Abstract
The structural reconstruction mechanism of lignin and cellulose-derived biochars during direct graphitization under ultra-high temperatures was intensively investigated. It was demonstrated that cellulose-derived char was almost composed of carbon microcrystallites, whereas lignin-derived char reserved some of its skeleton structures, and such structural difference played a vital role in the morphology of formed graphitic layers. The results illustrated that the graphitized lignin-derived sample under 2800 ℃ had graphitic degree of 89.53%, interlayer spacing of 0.3363 nm and electronic conductivity of 104.6 S cm−1, while cellulose-derived sample had graphitic degree of 76.74%, layer distance of 0.3374 nm, and electronic conductivity of only 48.8 S cm−1. Combined with the results of structural analysis of the chars derived from lignin and cellulose, it was inferred that the stable and aromatic ring containing skeleton structure in lignin was beneficial to the ring-enlarging reconstruction and the formation of large areas of continuous graphitic layers during graphitizing process, leading to high electronic conductivity. Meanwhile, the interwoven microcrystallites in cellulose-derived char strongly restricted the expanding of continuous lamellar graphitic areas even at such ultra-high temperature, causing the formation of turbostratic structure with numerous structural defects as well, and finally resulting in relatively lower electronic conductivity. This work is expected to provide theoretical guidance for preparing high-performance functional carbon materials from lignocellulosic biomass.
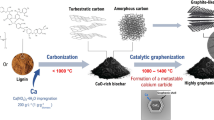
Graphical Abstract

Highlights
-
The different characteristics of lignin and cellulose resulted in the significant structural differences of the pyrolytic chars.
-
Large area of lamellar graphitic layers formed in lignin-derived graphitic carbon, of which the graphitization degree was as high as nearly 90%, leading to excellent electronic conductivity.
-
The structural differences of pyrolytic chars had a significant impact on the structural reconstruction under high temperature.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Owing to the characteristics of abundance, renewability and high carbon content (Carreira et al. 2023; Goswami et al. 2017), lignocellulosic resources have already been widely utilized for preparing numerous kinds of functional biochars. The functionalized biochars exhibited attractive potential applications in energy storage (Liu et al. 2019; Senthil and Lee 2021), and pollutant treatment (Ji et al. 2022; Qin et al. 2020; Zhao and Wang 2021). These biochars mainly include activated carbon (Phiri et al. 2019), carbon dots (Meng et al. 2019) and graphitic carbon/graphene (Abbas et al. 2018; Zhao et al. 2021), and especially, graphitic carbon/graphene has gained increasing attention due to its remarkable physical and chemical properties (Purkait et al. 2017). Nowadays, great efforts have been made to prepare lignocellulose-derived graphitic carbon/graphene, or so-called graphene-like materials via multiple pathways including salt-based method, template-based method and so on (Kong et al. 2020). However, the obtained carbon still had glassy-like structure and relatively low crystallinity, which restricts the enhancement of the physical and chemical characteristics.
Up to now, all kinds of methods for preparing lignocellulosic biomass-derived graphitic carbon are based on pyrolysis. Therefore, investigating the mechanism for the formation of char and graphitic structure is of great importance. As one kind of non-graphitizing carbon (Franklin 1951), biomass-derived char is always believed to be composed of interwoven graphite-like microcrystallites, which are resistant to structural transformation even under extremely high temperature (e.g., 2800 ℃). Therefore, numerous researches focused on the catalytic graphitization of biochars at obviously lower temperature with the assistance of other elements, generally some transition metals (Destyorini et al. 2021; Liu et al. 2012; Sevilla and Fuertes 2010; Thompson et al. 2015). Although those obtained results were exciting and the demanded temperature could be even lower than 1000 ℃, there were still some disadvantages that are difficult to overcome: graphitizing reaction can only occur at the contact positions between catalyst particles and carbon, and the areas without reacting with catalyst remain amorphous structure yet, leading to inhomogeneous products. Besides, the metallic catalyst particles can probably be wrapped by the formed graphitic shells, causing significant difficulty in purification by acid washing. To overcome these problems, direct graphitization at high temperature without metallic catalyst might be applicable, and increasing efforts have been made in recent years (Jiang et al. 2019; Kim et al. 2001; Rodríguez-Mirasol et al. 1996). For instance, graphitic film with ultra-high electronic conductivity (4500 S cm−1) was obtained from lignin/graphene oxide mixture via Joule heating process at 2500 K (Jiang et al. 2019), while “potato graphite” was prepared from biomass by laser irradiation (Banek et al. 2018). Therefore, high temperature played a key role in the structural transformation.
As mentioned above, char structure has a significant effect on graphitization, and therefore, it is important to investigate the structural characteristics of different biochars. It has been pointed out that the three major components of lignocellulosic resources: cellulose, lignin and hemicellulose, of which the content was 40–50%, 20–40% and 15–30%, respectively (McKendry 2002), are more likely to be pyrolyzed independently with negligible interactions, especially in woody biomass (Zhang et al. 2015). Besides, hemicellulose has low thermal stability due to its amorphous and branched structure (Wang et al. 2016; Yang et al. 2007), and in our pretests, xylan, which is usually utilized as the representative of hemicellulose (Dong et al. 2019), leaves negligible solid products after pyrolysis at 500 ℃ in a sealed crucible. Thus, we hold the opinion that lignin and cellulose make major contribution for the formation of biochars. However, the significant structural difference of lignin and cellulose might result in totally different mechanisms of structural transformation during pyrolysis and graphitization processes. Although the research focusing on graphitization of cellulose and lignin under high temperature is still rarely seen nowadays due to the harsh experimental conditions, it is worthwhile to investigate the mechanism of their structural transformation during graphitizing process in order to better reveal the high-value transformation and utilization of biomass resources.
