Abstract
Modified biochar with higher electron transport and adsorption capabilities could significantly improve the performance of anaerobic ammonia oxidation (anammox). However, there are few related investigations on the reinforcement of anammox through iron-modified Enteromorpha prolifera biochar (IMEPB). In this study, with the addition of the IMEPB in the anammox system, the enhancing process of anammox performance was studied, the improving feasibility of anammox was evaluated, and the reinforcing mechanism of anammox was elucidated. The results showed that the optimal iron−charcoal ratio (Fe:C) and IMEPB dosage were 1:10 and 10 g L−1, respectively. Under the optimal conditions, when the nitrogen loading rate gradually increased to 0.557 (kg m−3 day−1), the nitrogen removal efficiency and nitrogen removal rate of the anammox process supplemented with IMEPB increased by 11%, and the specific anammox activity increased by 23.8%. Compared with the control, the secretion of extracellular polymeric substances (EPS) of anammox bacteria supplemented IMEPB increased by 24.4%, greatly improving the stability of the anammox system. Meanwhile, EPS secretion further promoted the microbial activity of anammox bacteria, achieving a 19% increase in the abundance of Candidatus Brocadia. These findings demonstrate the potential mechanism of IMEPB in improving anammox, provide new insights into recycling E. prolifera, and provide a novel reinforcement strategy for anammox. In the future, adding IMEPB may be a vital measure for the practical application of anammox in coastal areas.
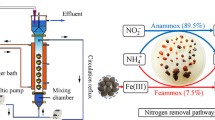
Graphical Abstract

Highlights
-
1.
The optimum Fe:C (1:10) and dosage (10 g L−1) of the IMEPB were obtained.
-
2.
The NRE and NRR increased by 11% and the Candidatus Brocadia increased by 20% in the anammox with adding IMEPB.
-
3.
A novel biochar-based strategy was established for the anammox reinforcement.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Anaerobic ammonia oxidation (anammox), an autotrophic biological process with novel nitrogen removal technology applications, has been capturing the interest of many scholars since it was discovered in 1995 (Strous et al. 1999). Under anaerobic conditions, anaerobic ammonium-oxidizing bacteria (AnAOB) can directly convert ammonium (NH4+-N) to nitrogen gas (N2) using nitrite (NO2−-N) as an electron acceptor (Liu et al. 2020; Yuan et al. 2022). Compared with traditional biological nitrogen removal processes (nitrification and denitrification), anammox has the advantages of not requiring a carbon source, reducing aeration by 63% and producing little sludge. Thus, anammox is widely acknowledged as an energy-saving and environmental-protecting process (Tang et al. 2011; Jin et al. 2013). However, AnAOB is sensitive to the growth conditions, such as high nitrogen load, fluctuating operating conditions (pH, temperature), and heavy metals in wastewater, which all restrain the AnAOB activity (Jin et al. 2012; Xu et al. 2020). These factors also can impede the extensive application of anammox in practical engineering. In recent years, many strategies or measures have been implemented to improve both the stability and resistance of anammox systems, for example, facilitating the granulation of anammox sludge (Kang et al. 2019), or attaching embedded carriers (Wang et al. 2022a, b, c). Adding enhancers is another common method that can significantly improve the performance of anammox. Various metal oxide nanoparticles, such as zinc oxide and copper oxide, have been widely used as enhancers in anammox systems (Zhang et al. 2018a, b). Nevertheless, the residues of these enhancer agents will generate extra pollutants in the treatment processes. Therefore, a pro-environment and high-efficiency enhancer has become urgent.
Biochar is a carbon-rich solid produced by pyrolyzing organic plant or animal material at high temperatures without oxygen (Liu et al. 2022). It has excellent characteristics in terms of specific surface area, micropore volume, and electron transfer (Liu et al. 2022; Wang et al. 2022a, b, c), thereby presenting a high adsorption capacity for pollutants in wastewater. Moreover, biochar can be modified to obtain a higher hydrophobic property and stronger adsorption capacity (Liu et al. 2022). Liu et al. (2022) performed a simple traditional acid–base modification on rice-husk biochar, resulting in the adsorption capacity of the modified biochar significantly higher than the unmodified biochar. However, this traditional modification method will harm water bodies. Notably, iron with different valence states has been widely used in biochar modification. Easton et al. (2015) reported that biochar modified with iron hydroxide could promote the removal of nitrate and reduce the emission of greenhouse gases (N2O) in the denitrification process. In addition, zero-valent iron-modified biochar can enhance microbial abundance and biological nitrogen removal (Jia et al. 2020). Importantly, iron itself participates in the global nitrogen cycle (Jia et al. 2020). Regrettably, modified biochar has not been widely used in the biological removal of nitrogen. This is due to the fact that raw materials for biochar production are mostly from inland plants, such as bamboo and corn, which restricts its use in coastal areas (Li et al. 2021). Algae, as an important biological resource in the ocean, has the advantages of high carbon content and low ash content compared with inland plants (Wang et al. 2018). Li et al. (2021) found that compared with common inland plants, algae-based biochar has better pore characteristics. In recent years, the explosion of the alga Enteromorpha prolifera in coastal areas (such as Qingdao, China) has not been properly addressed, destroying the natural marine ecological environment and consuming many social resources. However, few studies have reported the application of E. prolifera biochar (EPB) in biological nitrogen removal processes, and EPB with iron modification to reinforce the anammox process is rarely reported. Simultaneously, the mechanism of iron-modified E. prolifera biochar (IMEPB) in the long-term operation of anammox systems is unclear. Therefore, to reinforce the anammox and elucidate the reinforcement mechanism, this study established a novel control strategy by adding IMEPB.
In this study, with added IMEPB, the long-term operation of the anammox system and the reinforcement mechanism of anammox were investigated. To reveal the variation in the anammox system, the specific anammox activity (SAA), extracellular polymeric substances (EPS) and microbial community structure were determined. The purpose of this study was to (1) demonstrate the reinforcement effect of IMEPB on anammox; (2) clarify the optimal iron−charcoal ratio (Fe:C) and dosage of IMEPB; (3) reveal the microbial succession of the anammox system under an increasing nitrogen loading rate (NLR); and (4) establish a novel strategy for anammox with IMEPB as an enhancer.
