Res 34(6):1897–1911. https://doi.org/10.1016/S0043-1354(99)00330-9
Pintathong P, Richardson DJ, Spiro S, Choorit W (2009) Influence of metal ions and organic carbons on denitrification activity of the halotolerant bacterium, Paracoccus pantotrophus P16 a strain from shrimp pond. Electron J Biotechnol. https://doi.org/10.2225/vol12-issue2-fulltext-12
Rezania B, Cicek N, Oleszkiewicz J, a. (2005) Kinetics of hydrogen-dependent denitrification under varying pH and temperature conditions. Biotechnol Bioeng 92(7):900–906. https://doi.org/10.1002/bit.20664
Ritter K, Odziemkowski MS, Gillham RW (2002) An in situ study of the role of surface films on granular iron in the permeable iron wall technology. J Contam Hydrol 55(1):87–111. https://doi.org/10.1016/S0169-7722(01)00187-5
Rivett MO, Buss SR, Morgan P, Smith JWN, Bemment CD (2008) Nitrate attenuation in groundwater: A review of biogeochemical controlling processes. Water Res 42(16):4215–4232. https://doi.org/10.1016/j.watres.2008.07.020
Sadeq M, Moe CL, Attarassi B, Cherkaoui I, ElAouad R, Idrissi L (2008) Drinking water nitrate and prevalence of methemoglobinemia among infants and children aged 1–7 years in Moroccan areas. Int J Hyg Environ Health 211(5):546–554. https://doi.org/10.1016/j.ijheh.2007.09.009
Salam MA, Fageeh O, Al-Thabaiti SA, Obaid AY (2015) Removal of nitrate ions from aqueous solution using zero-valent iron nanoparticles supported on high surface area nanographenes. J Mol Liq 212:708–715. https://doi.org/10.1016/j.molliq.2015.09.029
Sarkar S, Banerjee A, Halder U, Biswas R, Bandopadhyay R (2017) Degradation of synthetic azo dyes of textile industry: a sustainable approach using microbial enzymes. Water Conserv Sci Eng 2(4):121–131. https://doi.org/10.1007/s41101-017-0031-5
Shehab N, Li D, Amy GL, Logan BE, Saikaly PE (2013) Characterization of bacterial and archaeal communities in air-cathode microbial fuel cells, open circuit and sealed-off reactors. Appl Microbiol Biotechnol 97(22):9885–9895. https://doi.org/10.1007/s00253-013-5025-4
Shen Z, Zhou Y, Hu J, Wang J (2013) Denitrification performance and microbial diversity in a packed-bed bioreactor using biodegradable polymer as carbon source and biofilm support. J Hazard Mater 250–251:431–438. https://doi.org/10.1016/j.jhazmat.2013.02.026
Shi L-D, Gao T-Y, Wei X-W, Shapleigh JP, Zhao H-P (2022) pH-dependent hydrogenotrophic denitratation based on self-alkalization. Environ Sci Technol. https://doi.org/10.1021/acs.est.2c05559
Si Z, Song X, Wang Y, Cao X, Wang Y, Zhao Y, Ge X, Sand W (2020) Untangling the nitrate removal pathways for a constructed wetland- sponge iron coupled system and the impacts of sponge iron on a wetland ecosystem. J Hazard Mater 393:122407. https://doi.org/10.1016/j.jhazmat.2020.122407
Wang W, Jin Z, Li T, Zhang H, Gao S (2006) Preparation of spherical iron nanoclusters in ethanol–water solution for nitrate removal. Chemosphere 65(8):1396–1404. https://doi.org/10.1016/j.chemosphere.2006.03.075
Wang L, Gao C, Yang K, Sheng Y, Xu J, Zhao Y, Lou J, Sun R, Zhu L (2021a) Effects of biochar aging in the soil on its mechanical property and performance for soil CO2 and N2O emissions. Sci Total Environ 782:146824. https://doi.org/10.1016/j.scitotenv.2021.146824
Wang S, Yu H, Su Q, Zuo J (2021b) Exploring the role of heterotrophs in partial nitritation-anammox process treating thermal hydrolysis process - anaerobic digestion reject water. Biores Technol 341:125762. https://doi.org/10.1016/j.biortech.2021.125762
Wang J, Huang JJ, Zhou Y, Liao Y, Li S, Zhang B, Feng S (2022) Synchronous N and P removal in carbon-coated nanoscale zerovalent iron autotrophic denitrification─the synergy of the carbon shell and P removal. Environ Sci Technol 56(18):13314–13326. https://doi.org/10.1021/acs.est.2c02376
Wu X, Meng L, Wang Q, Zhang W, Wang Y (2019) Highly flexible and large areal/volumetric capacitances for asymmetric supercapacitor based on ZnCo2O4 nanorods arrays and polypyrrole on carbon cloth as binder-free electrodes. Mater Lett 234:1–4. https://doi.org/10.1016/j.matlet.2018.09.053
Xing W, Li D, Li J, Hu Q, Deng S (2016) Nitrate removal and microbial analysis by combined micro-electrolysis and autotrophic denitrification. Biores Technol 211:240–247. https://doi.org/10.1016/j.biortech.2016.03.044
Yang F, Shi B, Gu J, Wang D, Yang M (2012) Morphological and physicochemical characteristics of iron corrosion scales formed under different water source histories in a drinking water distribution system. Water Res 46(16):5423–5433. https://doi.org/10.1016/j.watres.2012.07.031
Yang Q, Zhao N, Wang H, Huang B, Yan Q (2020) Electrochemical and biochemical profiling of the enhanced hydrogenotrophic denitrification through cathode strengthening using bioelectrochemical system (BES). Chem Eng J 381:122686. https://doi.org/10.1016/j.cej.2019.122686
Zhang J, Hao Z, Zhang Z, Yang Y, Xu X (2010) Kinetics of nitrate reductive denitrification by nanoscale zero-valent iron. Process Saf Environ Prot 88(6):439–445. https://doi.org/10.1016/j.psep.2010.06.002