Owing to the existing weakness in understanding the characteristics of the pyrolytic chars from lignocellulosic materials, and the effect of biochar structure on graphitizing under high temperature, the aim of this work is to investigate the mechanism of structural transformation during graphitization of lignin and cellulose-derived chars under temperature ranging from 1600 to 2800 ℃ via comparing the lattice, morphology and electronic conductivity of original chars and obtained samples, and the key point is to get better knowledge of the relationship between char structure and the corresponding graphitizing effect. This work is helpful for the further high-value utilization of lignocellulosic resources.
2 Experiment
2.1 Preparation of lignin and cellulose-derived chars
Microcrystalline cellulose, of which the average particle size was 50 μm, was obtained from Aladdin (Shanghai, China); Dealkaline lignin, of which the average particle size was 20 μm, was supplied by Tokyo Chemical Industry (Tokyo, Japan). According to thermalgravimetric (TG) results in former reports, the pyrolysis of cellulose almost ended before 500 ℃, and lignin accomplished majority of the weight lost at 500 ℃ as well (Wang et al. 2014; Dong et al. 2019). Therefore, 500 ℃ was suitable for obtaining chars from lignin and cellulose. In this work, lignin and cellulose were added in quartz boats, respectively, and carbonized from room temperature to 500 ℃ at a constant heating rate of 10 ℃ min –1 in a tubular furnace under nitrogen atmosphere, and kept for 1 h. The obtained chars were kept in a desiccator after cooling to room temperature. Besides, the TG spectra for lignin, cellulose, and the element analysis of both precursors and biochars are exhibited in Supporting Information.
2.2 Selective oxidation of chars
To investigate the structures of chars derived from lignin and cellulose in order to discover the relationship between char structure and graphitization effect, the selective oxidation process was adopted as reported by Zuo’s group (Miao et al. 2018). In short, 4 g lignin or cellulose-derived char was added into 100 mL oxidative acid mixture (nitric acid/sulphuric acid or nitric acid/perchloric acid) at boiling point for 1 h, followed by neutralization using ammonia solution after cooling to room temperature, and finally, large granulates, carbon microcrystallites and carbon nanoparticles could be collected by centrifugation and ultrafiltration, respectively. For convenience, only nitric acid/sulphuric acid mixture was utilized for all the experiments. All the obtained products were weighted after freeze-drying.
2.3 Preparation of graphitic carbons under ultra-high temperature
The obtained chars were transferred into hermetic graphite crucibles before further calcinated at 1600, 1800, 2000, 2200, 2400, 2600 and 2800 ℃, respectively, in an ultra-high temperature furnace under argon atmosphere. Both carbonization and graphitization processes were carried out at heating rate of 10 ℃ min –1, and all samples were held at the destination temperatures for 1 h. All the so-prepared samples were collected after cooling to room temperature followed by storing in a desiccator. For convenience, lignin or cellulose-based products were defined as L-X and C-X, respectively, where X represents the calcination temperature.
2.4 Structural characterization and electrical characteristics tests
Both as-prepared chars were characterized by high-resolution transmission electron microscopy (HRTEM), Fourier transform infrared spectrometer (FTIR), and X-ray photoelectron spectroscopy (XPS), and X-ray diffraction (XRD), Raman spectra, XPS and HRTEM were utilized for analyzing the graphitized samples (especially treated at 2800 ℃). For observing the morphology of the chars and graphitized products, all samples were well dispersed in alcohol with the assistance of ultrasonic, followed by coating to copper grids (Gilder Grids Ltd., Grantham, UK), which were further placed into a JEM2100 HRTEM, and operated at 200 kV accelerating voltage. FTIR spectra in the range of 525 to 4000 cm−1 were obtained from a Nicolet iS50 spectrometer (Thermo Fisher Scientific, Waltham, USA), of which the resolution factor was selected to be 1 cm−1. XPS data were recorded on a Kratos Axis Ultra DLD spectrometer (Kratos Analytical Co., Ltd, Manchester, UK). XRD analysis was carried out on a Bruker D8 operated at 40 kV and 20 mA with Cu Kα radiation (λ = 0.15406 nm) and with measuring angles ranging from 10° to 80°, and the scan rate was 5°min –1; Raman spectra/selected area mapping analysis was measured in the range of 50 to 3500 cm−1 on a DXR532 laser Raman spectrometer (equipped with a DXR2xi Raman Imaging Microscope), of which the excitation laser source was emitted at 532 nm. The electrical conductivity of all graphitized products was tested on a ST2722 powder resistivity tester (Jing Ge Electronic Co., Ltd, Suzhou, China) under a pressure of 20 MPa, and commercial conductive carbon black (XC-72R, Cabot Corporation, USA) was utilized as a benchmark; The electrochemical impedance spectra (EIS) of the graphitized samples at 2800 ℃ are shown in the Additional file 1: Supporting Information.