2 Materials and methods
2.1 Preparation of EPB and IMEPB
The E. prolifera used for biochar preparation was obtained from Mai Dao (the coastal area of Qingdao City, Shandong Province, China). After washing, the algae were dried to a constant weight at 45 °C and then crushed. Under anaerobic conditions maintained by nitrogen (N2), the dried and crushed E. prolifera was carbonized in a muffle furnace for 2 h at 400 °C to obtain the unmodified E. prolifera biochar (EPB). The obtained EPB was cooled to room temperature in the muffle furnace, then screened with a 30-mesh sieve and stored in a Ziplock bag.
To prepare the iron-modified E. prolifera biochar (IMEPB), an appropriate amount of the EPB was washed in 200 mL of 1 mol L−1 hydrochloric acid (HCl) solution for 2 h in an oscillating chamber (150 rpm), and 5 g of EPB was rinsed with distilled water until neutral (pH 6.5 ± 0.2). Different amounts of acid-washed EPB (0.603 g, 2.413 g, 4.826 g, 12.065 g and 24.130 g) were dissolved in 200 mL FeCl3·6H2O to create IMEPB with iron−charcoalratios (Fe:C) of 1:40, 1:10, 1:5, 1:2 and 1:1, respectively. Then, all mixtures were placed again in an oscillating chamber (150 rpm) for 2 h, followed by suction filtration with a vacuum pump. Finally, they were washed with distilled water to neutral and dried at 80 °C, ultimately obtaining the IMEPB.
The chemical composition of the EPB and IMEPB is provided in Additional file 1: Table S1. Scanning electron microscopy (SEM) images shown in Additional file 1: Figs. S1 and S2 reveal the X-ray diffraction (XRD) and Fourier transform infrared (FTIR).
2.2 Establishment of an anammox process strategy reinforced by IMEPB
The anammox sludge for batch tests was obtained from a sequencing batch reactor (SBR) at the laboratory, which was made of polymethyl methacrylate (PMMA), and the working volume was 8 L. The SBR was operated for more than 3 years with one 24-h cycle per day (5 min for inlet, 23 h for reaction, 5 min for outlet, and 50 min for idling). The SBR was kept dark with aluminum foil, and an electronic stirrer was built in to keep the sludge suspended. The heater maintained the internal temperature at about 32 °C, while the pH inside the SBR was kept at about 7.5.
2.2.1 Operation effect of the anammox process with different Fe:C ratios
To obtain the best-reinforced effect of the IMEPB, the operation of anammox under different Fe:C ratios was investigated through batch tests. First, anammox sludge was taken out of the SBR. Next, it was rinsed three times with distilled water, and then evenly divided into five 500 mL conical flasks. Meanwhile, N2 was used to maintain an anaerobic environment. Identical conditions maintained for each conical flask in the constant temperature air bath shock chamber, and the sampling interval was 2 h. The concentrations of NH4+-N, NO2−-N and nitrate (NO3−-N) in influent and effluent were measured when the reaction finished.
2.2.2 Operation effect of anammox with different dosages of IMEPB
The IMEPB dosage will also affect the anammox process. Therefore, the optimal dosage was determined via batch tests. Different concentrations (0 g L−1, 5 g L−1, 10 g L−1, and 20 g L−1) were set in four identical 500 mL conical flasks, and the sampling interval was 1 h. The concentrations of NH4+-N, NO2−-N and NO3−-N in influent and effluent were measured when the reaction finished.
2.2.3 Influence of IMEPB addition on long-term operation of anammox
Long-term operation of anammox was conducted under the condition that the IMEPB maintained the optimum Fe:C ratio and dosage. To observe the reinforced effect of IMEPB on anammox, three groups of reaction systems were set up, namely the control (CK), EPB addition (BC), and IMEPB addition (FeBC). The experiment was carried out in conical flasks with an effective volume of 0.5 L. The mixed liquid suspended solids (MLSS) maintained at 3260 mg L−1 and the mixed liquid volatile suspended solids (MLVSS) maintained at about 1994 mg L−1. As the anammox activity increased, the substrate concentration gradually increased (Table 1). Similarly, the water quality of the three reaction systems was measured daily.
2.3 Measurement of important indicators
2.3.1 Extraction and measurement of EPS
EPS was extracted from the granular sludge using an improved thermal extraction method. 10 mL Granular sludge (10 mL) was collected and placed into a 50 mL centrifuge tube, rinsed three times with distilled water, and centrifuged for 10 min in a high-speed refrigerated centrifuge (MIKRO 220R) at 3262 × g. After the supernatant was poured out, the residue was cleaned three times with phosphate buffered saline (PBS) and then kept at a constant volume of 10 mL. Next, the residue was heated in a water bath (THZ-82A) at 60 °C for 30 min and then centrifuged at 13,050×g for 15 min. After centrifugation, the supernatant was filtered using a 0.45 μm polyethersulfone membrane (PES), and the protein (PN) and polysaccharide (PS) fractions were reserved for measurement. Protein was measured by the Coomassie blue method using bovine serum albumin as the standard substance (Bradford 1976) and polysaccharide was determined by the anthrone method using glucose as the standard substance (Lever 1972).
2.3.2 Measurement of SAA
At the end of each stage of the anammox system, SAA was measured. The SAA batch test was conducted in an enclosed serum flask (100 mL effective volume) containing 5 g (wet weight) anammox sludge. The measurement of each sample was repeated three times. Before the experiment, the serum flask was purged with high purity N2 gas to remove oxygen. A constant temperature air bath shock chamber (180 rpm) was used to maintain the temperature at 34 ± 1 °C under dark conditions. The sampling interval was 1 h. SAA was calculated by the ratio of the reduction rate of NH4+-N and NO2−-N in the system to MLVSS.