Zhang Y, Li Y, Li J, Hu L, Zheng X (2011) Enhanced removal of nitrate by a novel composite: Nanoscale zero valent iron supported on pillared clay. Chem Eng J 171(2):526–531. https://doi.org/10.1016/j.cej.2011.04.022
Zhang Y, Douglas GB, Kaksonen AH, Cui L, Ye Z (2019) Microbial reduction of nitrate in the presence of zero-valent iron. Sci Total Environ 646:1195–1203. https://doi.org/10.1016/j.scitotenv.2018.07.112
Zhang Y, Jiao X, Liu N, Lv J, Yang Y (2020) Enhanced removal of aqueous Cr(VI) by a green synthesized nanoscale zero-valent iron supported on oak wood biochar. Chemosphere 245:125542. https://doi.org/10.1016/j.chemosphere.2019.125542
Zhang M, Li Z, Häggblom MM, Young L, Li F, He Z, Lu G, Xu R, Sun X, Qiu L, Sun W (2021) Bacteria responsible for nitrate-dependent antimonite oxidation in antimony-contaminated paddy soil revealed by the combination of DNA-SIP and metagenomics. Soil Biol Biochem 156:108194. https://doi.org/10.1016/j.soilbio.2021.108194
Zhang S, Kong Z, Wang H, Yan Q, Vayenas DV, Zhang G (2022) Enhanced nitrate removal by biochar supported nano zero-valent iron (nZVI) at biocathode in bioelectrochemical system (BES). Chem Eng J 433:133535. https://doi.org/10.1016/j.cej.2021.133535
Zhao Y, Feng C, Wang Q, Yang Y, Zhang Z, Sugiura N (2011) Nitrate removal from groundwater by cooperating heterotrophic with autotrophic denitrification in a biofilm–electrode reactor. J Hazard Mater 192(3):1033–1039. https://doi.org/10.1016/j.jhazmat.2011.06.008
Zhong D, Liao X, Liu Y, Zhong N, Xu Y (2018) Enhanced electricity generation performance and dye wastewater degradation of microbial fuel cell by using a petaline NiO@ polyaniline-carbon felt anode. Biores Technol 258:125–134. https://doi.org/10.1016/j.biortech.2018.01.117
Zhou D, Liang X, Wang J, Wang S, Li X, Ning Y (2021) Study on the regulatory mechanism of the earthworm microbial community in vitro and in vivo under cadmium stress. Environ Pollut 279:116891. https://doi.org/10.1016/j.envpol.2021.116891
Zhou X, Zhang C, Li Y, Xiong X, Wang Y, Rong S (2022) Promoted microbial denitrification and carbon dioxide fixation via photogenerated electrons stored in novel core/shell memory photocatalysts in darkness. Chemosphere 303:135259. https://doi.org/10.1016/j.chemosphere.2022.135259
Zou S, Yao S, Ni J (2014) High-efficient nitrogen removal by coupling enriched autotrophic-nitrification and aerobic-denitrification consortiums at cold temperature. Biores Technol 161:288–296. https://doi.org/10.1016/j.biortech.2014.03.066
Acknowledgements
We would like to thank Petar Ruler (HNC Mechanical and Production Eng) for his critical review of the manuscript and helpful suggestions.
Funding
This work was supported by the project from Science and Technology Department of Yunnan Tobacco Cooperation (#202020530000241006) and the key project from Zhejiang Science and Technology Bureaux (#2023C02002).
Author information
Authors and Affiliations
Contributions
RW: designed and carried out the experiment, analyzed the data, and wrote the manuscript. PJ: helped revise the manuscript. NB: helped revise the manuscript. XZ: supervision, writing—review & editing. HW: review & editing. MP: helped conduct EIS determination. JL: writing—review & editing. LC: writing—review & editing. JL: writing—review & editing. MH: writing—review & editing. YC: writing—review & editing. XY: Writing—review & editing. KD: writing—review & editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Baoshan Xing.
Supplementary Information
Additional file 1. Text S1.
Manufacturing process and parameters of CMC. Text S2. Sampling methods for microorganisms. Text S3. Determination procedure of electrochemical tests. Text S4. Main reactions of chemical denitrification involving iron. Text S5. Equation of the heterotrophic denitrification pathway. Fig. S1. The NO2--N and NH4+-N concentration in effluent of 2-MPDR and M-MPDR during the continuous operation (stages 1–3). Fig. S2. Relative content of surface elements on CMC in 2-MPDR (a) and V-MPDR (b) by EDS. Fig. S3. The Denitrification contribution rate tests for 2-MPDR. a Iron concentrations determined in CD, AD and HD through ICP-OES, b the linear fitting of the relationship between CMC addition and nitrate removal rate in CD. Fig. S4. Relative abundance of bacterial community at the phylum level in C-MPDR, 1-MPDR, 2-MPDR, M-MPDR and V-MPDR. Fig. S5. The principal component analysis (PCA) of microbial community grouped by C-MPDR, 1-MPDR, 2-MPDR, M-MPDR and V-MPDR. Fig. S6. The relative abundances of denitrification functional genes encoding for NR, NAR, NIR, NCR and NOR in C-MPDR, 1-MPDR, 2-MPDR, M-MPDR and V-MPDR.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, R., Jeyakumar, P., Bolan, N. et al. Enhanced denitrification driven by a novel iron-carbon coupled primary cell: chemical and mixotrophic denitrification. Biochar 6, 5 (2024). https://doi.org/10.1007/s42773-023-00274-2
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00274-2