There are some important equations for calculating the lattice parameters of graphitic carbon according to XRD patterns. The Bragg Eq. (1), Scherrer Eq. (2) and Maire–Mering Eq. (3), which are utilized for calculating the lamellar spacing, crystallite size and graphitization degree, respectively, are listed below:
where d is the lamellar spacing; θ is the scattering angle; n is the integer (usually 1); λ is the radiation wavelength; L is the crystallite size in the prismatic direction (Lc) or in the basal plane (La); β is the full width at half maximum (FWHM); K is a dimensionless constant, usually assigned to be 1.84 and 0.89 for calculating La and Lc, respectively (Lim et al. 2020); g is the graphitization degree.
3 Results and discussion
3.1 Structural characterization of chars
The morphology and obtained lignin and cellulose-derived chars were characterized by HRTEM, XPS and FTIR as shown in Fig. 1. No distinct crystallized structure in lignin-derived char could be found as shown in Fig. 1a, indicating that during pyrolysis, lignin transformed to typical amorphous carbon structure that had already been described (Farid and Andou. 2022), which might be ascribed to the thermal-stable three-dimensional phenylpropanoid skeleton. On the other hand, as seen in Fig. 1b, cellulose-derived char had distinct interwoven graphite-like carbon microcrystallites as the arrows show, of which the size was around 5 nm, and these anisotropic microcrystallites were accumulated with 10–20 short layers. Therefore, cellulose-derived char was the typical non-graphitizing carbon as presented by Franklin (1951).
FTIR was adopted for investigating surveying the transformation of functional groups during pyrolysis, and the spectra are illustrated in Fig. 2. It is clear in Fig. 2a that the characteristic peaks at 3434 and 2914 cm−1, which were assigned to phenolic hydroxyl and methyl/methylene groups, could still be observed in the char, indicating that such functional groups were preserved at 500 ℃ owing to the thermal-stable phenylpropanoid units. Besides, it was noticeable that the peaks mainly assigned to aromatic skeletal vibration or syringyl ring breathing with C–O stretching within the range of 1100 to 1700 cm−1 (Sharma et al. 2004) mostly diminished, indicating the removal of partial substituents. On the contrary, in the spectrum of cellulose-derived char as shown in Fig. 2b, it is obvious that most of the major peaks assigned to hydroxyl or methyl/methylene groups totally disappeared after pyrolysis, indicating that such functional groups could be easily removed at 500 ℃ because these groups are aliphatic. Meanwhile, the newly emerged peak at 872 cm−1 indicated the formation of aromatic structure in the char.
C 1s spectra for lignin and cellulose-derived char are shown in Fig. 3. It was clear that carbon atoms in both chars existed in the form of C=C, C–C and C=O bonds which corresponded to peaks centered at 284.4 eV, 285.5 eV and 288.7 eV, respectively. According to the results from element analysis, lignin and lignin-derived chars had high carbon content and relatively lower hydrogen and oxygen content as shown in Additional file 1: Table S1, and it was reasonable that lignin-derived char had more C=C bonds (74.03%) than cellulose-derived char (64.95%). Furthermore, cellulose-derived char had obviously higher C–C group content (26.41%), and this result corresponded to the higher hydrogen and oxygen content as listed in Additional file 1: Table S1. The higher C–C bonds content might be attributed to the abundant amorphous carbon components that link the microcrystallites via chemical bonding (Franklin 1951). These amorphous components resulted in the chaotic arrangement of the microcrystallites, and could probably enhance the difficulties in forming continuous and regular lamellar graphitic structure under high temperature.
According to the previous report from Zuo’s group (Miao et al. 2018), the oxidized char could be separated into 3 parts with different particle sizes: large granulates, microcrystallites and fluorescent carbon nanoparticles, and the products were obtained via de-crosslinking by selectively oxidizing the amorphous structure between microcrystallites. Therefore, this method is suitable for charcoal composed of microcrystallites which are bonded with amorphous carbon component. The yield of the 3 kinds of particles by oxidizing lignin and cellulose-derived char is listed in Table 1. For lignin-derived char, all the mentioned products could be obtained, and the yields of large granulates, microcrystallites and carbon nanoparticles were 18.57%, 56.36% and 0.44%, respectively. On the other hand, only microcrystallites and carbon nanoparticles could be obtained after oxidizing cellulose-derived char, and noticeably, the yields of microcrystallites and carbon nanoparticles were 71.83% and 0.92%, respectively, distinctively higher than lignin-derived products. The overall experimental data were similar to the previous work in which coconut shell-derived activated carbon was investigated (Zuo et al. 2018, 2020, 2021, 2022). Interestingly, lower large granulates percentage (around 7%) was detected in that report than in our result from lignin-derived char (18.57%), indicating that the stable large granulates were almost derived from lignin. Therefore, we believe that lignin-derived char preserved partial stable skeleton during pyrolysis while cellulose-derived char totally transferred to tiny graphite-like microcrystallites.
For further structural comparison, the morphology of oxidized products—carbon nanoparticles, microcrystallites and large granulates was observed by HRTEM as illustrated in Fig. 4. The carbon nanoparticles and microcrystallites from lignin and cellulose had similar morphology, and therefore, the TEM images of carbon nanoparticles from lignin and microcrystallites from cellulose are exhibited below. The nanoparticles had size of less than 10 nm as shown by the arrow in Fig. 4a; Clearly microcrystallites with regular lamellar structure can be seen in Fig. 4b, and although the microcrystallites had aggregated before observation, they were obviously more ordered, and were no longer interwoven as compared with Fig. 1b. Large granulates with distinct size of larger than 500 nm, as shown in Fig. 4c, could only be obtained from lignin-derived char after such severe oxidative treatment, indicating that the aromatic ring-containing skeleton of lignin had higher thermal and chemical stability than the glucose structure of cellulose, and this was proved by TG analysis as seen in Additional file 1: Fig. S1.