2.4 Synthetic wastewater and analytical methods
The synthetic wastewater composition used in the SBR was as follows: 191 mg L−1 NH4Cl (50 mg L−1 NH4+-N), 325 mg L−1 NaNO2 (66 mg L−1 NO2−-N), 27 mg L−1 KH2PO4 (6 mg L−1 PO43−-P), 1.2 g L−1 MgSO4·7H2O, 1 g L−1 KHCO3, 136 mg L−1 CaCl2; 1 mL trace elements I and II. The composition of trace elements I was as follows: 5 g L−1 EDTA and 5 g L−1 FeSO4·7H2O. The composition of trace elements II was as follows: 15 g L−1 EDTA, 0.014 g L−1 H3BO4, 0.99 g L−1 MnCl2·4H2O, 0.25 g L−1 CuSO4·5H2O, 0.43 g L−1 ZnSO4·7H2O, 0.21 g L−1 NiCl2·6H2O, 0.22 g L−1 NaMoO4·2H2O and 0.24 g L−1 CoCl2·6H2O.
NH4+-N, NO2−-N and NO3−-N were analyzed with a UV spectrophotometer (UV-5200) using standard methods (APHA 2012). Chemical oxygen demand (COD) was analyzed using a COD quick-analysis apparatus (Lian-Hua Tech. Co., Ltd., 5B-3A, China). MLSS and MLVSS were also analyzed using standard methods (APHA 2012). The dissolved oxygen (DO) concentration, pH and temperature were measured with a pH/DO meter (WTW Company, WTW 3620 IDS, Germany).
The long-term nitrogen removal efficiency (NRE) and nitrogen removal rate (NRR) were calculated according to Eqs. (1) and (2) (Xu et al. 2020), respectively.
where NH4+-Ninf and NO2−-Ninf are the initial anoxic ammonium and nitrite concentrations (mg L−1), respectively; and NH4+-Neff and NO2−-Neff are the ammonium and nitrite concentrations (mg L−1)), respectively.
2.5 High-throughput sequencing
Activated sludge samples were taken from the CK, BC and FeBC systems after 110 days of operation. These samples were characterized for microbial community structure variations by high-throughput sequencing. The DNA extracts were checked on 1% agarose gel, and the DNA concentrations and purity were determined with a NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, USA). Primer pairs 338F (5ʹ-ACTCCTACGGGAGGCAGCAG-3ʹ) and 806R (5ʹGGACTACHVGGGTWTCTAAT-3ʹ) were used to PCR amplify the V3-V4 hypervariable region of the bacterial 16S rRNA gene. The amplification products were sequenced on an Illumina MiSeq PE250 platform (Illumina, USA) by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw data for the CK, BC and FeBC systems have been uploaded to the NCBI Sequence Read Archive (SRA) under accession numbers SRR20114949, SRR20114950 and SRR20114951, respectively.
3 Results and discussion
3.1 The characterization of EPB and IMEPB
SEM displays the morphology of EPB (Additional file 1: Fig. S1a, b) and IMEPB (Additional file 1: Fig. S1b, c). As shown in Additional file 1: Figure S1, compared with the EPB, IMEPB possessed a highly porous structure with a square-shaped pore structure, close and regular arrangement. In addition, iron uniformly covered the surface and pores of biochar, making IMEPB have a better pore structure and larger specific surface area.
XRD patterns of EPB and IMEPB are shown in Additional file 1: Fig. S2a. The XRD spectra of the EPB reveal that it contained a certain amount of mineral elements such as calcium, and sodium. For instance, the peaks at 2θ = 29° suggest that calcium carbonate was detected, and the appearance of sodium nitrite at 2θ = 46°. Contrarily, the XRD spectra of the IMEPB reveal no mineral elements. Furthermore, the peaks at 2θ = 35° (Fe3O4) (Shalali et al. 2022) are significantly enhanced, implying that iron was partially oxidized during preparation or preservation. Similarly, Jia et al. (2020) used iron-modified biochar to enhance the nitrogen removal efficiency in a constructed wetland and found that iron oxide appeared on the biochar surface, but the peak intensity of the XRD pattern was different, which may be caused by different iron loading.
Figure S2b shows the FTIR of the EPB and IMEPB. EPB and IMEPB had characteristic absorption peaks of –OH at 3414 cm−1 and 3417 cm−1, respectively (Keiluweit et al. 2010). In addition, the stretching vibration peak of C=O was found at 1611 cm−1 and 1617 cm−1, respectively (Wang et al. 2013), which indicated that both biochars contained aromatic. The characteristic peak of EPB at 1439 cm−1 was related to the C=C group and IMEPB showed the characteristic peak of carbohydrate and aliphatic –CH2 group at 1383 cm−1 (Cole et al. 2019). Moreover, the characteristic peaks of EPB and IMEPB at 1112 cm−1 and 1109 cm−1 were caused by the stretching vibration of C–O (Cole et al. 2019). The absorption peak intensity of EPB at 1112 cm−1 was significantly lower than that of IMEPB, which indicated that C–O functional groups would decrease during the modification process. In summary, in the modification process of EPB, the type of functional groups will not change obviously, but the addition of iron will increase the content of functional groups.
3.2 Analysis of the reinforcement effect of the anammox system with IMEPB
3.2.1 Effect of different Fe:C ratios on the anammox process
The effect of the anammox operation process was investigated with different Fe:C ratios (control group; 1:1; 1:2; 1:5; 1:10; 1:40), which are represented by systems BK and A–E, respectively. As shown in Fig. 1, the removal efficiencies of NH4+-N and NO2−-N from high to low were: system D (1:10) > E (1:40) > C (1:5) > B (1:2) > A (1:1) > CK. This result indicated that the addition of IMEPB could improve the NRE of the anammox process and the Fe:C ratio directly affected the reinforced effect of IMEPB.
Nitrogen removal performance of the six reactors under variational Fe:C ratios. a variations of NH4+-N concentrations; b variations of NO2−-N concentrations; c variations of NO3−-N concentrations; d nitrogen removal efficiency (NRE); BK, A, B, C, D and E represent the anammox system with the iron-charcoal ratios (Fe:C) of 0,1:1,1:2,1:5,1:10 and 1:40 biochar additions, respectively
Specifically, in system BK, the NH4+-N, and NO2−-N concentrations decreased from 51.4 mg L−1 and 71.1 mg L−1 to 16.5 mg L−1 and 22.6 mg L−1, respectively. Conversely, in system A (1:1), the removal efficiency of NH4+-N was slightly higher than that in system BK (70.9%), and the removal rate of NO2−-N in system A reached 77.9%. In system B (1:2) and C (1:5), NH4+-N decreased from 51.4 mg L−1 and 47.3 mg L−1 to 10.5 mg L−1 and 5.8 mg L−1, respectively, and the effluent NO2−-N concentrations were 12.2 mg L−1 and 10.6 mg L−1, respectively. In system D (1:10), the NH4+-N and NO2−-N were removed completely within the operation cycle, and the optimal total nitrogen (TN) removal efficiency (80.8%) was maintained. These results indicated that the Fe:C ratio affected the reinforcement effect of IMEPB, and that anammox was reinforced optimally with an Fe:C of 1:10. The reason for this is that with excessive iron loaded on the surface of the biochar, the surface active sites of the material will be reduced. Meanwhile, the agglomeration particles will also block the porous structure of the material, affecting the chemical migration pathway of electrons. Thus, the reinforced effect of anammox in the presence of IMEPB was weakened (Wei et al. 2018). In conclusion, the optimal Fe:C (1:10) of the IMEPB was obtained in the experiment.