3.2 Characterization of graphitized samples
XRD is of great importance for measuring the crystal structure of graphitic carbon due to its convenience of supplying lattice parameters such as interplanar spacing, layer stacking distance, longitudinal size of structural distance, and the degree of graphitization can be calculated (Li et al. 2007). XRD patterns of all samples after calcination are shown in Fig. 5a and b. It was obvious that the characteristic peak centered at around 26°, corresponding to (002) reflection of graphitic structure, appeared when calcinating temperature was higher than 2000 ℃ for both cellulose and lignin-derived samples, indicating that the occurrence of structural transformation required temperature higher than 2000 ℃, especially at temperatures ranging from 2200 to 2600 ℃ due to the most obvious increasement of (002) peak intensity. Besides, it was clearly seen that the intensity of (002) peaks of C-2600 and C-2800 appeared almost the same, while that of L-2800 was obviously higher than L-2600, indicating that high temperature benefits the formation of graphitic structure in lignin-derived structure rather than in cellulose-derived structure. For better comparison, the patterns of C-2200/L-2200 and C-2800/L-2800 were shown in Fig. 5c and d, respectively. It was clear that in the same heating temperature, lignin-derived products had obviously higher and sharper (002) peak than cellulose-derived products, especially for the comparison of the products obtained at 2800 ℃ as exhibited in Fig. 5d.
The lamellar spacing, Lc values and graphitization degree calculated according to the above equations, and the FWHM values of (002) peak for all lignin and cellulose-derived samples are listed in Table 2. Obviously, for both lignin and cellulose-derived products, the higher calcination temperature was, the smaller lamellar spacing, FWHM and the higher Lc value/graphitization degree of the products had. It was clearly seen that calcinating at temperatures above 2200 ℃ was essential for lamellar spacing decreasing to lower than 0.3440, which resulted in a positive value of g according to Eq. (2). Furthermore, lignin-derived products had distinctly lower lamellar spacing, FWHM and higher Lc value/graphitization degree than cellulose-derived products obtained under the same condition. For instance, the lamellar spacing and FWHM of L-2200/L-2800 were 0.3405 nm/0.3363 nm and 6.256 × 10–3 rad/3.961 × 10–3 rad, and the graphitization degrees of L-2200 and L-2800 were calculated to be 40.70% and 89.53%, respectively; On the contrary, the lamellar spacing and FWHM of C-2200/C-2800 were 0.3435 nm/0.3374 nm and 12.750 × 10–3 rad/5.639 × 10–3 rad, and the corresponding graphitization degrees were calculated to be only 5.81% and 76.74%. Compared with the data from lignocellulosic samples via catalytic graphitization (Sevilla and Fuertes 2010), the graphitized lignin and cellulose under ultra-high temperature had much better lattice data, especially for L-2800, of which the lamellar spacing was very close to graphite (0.3354 nm). Therefore, we believe that temperature played a vital role in graphitization rather than metallic catalysts.
In the Raman spectra of carbons, there are generally two distinct peaks centered at around 1360 cm−1, 1580 cm−1, which are defined as D and G band. These two peaks are attributed to local structural defects/disorder and graphitic structures, respectively (Shin et al. 2012). Besides, for graphitic carbons with high degree of sheet stacking, another characteristic peak labelled 2D band appears at around 2680 cm−1 (Schuepfer et al. 2020), and this is the overtone of the D band (Ferrari and Basko 2013). The D band is sensitive to structural defects, and therefore, the intensity or area ratio of these two peaks, ID/IG, is of great importance for evaluating graphitic structure (Wang et al. 1990). Lower ID/IG ratio indicates lower defect content and higher regularity, and vice versa (Tai et al. 2006). For better investigation, Raman mapping was utilized for measuring the Raman spectra in a selected area, and the ID/IG ratio distribution could be clearly seen. Figure 6 displays the Raman mapping results of C-2800 and L-2800, and in the mapping images, different colors correspond to different ID/IG ratios. As displayed in Fig. 6a, the mapping image of L-2800 contained large blue-colored areas which corresponded to the ID/IG ratio lower than 0.1, indicating the existence of extensive highly graphitic structure. The Raman spectrum at point A in red area illustrated in Fig. 6b had an ID/IG value of 0.41, while exhibited in Fig. 6c, the ID/IG value of point B in blue area was 0.08, which was even lower than the tested graphite samples (Kumar et al. 2013; Mori et al. 2018). Meanwhile, majority of the area in the mapping image of C-2800 is displayed as green color, which corresponded to ID/IG value ranging from 0.4 to 0.8, as illustrated in Fig. 6(d), indicating that there were more lattice defects. Besides, as demonstrated in Fig. 6e and f, the ID/IG value even at point C in the red area was as high as 0.90, and the value was 0.32 at point D in the blue area. The ID/IG values of both L-2800 and C-2800 were obviously lower than most reported lignocellulosic biomass-derived graphitic materials (Farid and Andou 2022), indicating the high crystallinity of the obtained samples.
Not only are the D and G bands vital for measuring graphitization, but also the 2D band is valuable as well. It was interesting that the 2D band had lower intensity than G band for L-2800 as seen in Fig. 6b and c, while the 2D band was the strongest peak in the spectrum for C-2800 as displayed Fig. 6e and f. According to previous reports, the shape of 2D band indicated the nature of the sample: the narrow and strong shape corresponded to regular structure even if the crystalline domain was not very large, while the broad and weak shape indicated the absence of order in the sample (Escribano et al. 2001). The FWHM values of 2D band for L-2800 were measured to be 69.05 and 64.39 cm−1 in Fig. 6b and c, respectively, while for C-2800, the 2D band had FWHM values of 60.82 and 36.90 cm−1 in Fig. 6e and f. Hence, it could be deduced that there were some tiny crystalline domains with relatively high structural regularity, and these tiny domains might be formed from some well-organized microcrystallites in the char after graphitization progress. However, these crystalline domains could not extend well due to the chaotic arrangement of microcrystallites, and could hardly be beneficial to improve the overall crystallinity.
The distribution of ID/IG values calculated from the mapping images of all the graphitized samples are listed in Fig. 7. It was clear that the area ratio of low ID/IG value increased with higher calcinating temperature, especially at temperatures above 2200 ℃. For lignin-derived samples, as displayed in Fig. 7a, areas with ID/IG value lower than 0.8 and 0.4 appeared after calcinating at 1800 ℃ and 2000 ℃, respectively, and areas with ID/IG value lower than 0.1 increased drastically when calcinating temperature rose to above 2400 ℃, indicating that such high temperature was essential for the formation of large continuous conjugate structure. Especially, when calcinating temperature rose to 2800 ℃, the area ratio with ID/IG value lower than 0.1 reached 73.43%, and only 0.40% of the analyzed area had ID/IG value ranging from 0.4 to 0.8; Nevertheless, as seen in Fig. 7b, in the case of cellulose-derived samples, ID/IG value remained higher than 1.2 until calcinating temperature rose to above 1800 ℃, and only 14.91% of the analyzed area had ID/IG value lower than 0.4 even after heating at 2800 ℃. Therefore, forming large area of graphitic layers with few defects in lignin-derived char was much easier than in cellulose-derived char, and this conclusion is consistent with the XRD results above.
C 1s spectrum is of great importance for investigating the ratios of carbon atoms with different chemical bonding states. Herein, the C 1s spectra of L-2800 and C-2800 are compared in Fig. 8. The obvious similarity was that both spectra could be separated, including 3 major peaks centered at 284.8 eV, 285.8 eV and 290.0 eV, which attributable to C=C group, C–C group and π–π* shakeup satellite (Gao et al. 2009) , respectively, and therefore, there were only 2 kinds of carbon atoms in both L-2800 and C-2800. From peak separation and area analysis, it was concluded that the content of C=C group was 83.2% for L-2800 and 75.2% for C-2800, along with C–C group content of 16.8% for L-2800 and 24.8% for C-2800. Compared with Fig. 3, it was clear that after calcination, the ratio of C=C group increased distinctly while C=O group was completely removed. Besides, the appearance of π–π* shakeup satellite peak indicated the increase of conjugated aromatic structure. Noticeably, although the ratio of C=C group for L-2800 and C-2800 had no significant difference, the graphitization effect was obviously different according to XRD and Raman results mentioned above. Therefore, the graphitization effect depended on the amount of conjugated sp2 carbon atoms, especially fused aromatic rings rather than simple C=C bonds, and it could be confirmed that compared with C-2800, more sp2 carbon atoms existed in the form of fused aromatic rings in L-2800, leading to larger conjugated areas and less structural defects.
HRTEM was adopted for in-depth investigation of the morphology and crystallinity, and the images of L-2800 and C-2800 are demonstrated in Fig. 9. The overall appearance of L-2800 was planar structure seen in Fig. 9a, and clearly laminar structure with 10–20 layers at the edge areas could be observed as exhibited in Fig. 9b. TEM electron diffraction image of L-2800 displayed bright diffraction rings and scattered diffraction spots as presented in Fig. 9c, suggesting that L-2800 was primarily composed of polycrystalline graphite. On the other hand, distinct twining structure could be observed in C-2800 as shown in Fig. 9d and e, and such structure was categorized as turbostratic graphite (Wang et al. 2017). Besides, in TEM electron diffraction image of C-2800, only diffraction rings could be seen, indicating that C-2800 had lower graphitic crystallinity compared with L-2800. According to the structural comparison of lignin and cellulose-derived chars mentioned above, it could be concluded that the remained stable skeleton structures in lignin-derived char were beneficial to reconstruction and formation of continuous graphitic areas, whereas the massive interwoven microcrystallites existing in cellulose-derived char could hardly transform into long-range ordered structure, but rather form to such turbostratic structure via limited reconstruction even under high temperature.