3.2.2 Effect of different IMEPB dosages on the anammox process
When the dosage was 10 g L−1, the anammox process achieved the optimal reinforcement effect. As shown in Fig. 2, with the increasing dosage of IMEPB, the NRE of the anammox system showed a trend of first increasing and then decreasing.
The IMEPB dosage played an important role in the reinforced anammox process. Therefore, the operation effect of anammox under different dosages of IMEPB was investigated. Specifically, when the effluent NH4+-N was 1.1 mg L−1, the removal efficiency of NO2−-N reached 100% and the TN removal efficiency reached 78.74%. Compared with BK, the NRE was improved by about 20%. In contrast, when the IMEPB dosage was 20 g L−1, the removal efficiencies of NH4+-N and NO2−-N decreased in the anammox system. These results indicated that IMEPB addition could promote the nitrogen removal effect of the anammox process. And the optimal dose of IMEPB was 10 g L−1. It was reported that when using coconut shell biochar as a fortifier in an expanded granular sludge bed (EGSB) reactor, the NRE of anammox with 5% biochar was better than that of of anammox with 10% and 15% biochar (Adams et al. 2021). This indicates that the dosage of biochar is an important factor in the reinforced anammox process and this is consistent with the conclusion reached in this experiment.
3.2.3 Effect of IMEPB addition on anammox reinforcement
When comparing with the absence of biochar and adding EPB, the addition of IMEPB could effectively improve the NRE and resistance of the anammox system with the NLR increasing under the optimal Fe:C ratio (1:10) and dosage (10 g L−1). This provided a novel reinforcement strategy for the anammox process. To more clearly study the process of IMEPB reinforced anammox and verify the results of the optimal Fe:C ratio (1:10) and dosage (10 g L−1), the long-term operation effect of the anammox system was investigated. Meanwhile, a novel reinforcement strategy was tested: adding IMEPB. In this experiment, three anammox systems were set, namely CK (without biochar), BC (with EPB) and FeBC (with IMEPB). The results are shown in Fig. 3.
Nitrogen removal performance of the anammox system with the increased nitrogen loading rate (NLR). a variations of NH4+-N concentrations; b variations of NO2−-N concentrations; c variations of NO3−-N concentrations and NRE; d NRR and NLR. CK, BC and FeCK represent the anammox system without biochar, Enteromorpha prolifera and iron-modified Enteromorpha prolifera biochar additions, respectively
The operation process was divided into five phases corresponding to the NLR of 0.139–0.557 (kg m−3 day−1). Specifically, in phase I (1–30 days), the influent NH4+-N and NO2−-N were 30 mg L−1 and 36 mg L−1, respectively. In this stage, as the NRE increased, the HRT of 11 h gradually decreased to 6 h and the NRE of each reactor reached 100% by the end of the phase. In phase II (31–50 days), EPB (BC) and IMEPB (FeBC) were added to two reactors on day 30, and the influent NH4+-N and NO2−-N increased to 50 mg L−1 and 66 mg L−1, respectively. With the increased NLR of the anammox system, the effluent water quality of each reactor notably decreased. The NRE of the FeBC system (about 85%) was significantly higher than that of CK (about 75%) and BC (about 80%), and the NRE of the FeBC system recovered to 100% first. These results indicated that adding EPB could enhance performance to resist an elevated NLR in the anammox system, and the improvement effect of IMEPB was optimal. In phases III (51–70 days) and IV (71–90 days), the NLR increased from 0.232 (kg m−3 day−1) to 0.464 (kg m−3 day−1). As shown in Fig. 3, with the increased NLR, the effluent water quality of each system deteriorated over a short term, and afterward returned to normal. It is worth noting that at the end of phase III, the NO2−-N removal efficiency of each system still reached 100%, but the NH4+-N was only completely removed in the FeBC system, indicating that the addition of IMEPB could best promote the resistance of the anammox. This was because the IMEPB had a superior surface area and functional group properties, and the AnAOB could obtain better protection. In phase V (91–110 days), the NLR increased to 0.557 (kg m−3 day−1). With the NLR increasing to a higher level, after recovery, the NRE of CK could only reach 85.38%, and the NRR was 0.476 (kg m−3 day−1). That was because the high concentration of NO2−-N (> 100 mg L−1) resulted in irreversible inhibition of the anammox process (Lotti et al. 2012). Conversely, the NRE of BC and FeBC reached 88.36% and 96.58%, respectively, while the NRR was 0.492 (kg m−3 day−1) and 0.538 (kg m−3 day−1), respectively. Notably, in the FeBC system, the removal effect of NO2−-N was better than that of NH4+-N, and the effluent NO3−-N was higher. This phenomenon could be explained by the electric neutralization theory. With the cation Fe3+ in the system increasing the positive charge content, the cations are more inclined to attract NO2−-N anion for electron transfer (Jia et al. 2020).
3.3 Effect of IMEPB addition on SAA
With the increased NLR, IMEPB addition could improve the SAA of the anammox system. When the NLR was 0.464 (kg m−3 day−1), the SAA of the anammox system was highly improved by 25.9% compared with the control (P < 0.05). Therefore, IMEPB could effectively improve the activity of the anammox process under optimal conditions (Fe:C = 1:10 and a 10 g L−1 dosage). SAA is an important parameter reflecting the metabolic capacity of AnAOB, and can directly demonstrate the nitrogen removal capacity of the anammox system (Wang et al. 2022a, b, c). Therefore, it is necessary to investigate the SAA during anammox reinforcement. Figure 4 shows variation in the SAA with the increasing NLR of CK, BC and FeBC. With an increasing NLR, the SAA in all reactors showed a gradually increasing trend. However, the highest SAA was observed in the FeBC reactors throughout, which is consistent with the results discussed in Sect. 3.1 (with the highest NRR and NRE in FeBC).