3.3 Electrical conductivity test for graphitized samples
It is well known that the integrated benzene rings in graphitic structure form giant π bonds where the electrons are delocalized and movable, and this characteristic endows graphitic carbon electrical conductivity. Therefore, graphitization degree strongly affects the electrical conductivities of carbon materials (Liu et al. 2012). The electrical conductivity makes graphitic carbon material an electrical conductor. Therefore, the electrical conductivity of all the graphitized products under a pressure of 20 MPa was tested, and the results are shown in Fig. 10. It was clear that lignin-derived products had obviously higher conductivity than cellulose-derived products graphitized at the same temperature. With the rising of calcination temperature from 1600 to 2800 ℃, the conductivity of lignin-derived products increased from 41.1 to 104.6 S cm−1, while that of cellulose-derived products only increased from 32.8 to 48.8 S cm−1. Besides, it was noticeable that the conductivity of lignin-derived products was enhanced drastically when calcination temperature rose to higher than 2400 ℃, indicating that for lignin-derived char, such high temperature played a significant role in structural reconstruction, forming large continuous areas of graphitic layers and eliminating lattice defects. On the other hand, the rising of conductivity of cellulose-derived products was inconspicuous even calcinated under 2800 ℃, suggesting that the effect of rising temperature on structural transformation and removing lattice defects was unsatisfying for cellulose-derived char due to its extremely stable interwoven microcrystallites. The difference of electrical conductivity further proved that the highly graphitized carbon material with few lattice defects and high electrical conductivity could be obtained from lignin rather than cellulose. The obtained samples via high-temperature graphitization had significantly higher electrical conductivities than the reported data. For instance, the sample derived from coconutshell-activated carbon via catalytic graphitization at 1000 ℃ had electrical conductivity of only 7.4 S cm−1 (Liu et al. 2012). Additionally, the conductivities of both L-2600 and L-2800 were obviously higher than commercial conductive carbon black (28.2 S cm−1), exhibiting great application prospect in battery, electrocatalysis and electromagnetic shielding. Furthermore, both L-2800 and C-2800 had similar EIS spectra as seen in Additional file 1: Fig. S2, indicating low electrical resistance. Future research will focus on exploiting potential application value of the prepared highly graphitized carbon from lignin.
4 Conclusion
According to the comparison of pyrolytic chars from lignin and cellulose, it was discovered that during pyrolysis, lignin-derived char was amorphous carbon, and contained partial of original skeleton owing to the stable phenylpropanoid units, while cellulose-derived char was almost composed of interwoven microcrystallites. Such significant distinction resulted in different performance for structural reconstruction during graphitization progress under ultra-high temperature: after calcination under 2800 °C, lignin-derived product formed continuous lamellar graphitic layers with high crystallinity and few structural defects, and the degree of graphitization was calculated to be 89.53%, which endowed electrical conductivity of 104.6 S cm−1 under 20 MPa; under the same treating condition, cellulose-derived product only had graphitization degree of 76.74% along with higher structural defect content, which resulted in obviously lower electrical conductivity of only 48.8 S cm−1 under 20 MPa. It was deduced that the possible underlying reason for such obvious dissimilarity was that the thermally and chemically stable skeleton in lignin played a significant role in generating continuous lamellar graphitic structure, while interwoven microcrystallites existing in cellulose-derived char could hardly transform into regular lamellar layers even under ultra-high temperature, leading to more structural defects and lower electrical conductivity. Although the interwoven microcrystallites in cellulose-derived char had negative effect on the formation of large-sized graphitic layers, according to the result of selective-oxidation experiments, it is expectable that nano-sized graphitic carbon might be obtained from the de-crosslinked microcrystallites after ultra-high temperature calcination, and further research is in progress.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Abbas A, Mariana L, Phan A (2018) Biomass-waste derived graphene quantum dots and their applications. Carbon 140:77–99. https://doi.org/10.1016/j.carbon/2018.08.016
Banek NA, Abele DT, McKenzie KR, Wagner MJ (2018) Sustainable conversion of lignocellulose to high-purity, highly crystalline flake potato graphite. ACS Sustain Chem Eng 6:13199–13207. https://doi.org/10.1021/acssuschemeng.8b02799
Carreira ARF, Veloso T, Macário IPE, Pereira JL, Ventura SPM, Passos H, Coutinho JAP (2023) The role of biomass elemental composition and ion-exchange in metal sorption by algae. Chemosphere 314:137675. https://doi.org/10.1016/j.chemosphere.2022.137675