Specifically, in phase I, the SAA of each system was consistent because the biochar was not added. In phase II, the SAA of FeBC (0.326 ± 0.01 g N g VSS−1 day−1) and BC (0.293 ± 0.01 g N g VSS−1 day−1) increased by 18.9% and 6.9%, respectively, compared with CK (0.293 ± 0.01 g N g VSS−1 day−1). Similarly, in phase III, the SAA of the FeBC (0.413 ± 0.01 g N g VSS−1 day−1) and BC (0.404 ± 0.05 g N g VSS−1 day−1) systems increased by 11.6% and 9.2%, respectively, compared with CK (0.37 ± 0.05 g N g VSS−1 day−1). The SAA of the FeBC system showed significant improvement in the two phases (P < 0.05), indicating that the addition of IMEPB could effectively improve the AnAOB activity. However, Xu et al. (2020) illustrated that with the higher NLR, the SAA of an anammox system with added biochar decreased by 10.3% compared with the control. This difference was caused by the different temperatures of the biochar during the firing process and biochar without modification. In phase IV, the SAA of the FeBC (0.642 ± 0.02 g N g VSS−1 day−1) and BC (0.607 ± 0.02 g N g VSS−1 day−1) systems increased by 25.9% and 19.0%, respectively, compared with CK (0.51 ± 0.03 g N g VSS−1 day−1). In phase V, the SAA of FeBC (0.665 ± 0.02 g N g VSS−1 day−1) and BC (0.622 ± 0.03 g N g VSS−1 day−1) increased by 23.8% and 15.8%, respectively, compared with CK (0.537 ± 0.02 g N g VSS−1 day−1). The SAA of BC and FeBC showed significant improvements in the two phases (P < 0.05). These results indicate that the higher the NLR, the more obvious the protective effect of biochar on AnAOB and the stronger the improvement effect of modified biochar on the anammox system. This demonstrates precisely why the FeBC system can maintain the highest NRE and NRR throughout the operation process. Furthermore, Wang et al. (2022a, b, c) illustrated that the addition of biochar can improve the activity of the anammox system. In addition, compared with the control, the SAA of the AnAOB with redox-active biochar increased by 51%. This is consistent with the conclusion of the experiment, and the reason for the difference in the strengthening effect is the difference in NLR.
3.4 Variation in EPS
The addition of IMEPB could significantly promote the secretion of EPS and the protein to polysaccharide ratio (PN/PS) of AnAOB. During the long-term operation of the anammox system, EPS secretion and the PN/PS were significantly increased by the addition of IMEPB, compared with the control the system with EPB addition. EPS mainly contains proteins and polysaccharides, which can defend against environmental damage to AnAOB (Xu et al. 2018). It is also reported that EPS plays a crucial role in improving the activity and metabolic capacity of the anammox process (Xu et al. 2022). Furthermore, the different PN/PS will directly affect the precipitation performance of anammox sludge (Xu et al. 2018). Figure 5 shows the total EPS and the change in the PN/PS of each phase in all reactors.
Figure 5a illustrates the changes in PN and PS in the EPS. On day 30 without biochar added to each reactor, the EPS content was consistent at 126.1 mg g VSS−1 (CK), 134.3 mg g−1 VSS−1 (BC) and 128.6 mg g−1 VSS−1 (FeBC). On day 50, the total EPS in BC and FeBC increased by 5.6% and 24.7%, respectively (P < 0.05), compared with CK (190.1 mg g−1 VSS−1). The results showed that the addition of IMEPB could promote the AnAOB to secret EPS, thereby reducing the negative effects caused by the increased NLR. On day 70, the total EPS in BC and FeBC increased by 17.1% and 24.4%, respectively (P < 0.05). On day 90, the highest concentrations of proteins, polysaccharides and EPS were obtained (275.6, 26.3, and 301.9 mg g−1 VSS−1, respectively). Subsequently, the EPS content in each reaction system showed a decreasing trend at 110 days. The results showed that over the long-term operation process (the 90 days), the addition of IMEPB could significantly promote the secretion of proteins and polysaccharides, and the increase in protein was obvious. This is a self-protection mechanism of microorganisms; when the external environment changes, AnAOB secretes EPS as a means to protect themselves, reducing the damage (Gao et al. 2012; Xu et al. 2018). EPS secretion also benefits the aggregation of microorganisms and sludge granulation, improving the activity of AnAOB. In a previous report, the higher the AnAOB activity, the higher the secreted EPS (Jia et al. 2017), which is consistent with the variation of EPS in this study.
The PN/PS is shown in Fig. 5b. After 90 days of operation, the PN/PS in BC (8.3) and FeBC (10.5) increased significantly compared to CK (6.7). Guo et al. (2022) also reported that an increase in the PN/PS can promote the granulation of anammox sludge, thus enhancing the stability of the anammox system. In addition, the greater secretion of proteins with special functional groups also can boost anammox activity (Xu et al. 2018). A high PN/PS has been reported to reduce the strength and settlement performance of granular sludge, and excessive protein secretion can increase the water viscosity, thereby reducing the reaction rate (Tang et al. 2011). Due to the total EPS secretion being much lower, performance degradation did not appear in the experiment. However, in practical application, the excessive extracellular protein secretion by AnAOB should be paid attention to.
3.5 Microbial community variation in the reinforced anammox process
Three anammox-activated sludge samples (CK, BC and FeBC) were subjected to high-throughput sequencing to investigate variation in the microbial communities during reinforcement. The coverage, sequenced reads and microbial community diversity obtained in the sequencing process are presented in Table 2. The high coverage indicates that the three sequencing samples can be used to characterize changes in microbial communities. Among the three microbial samples, FeBC (with IMEPB) had the highest sequenced reads (41,264). The number of sequenced reads for CK (without biochar) and BC (with EPB) were 39,931 and 40,383, respectively. The change in community diversity was reflected by the changes in ACE, Chao, Shannon and Simpson indices (Table 2). The ACE and Chao indices displayed a gradual increase, which was attributed to a stronger protection capacity (adsorption) of IMEPB for AnAOB (Li et al. 2020). The Shannon and Simpson indices for BC were better than those of CK and FeBC. This suggested that supplementing EPB could protect the entire microbial community during the long-term operation, while IMEPB had a more protective effect on anammox functional bacteria.
Figure 6a shows the relative abundance of the microbial community at the phylum level. In all samples, the main phyla were Chloroflexi, Proteobacteria, Planctomycetota, Actinobacteriota, Nitrospirota and Acidobacteriota, which are commonly reported during the anammox process (Bhattacharjee et al. 2017; Chen et al. 2019, 2021; Cao et al. 2022; Liu et al. 2022). However, their relative abundances revealed significant differences in each system due to appending EPB and IMEPB. Chloroflexi, an anaerobic microorganism, plays an important role in NH4+-N transformation, and its filamentous properties can promote the granulation of AnAOB (Chen et al. 2021). It has been demonstrated that Chloroflexi consumes organic compounds released by the death of other bacteria to coexist with anammox bacteria (Park et al. 2021). Compared to CK (35.4%), the abundance of Chloroflexi decreased by 5% in BC (30.4%), but was consistent with FeBC (35.3%). This result illustrated that IMEPB could maintain the granulation of anammox sludge. Proteobacteria was consistently present in each system (CK, 16.6%; BC, 17.3%; FeBC, 15.0%), which is an important reason for the anammox process maintaining a high NRE. However, a recent study found that Proteobacteria was the dominated bacteria in an anammox system using activated carbon as the carrier (Cao et al. 2022). The difference may be caused by the distinction between modified biochar and activated carbon. Planctomycetota is a functional anammox bacterium, the abundance of which can reflect the activity of AnAOB (Bhattacharjee et al. 2017). Compared with CK and BC, the abundance of Planctomycetota in the FeBC increased by about 20% and 10%, respectively. This result suggested that the addition of IMEPB directly facilitated the enrichment of anammox bacteria, and elucidated the reason that the anammox process in FeBC maintained an optimal nitrogen removal effect. Notably, Nitrospirota, as a nitrifying bacterium, accounted for 15.0% in the CK, but decreased to 1.82% and 1.62% in BC and FeBC, respectively. This phenomenon may be because the connection between dissolved oxygen and biochar internal bacteria was impeded, severely inhibiting the reproduction of aerobic bacteria.
A heat map (row normalization and cluster analysis) at the genus level (Fig. 6b) shows a obvious difference in the microbial community structure in the three systems. The abundance of Candidatus Brocadia was 1.2% in CK, increasing to 13.5% and 20.6% in BC and FeBC, respectively. Meanwhile, Candidatus Brocadia was the dominating genus in FeBC. It has been confirmed that Candidatus Brocadia, the functional bacteria of the anammox, is the important reason for anammox converting NH4+-N and NO2−-N (Bhattacharjee et al. 2017). The widespread distribution of Candidatus Brocadia explains the reason why anammox in FeBC could recover quickly to optimal activity when the NLR increased. This result indicated that the IMEPB played a non-negligible role in increasing the activity of anammox functional bacteria. Guo et al. (2022) found that in an anammox system with biochar addition, apart from Candidatus Brocadia, Candidatus Kuenenia also played a crucial role in the nitrogen removal process. This difference is due to the anammox system of Guo et al. (2022) covering a large range of organic matter. The analysis showed that Candidatus Kuenenia prefers a high organic matter. Moreover, biochar was reported to increase the abundance of denitrifying bacteria in an anammox system (Wang et al. 2022a, b, c). However, no such phenomenon was observed in the present study. Interestingly, the relative abundances of Limnobacter and Defluviimonas were low in both CK and BC, but a significant increase occurred in FeBC amended with IMEPB.
3.6 Possible mechanism of IMEPB reinforcing anammox process
A possible mechanism for IMEPB reinforcing anammox process was proposed with the obtained results (Fig. 7). Firstly, the IMEPB built a favored environment for the enrichment of anammox bacteria (such as Candidatus Brocadia) based on high-throughput sequencing (Fig. 6). Subsequently, with the gradual increase of NLR, adding IMEPB promoted the enriched anammox bacteria to secrete EPS (Fig. 5) to boost their resistance, improving SAA (Fig. 4), NRE and NRR (Fig. 3). Secondly, with adhering iron to IMEBP, the reduction of iron generated electrons, and accelerated the electron transfer of anammox bacteria, enhancing the nitrogen removal efficiency. Wang et al. (2022a, b, c) elaborated on this mechanism from the genetic metabolic perspective. Finally, IMEPB can adsorb heavy metals (Mn2+, Cu2+) in water bodies, eroding the damage caused by heavy metals for anammox bacteria (Wang et al. 2022a, b, c). However, the adsorption and complexation performance of IMEPB for heavy metals should be taken into further research.
3.7 Comparison of the biochar reinforced anammox process and practical application of this study
As an efficient intensifier, biochar has been widely applied to reinforce the performance of anammox. Table 3 shows the related studies conducted using different biochar for anammox reinforcement. Adams et al. (2021) added 5% coconut biochar to an anammox system, achieving a rapid start-up of 46 days and 96% NH4+-N removal efficiency. Both Guo et al. (2022) and Wang et al. (2022a, b, c) investigated the effect of bamboo biochar on anammox. Guo et al. (2022) reported that with an increasing COD from 50 mg L−1 to 150 mg L−1, TN removal in the reactor with biochar addition improved by 3.1% ~ 6.4%. Wang et al. (2022a, b, c) showed that the SAA of anammox with biochar addition increased by 51% compared to the control. In this study, IMEPB was applied for the first time to reinforce the anammox performance, which greatly improved the resistance of anammox and provided a new idea for the effective utilization of E. prolifera. Importantly, a novel biochar-based reinforcement strategy was established for anammox.