Destyorini F, Yudianti R, Irmawati Y, Hardiansyah A, Hsu Y, Uyama H (2021) Temperature driven structural transition in the nickel-based catalytic graphitization of coconut coir. Diam Relat Mater 117:108443. https://doi.org/10.1016/j.diamond.2021.108443
Dong Z, Liu Z, Zhang X, Yang H, Li J, Xia S, Chen Y (2019) Pyrolytic characteristics of hemicellulose, cellulose and lignin under CO2 atmosphere. Fuel 256:115890. https://doi.org/10.1016/j.fuel.2019.115890
Escribano R, Sloan JJ, Siddique N, Sze N, Dudev T (2001) Raman spectroscopy of carbon-containing particles. Vib Spectrosc 26:179–186. https://doi.org/10.1016/S0924-2031(01)00106-0
Farid MAA, Andou Y (2022) A route towards graphene from lignocellulosic biomass: technicality, challenges, and their prospective applications. J Clean Prod 380:135090. https://doi.org/10.1016/j.jclepro.2022.135090
Ferrari AC, Basko DM (2013) Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat Nanotech 8:235–246. https://doi.org/10.1038/nnano.2013.46
Franklin RE (1951) Crystallite growth in graphitizing and non-graphitizing carbons. Proc R Soc Lond A 209:196–218. https://doi.org/10.1098/rspa.1951.0197
Gao W, Alemany LB, Ci L, Ajayan PM (2009) New insights into the structure and reduction of graphite oxide. Nat Chem 1:403–408. https://doi.org/10.1038/nchem.281
Goswami S, Banerjee P, Datta S, Mukhopadhayay A, Das P (2017) Graphene oxide nanoplatelets synthesized with carbonized agro-waste biomass as green precursor and its application for the treatment of dye rich wastewater. Process Saf Environ Prot 106:163–172. https://doi.org/10.1016/j.psep.2017.01.003
Ji J, Yuan X, Zhao Y, Jiang L, Wang H (2022) Mechanistic insights of removing pollutant in adsorption and advanced oxidation processes by sludge biochar. J Hazard Mater 430:128375. https://doi.org/10.1016/j.jhazmat.2022.128375
Jiang F, Yao Y, Natarajan B, Yang C, Gao T, Xie H, Wang Y, Xu L, Chen Y, Gilman J, Cui L, Hu L (2019) Ultrahigh-temperature conversion of biomass to highly conductive graphitic carbon. Carbon 144:241–248. https://doi.org/10.1016/j.carbon.2018.12.030
Kim D, Nishiyama Y, Wada M, Kuga S (2001) Graphitization of highly crystalline cellulose. Carbon 39:1051–1056. https://doi.org/10.1016/S0008-6223(00)00221-9
Kong X, Zhu Y, Lei H, Wang C, Zhao Y, Huo E, Lin X, Zhang Q, Qian M, Mateo W, Zou R, Fang Z, Ruan R (2020) Synethesis of graphene-like carbon from biomass pyrolysis and its applications. Chem Eng J. 399:125808. https://doi.org/10.1016/j.cej.2020.125808
Kuma N, Pandian R, Das PK, Ravindran TR, Dash S, Tyagi AK (2013) High-temperature phase transformation and low friction behaviour in highly disordered turbostratic graphite. J Phys D 46:395305. https://doi.org/10.1088/0022-3727/46/39/395305
Li ZQ, Lu CJ, Xia ZP, Zhou Y, Luo Z (2007) X-ray diffraction patterns of graphite and turbostratic carbon. Carbon 45:1686–1695. https://doi.org/10.1016/j.carbon.2007.03.038
Lim DJ, Marks NA, Rowles MR (2020) Universal Scherrer equation for graphene fragments. Carbon 162:475–480. https://doi.org/10.1016/j.carbon.2020.02.064
Liu Q, Gu J, Zhang W, Miyamoto Y, Chen X (2012) Biomorphic porous graphitic carbon for electromagnetic interference shielding. J Mater Chem 22:21183–21188. https://doi.org/10.1039/C2JM34590K
Liu WJ, Jiang H, Yu HQ (2019) Emerging applications of biochar-based materials for energy storage and conversion. Energ Environ Sci 12(6):1751–1779. https://doi.org/10.1039/C9EE00206E
McKendry P (2002) Energy production from biomass (part 1): overview of biomass. Bioresour Technol 83:37–46. https://doi.org/10.1016/S0960-8524(01)00118-3
Meng W, Bai X, Wang B, Liu Z, Lu S, Yang B (2019) Biomass-derived carbon dots and their applications. Energy Environ Mater 2:172–192. https://doi.org/10.1002/eem2.12038
Miao M, Zuo S, Zhao Y, Wang Y, Xia H, Tan C, Gao H (2018) Selective oxidation rapidly decomposes biomass-based activated carbons into graphite-like crystallites. Carbon 140:504–507. https://doi.org/10.1016/j.carbon.2018.09.018
Mori F, Kubouchi M, Arao Y (2018) Effect of graphite structures on the productivity and quality of few-layer graphene in liquid-phase exfoliation. J Mater Sci 53:12807–12815. https://doi.org/10.1007/s10853-018-2538-3
Phiri J, Dou J, Vuorinen T, Gane PAC, Maloney TC (2019) Highly porous willow wood-derived activated carbon for high-performance supercapacitors electrodes. ACS Omega 4:18108–18117. https://doi.org/10.1021/acsomega.9b01977
Purkait T, Singh G, Singh M, Kumar D, Dey RS (2017) Large area few-layer graphene with scalable preparation from waste biomass for high-performance supercapacitor. Sci Rep 7:15239. https://doi.org/10.1038/s41598-017-15463-w
Qin C, Wang H, Yuan X, Xiong T, Zhang J, Zhang J (2020) Understanding structure-performance correlation of biochar materials in environmental remediation and electrochemical devices. Chem Eng J 382:122977. https://doi.org/10.1016/j.cej.2019.122977
Rodríguez-Mirasol J, Cordero T, Rodríguez J (1996) High-temperature carbons from kraft lignin. Carbon 34:43–52. https://doi.org/10.1016/0008-6223(95)00133-6