Due to the E. prolifera outbreaks in coastal areas, ways of disposal and utilization of E. prolifera have attracted widespread attention. In this study, anammox showed higher NRE, NRR and EPS with an increased NLR under the optimal Fe:C ratio (1:10) and dosage (10 g L−1) of IMEPB. Therefore, the addition of IMEPB may be the most effective choice for improving the stability and nitrogen removal capacity of anammox. Thus, adding IMEPB not only improved the resistance and stability of anammox, but also provided an important theoretical basis for the application of the anammox process in coastal areas. Importantly, this work provided a new idea for resource utilization of E. prolifera. Furthermore, compared to inland plants, algae-based biochar has better pore size and surface area, and iron can absorb heavy metals and organic pollutants, thereby eliminating their negative impact on anammox.
4 Conclusion
This study investigated the reinforcement effect of IMEPB on the anammox process, proposing a novel biochar-based reinforcement strategy for the anammox process. The research found that the optimal Fe:C ratio and dosage of IMEPB were 1:10 and 10 g L−1, respectively. When the NLR was 0.557 (kg m−3 day−1), the NRE and NRR of anammox amended with IMEPB increased by 11% compared with the control. Furthermore, the addition of IMEPB increased EPS secretion and enhanced the SAA of the anammox process, thus improving the abundance of functional bacteria. Through multiple effects, the addition of IMEPB improved the stability and restorability of anammox under an increasing NLR. This study not only developed a novel reinforcement strategy for the anammox process but also inspired the resource utilization E. prolifera for the coastal areas. In the future, once IMEPB realizes quantity production, the reinforcement of the anammox process could use local materials in coastal areas.
Availability of data and materials
The datasets used or analyzed during the current study are available from the corresponding author on reasonable request.
References
Adams M, Xie J, Chang Y, Kabore AWJ, Chen C (2021) Start-up of Anammox systems with different biochar amendment: Process characteristics and microbial community. Sci Total Environ 790:148242. https://doi.org/10.1016/j.scitotenv.2021.148242
APHA (2012) Standard Methods for the Examination of Water and Wastewater, 21st edn. American Public Health Association, Washington, DC
Bhattacharjee AS, Wu S, Lawson CE, Jetten MSM, Kapoor V, Domingo JWS, McMahon KD, Noguera DR, Goel R (2017) Whole-community metagenomics in two different anammox configurations: process performance and community structure. Environ Sci Technol 51:4317–4327. https://doi.org/10.1021/acs.est.6b05855
Bradford MM (1976) A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding, Anal. Biochem. 72, 248–254. Anal Biochem 72:248–254
Cao J, Li N, Jiang J, Xu Y, Zhang B, Luo X, Hu Y (2022) Activated carbon as an insoluble electron shuttle to enhance the anaerobic ammonium oxidation coupled with Fe(III) reduction process. Environ Res 204:111972. https://doi.org/10.1016/j.envres.2021.111972
Chen C, Jiang Y, Liu J, Adams M, Chang Y, Guo M, Xie J, Xie J (2021) The structure of anammox granular sludge under varying long-term organic matter stress: Performance, physiochemical and microbial community. J Clean Prod 323:129117. https://doi.org/10.1016/j.jclepro.2021.129117
Chen Z, Meng Y, Sheng B, Zhou Z, Jin C, Meng F (2019) Linking exoproteome function and structure to anammox biofilm development. Environ Sci Technol 53:1490–1500. https://doi.org/10.1021/acs.est.8b04397
Cole EJ, Zandvakili OR, Xing B, Hashemi M, Herbert S, Mashayekhi HH (2019) Dataset on the effect of hardwood biochar on soil gravimetric moisture content and nitrate dynamics at different soil depths with FTIR analysis of fresh and aged biochar. Data Brief 25:104073. https://doi.org/10.1016/j.dib.2019.104073
Easton ZM, Rogers M, Davis M, Wade J, Eick M, Bock E (2015) Mitigation of sulfate reduction and nitrous oxide emission in denitrifying environments with amorphous iron oxide and biochar. Ecol Eng 82:605–613. https://doi.org/10.1016/j.ecoleng.2015.05.008
Gao F, Zhang H, Yang F, Qiang H, Zhang G (2012) The contrast study of anammox-denitrifying system in two non-woven fixed-bed bioreactors (NFBR) treating different low C/N ratio sewage. Bioresour Technol 114:54–61. https://doi.org/10.1016/j.biortech.2012.02.113
Guo M, Jiang Y, Xie J, Cao Q, Zhang Q, Mabruk A, Chen C (2022) Bamboo charcoal addition enhanced the nitrogen removal of anammox granular sludge with COD: Performance, physicochemical characteristics and microbial community. J Environ Sci (china) 115:55–64. https://doi.org/10.1016/j.jes.2021.07.010
Jia F, Yang Q, Liu X, Li X, Li B, Zhang L, Peng Y (2017) Stratification of Extracellular Polymeric Substances (EPS) for Aggregated Anammox Microorganisms. Environ Sci Technol 51:3260–3268. https://doi.org/10.1021/acs.est.6b05761
Jia W, Sun X, Gao Y, Yang Y, Yang L (2020) Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci Total Environ 740:139534. https://doi.org/10.1016/j.scitotenv.2020.139534
Jin R, Ma C, Yu J (2013) Performance of an Anammox UASB reactor at high load and low ambient temperature. Chem Eng J 232:17–25. https://doi.org/10.1016/j.cej.2013.07.059
Jin R, Yang G, Yu J, Zheng P (2012) The inhibition of the Anammox process: A review. Chem Eng J 197:67–79. https://doi.org/10.1016/j.cej.2012.05.014
Kang D, Yu T, Xu D, Zeng Z, Ding A, Zhang M, Shan S, Zhang W, Zheng P (2019) The anammox process at typical feast-famine states: Reactor performance, sludge activity and microbial community. Chem Eng J 370:110–119. https://doi.org/10.1016/j.cej.2019.03.111
Keiluweit M, Nico PS, Johnson MG, Kleber M (2010) Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ Sci Technol 44:1247–1253. https://doi.org/10.1021/es9031419