Schuepfer DB, Badaczewski F, Guerra-Castro JM, Hofmann DM, Heiliger C, Smarsly B, Klar PJ (2020) Assessing the structural properties of graphitic and non-graphitic carbons by Raman spectroscopy. Carbon 161:359–372. https://doi.org/10.1016/j.carbon.2019.12.094
Senthil C, Lee CW (2021) Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew Sust Energ Rev 137:110464. https://doi.org/10.1016/j.rser.2020.110464
Sevilla M, Fuertes AB (2010) Graphitic carbon nanostructures from cellulose. Chem Phys Lett 490:63–68. https://doi.org/10.1016/j.cplett.2010.03.011
Sharma RK, Wooten JB, Baliga VL, Lin X, Chan WG, Hajaligol MR (2004) Characterization of chars from pyrolysis of lignin. Fuel 83:1469–1482. https://doi.org/10.1016/j.fuel.2003.11.015
Shin K, Hong J, Lee S, Jang J (2012) High electrothermal performance of expanded graphite nanoplatelet-based patch heater. J Mater Chem 22:23404–23410. https://doi.org/10.1039/c2jm34196d
Tai FC, Lee SC, Wei CH, Tyan SL (2006) Correlation between ID/IG ratio from visible Raman spectra and sp2/sp3 ratio from XPS spectra of annealed hydrogenated DLC film. Mater Trans 47:1847–1852. https://doi.org/10.2320/matertrans.47.1847
Thompson E, Danks AE, Bourgeois L, Schnepp Z (2015) Iron-catalyzed graphitization of biomass. Green Chem 17:551–556. https://doi.org/10.1039/C4GC01673D
Wang Y, Alsmeyer DC, McCreery RL (1990) Raman-spectroscopy of carbon materials: structural basis of observed spectra. Chem Mater 2:557–563. https://doi.org/10.1021/cm00011a018
Wang J, Chen T, Wu J, Wu J (2014) A novel gaussian-DEAM-reaction model for the pyrolysis of cellulose, hemicellulose and lignin. RSC Adv 4:17513–17520. https://doi.org/10.1039/C4RA01445F
Wang S, Lin H, Zhang L, Dai G, Zhao Y, Wang X, Ru B (2016) Structural characterization and pyrolysis behavior of cellulose and hemicellulose isolated from softwood Pinus armandii franch. Energy Fuels 30:5721–5728. https://doi.org/10.1021/acs.energyfuels.6b00650
Wang J, Salim N, Fox B, Stanford N (2017) Anisotropic compressive behaviour of turbostratic graphite in carbon fibre. Appl Mater Today 9:196–203. https://doi.org/10.1016/j.apmt.2017.07.010
Yang H, Yan R, Chen H, Lee D, Zhang C (2007) Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 86:1781–1788. https://doi.org/10.1016/j.fuel.2006.12.013
Zhang J, Choi YS, Yoo CG, Kim TH, Brown RC, Shanks BH (2015) Cellulose-hemicellulose and cellulose-lignin interactions during fast pyrolysis. ACS Sustain Chem Eng 3:293–301. https://doi.org/10.1021/sc500664h
Zhao Y, Wang H (2021) Structure-function correlations of carbonaceous materials for persulfate-based advanced oxidation. Langmuir 37:13969–13975. https://doi.org/10.1021/acs.langmuir.1c02755
Zhao H, Cheng Y, Zhang Z, Zhang B, Pei C, Fan F, Ji G (2021) Biomass-derived graphene-like porous carbon nanosheets towards ultralight microwave absorption and excellent thermal infrared properties. Carbon 173:501–511. https://doi.org/10.1016/j.carbon.2020.11.035
Zuo S, Miao M, Zhao Y (2018) Graphitic crystallite-based carbon nanomaterial, and preparation method and application thereof. CN Patent ZL201780002578.2
Zuo S, Miao M, Zhao Y (2020) Graphitic crystallite-based carbon nanomaterial, and preparation method and application thereof. JP Patent 6762417
Zuo S, Miao M, Zhao Y (2021) Graphitic crystallite-based carbon nanomaterial, and preparation method and application thereof. U.S. Patent 11103850 B2
Zuo S, Miao M, Zhao Y (2022) Graphitic crystallite-based carbon nanomaterial, and preparation method and application thereof. EP Patent 3572375B1
Acknowledgements
The authors sincerely thank the financial support from Fundamental Research Funds of Research Institute of Chemical Industry of Forest Products, CAF (Grant No. CAFYBB2019SY031) and National Key R&D Program (Grant No. 2019YFB1503804). They would also like to acknowledge the support of XRD, Raman analysis from Instrument Analysis Center at Institute of Chemical Industry of Forest Product, and the support of XPS, HRTEM tests from Shiyanjia Lab (www.shiyanjia.lab).
Funding
This work was supported by Fundamental Research Funds of Research Institute of Chemical Industry of Forest Products, CAF (CAFYBB2019SY031) and the National Key R&D Program (No. 2019YFB1503804).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by CC, CH and MY. Characterization, data collection and analysis were accomplished by AW, GZ, BL and WX. The first draft of the manuscript was written by CC, KS and JL, and the manuscript was revised by KS and JJ. All authors commented on previous versions of the manuscript, and all authors had read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics appoval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling Editor: Hailong Wang.
Supplementary Information
Additional file 1. Table S1.
Elemental analysis of lignin, cellulose and biochars. Figure S1. TG curves of lignin (a) and cellulose (b). Figure S2. EIS spectra of L-2800 and C-2800.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, C., Sun, K., Huang, C. et al. Investigation on the mechanism of structural reconstruction of biochars derived from lignin and cellulose during graphitization under high temperature. Biochar 5, 51 (2023). https://doi.org/10.1007/s42773-023-00229-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00229-7