Lever M (1972) A new reaction for colorimetric determination of carbohydrates. Anal Biochem 47:273–279
Li S, Zhang M, Gao Y, Li H, Wang Q, Zhang L (2021) Preparation of a porous carbon from Enteromorpha prolifera with excellent electrochemical properties. New Carbon Mater 36:1158–1166. https://doi.org/10.1016/S1872-5805(21)60068-9
Li X, Wang C, Tian J, Liu J, Chen G (2020) Comparison of adsorption properties for cadmium removal from aqueous solution by Enteromorpha prolifera biochar modified with different chemical reagents. Environ Res 186:109502. https://doi.org/10.1016/j.envres.2020.109502
Liu T, Lawluvy Y, Shi Y, Ighalo JO, He Y, Zhang Y, Yap P (2022) Adsorption of cadmium and lead from aqueous solution using modified biochar: A review. J Environ Chem Eng 10:106502. https://doi.org/10.1016/j.jece.2021.106502
Lotti T, van der Star WRL, Kleerebezem R, Lubello C, van Loosdrecht MCM (2012) The effect of nitrite inhibition on the anammox process. Water Res 46:2559–2569. https://doi.org/10.1016/j.watres.2012.02.011
Park M, Kim J, Lee T, Oh Y, Nguyen VK, Cho S (2021) Correlation of microbial community with salinity and nitrogen removal in an anammox-based denitrification system. Chemosphere 263:128340. https://doi.org/10.1016/j.chemosphere.2020.128340
Shalali F, Cheraghi S, Taher MA (2022) A sensitive electrochemical sensor amplified with ionic liquid and N-CQD/Fe3O4 nanoparticles for detection of raloxifene in the presence of tamoxifen as two essentials anticancer drugs. Mater Chem Phys 278:125658. https://doi.org/10.1016/j.matchemphys.2021.125658
Tang C, Zheng P, Wang C, Mahmood Q, Zhang J, Chen X, Zhang L, Chen J (2011) Performance of high-loaded ANAMMOX UASB reactors containing granular sludge. Water Res 45:135–144. https://doi.org/10.1016/j.watres.2010.08.018
Wang S, Jiang D, Cao B, Qian L, Hu Y, Liu L, Yuan C, Abomohra AE, He Z, Wang Q, Zhang B (2018) Bio-char and bio-oil characteristics produced from the interaction of Enteromorpha clathrate volatiles and rice husk bio-char during co-pyrolysis in a sectional pyrolysis furnace: A complementary study. J Anal Appl Pyrolysis 135:219–230. https://doi.org/10.1016/j.jaap.2018.08.030
Wang W, Liu Q, Xue H, Wang T, Fan Y, Zhang Z, Wang H, Wang Y (2022a) The feasibility and mechanism of redox-active biochar for promoting anammox performance. Sci Total Environ 814:152813. https://doi.org/10.1016/j.scitotenv.2021.152813
Wang W, Wang T, Liu Q, Wang H, Xue H, Zhang Z, Wang Y (2022b) Biochar-mediated DNRA pathway of anammox bacteria under varying COD/N ratios. Water Res 212:118100. https://doi.org/10.1016/j.watres.2022.118100
Wang X, Yang H, Su Y, Liu X (2022c) Effect of the form of granular sludge and temperature on anammox immobilized fillers: From performance to microbial community analysis. Sci Total Environ 803:149754. https://doi.org/10.1016/j.scitotenv.2021.149754
Wang Y, Wang L, Fang G, Herath HMSK, Wang Y, Cang L, Xie Z, Zhou D (2013) Enhanced PCBs sorption on biochars as affected by environmental factors: Humic acid and metal cations. Environ Pollut 172:86–93. https://doi.org/10.1016/j.envpol.2012.08.007
Wei A, Ma J, Chen J, Zhang Y, Song J, Yu X (2018) Enhanced nitrate removal and high selectivity towards dinitrogen for groundwater remediation using biochar-supported nano zero-valent iron. Chem Eng J 353:595–605. https://doi.org/10.1016/j.cej.2018.07.127
Xu J, Li C, Shen Y, Zhu N (2022) Anaerobic ammonium oxidation (anammox) promoted by pyrogenic biochar: Deciphering the interaction with extracellular polymeric substances (EPS). Sci Total Environ 802:149884. https://doi.org/10.1016/j.scitotenv.2021.149884
Xu J, Wu X, Zhu N, Shen Y, Yuan H (2020) Anammox process dosed with biochars for enhanced nitrogen removal: Role of surface functional groups. Sci Total Environ 748:141367. https://doi.org/10.1016/j.scitotenv.2020.141367
Xu X, Liu G, Fan Q, Chen J, Wang Y, Zhang Y, Yang Y, Wang J, Zhang Y, Jiang H, Qi L, Wang H (2018) Effects of gibberellin on the activity of anammox bacteria. J Environ Manage 225:104–111. https://doi.org/10.1016/j.jenvman.2018.07.099
Yuan Y, Xie Y, Xu P, Li X (2022) Verification of inhibition effects of anoxic/aerobic alternation on NOB in nitrosation system under mainstream conditions. J Water Process Eng 45:102479. https://doi.org/10.1016/j.jwpe.2021.102479
Zhang X, Zhou Y, Xu T, Zheng K, Zhang R, Peng Z, Zhang H (2018a) Toxic effects of CuO, ZnO and TiO2 nanoparticles in environmental concentration on the nitrogen removal, microbial activity and community of Anammox process. Chem Eng J 332:42–48. https://doi.org/10.1016/j.cej.2017.09.072
Zhang Z, Cheng Y, Xu L, Bai Y, Xu J, Shi Z, Zhang Q, Jin R (2018b) Transient disturbance of engineered ZnO nanoparticles enhances the resistance and resilience of anammox process in wastewater treatment. Sci Total Environ 622–623:402–409. https://doi.org/10.1016/j.scitotenv.2017.12.016
Acknowledgements
The work was supported by the National Natural Science Foundation of China (Grant Number 51978348).
Funding
National Natural Science Foundation of China (Grant Number 51978348).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by [YT], [GC], [DG], [DY], [HL], [YW] and [QZ]. The first draft of the manuscript was written by [AX] and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Handling editor: Nabeel Khan Niazi
Supplementary Information
Additional file 1.
Supplementary figures (Fig. S1, S2) and tables (Table S1).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, A., Tian, Y., Chen, G. et al. A novel strategy for a reinforced anammox process with iron-modified Enteromorpha prolifera biochar. Biochar 5, 8 (2023). https://doi.org/10.1007/s42773-023-00206-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42773-023-00206-0