Abstract
Terrestrial and aquatic ecosystems are increasingly threatened by pesticide pollution resulting from extensive use of pesticides, and due to the lack of regulatory measures in the developing world, there is a need for affordable means to lessen environmental effects. This study aimed to investigate the impact of biochar amendment on the toxicity of imidacloprid to life-cycle parameters and biomarker responses of the earthworm Eisenia fetida. E. fetida was exposed to 10% biochar-amended and non-amended OECD artificial soils spiked with 0, 0.75, 1.5, 2.25 and 3 mg imidacloprid/kg for 28 days. An LC50 of 2.7 mg/kg was only computed in the non-amended soil but not in the biochar-amended soil due to insignificant mortality. The EC50 calculated in the non-amended soil (0.92 mg/kg) for reproduction (fertility) was lower than the one computed in the biochar amended (0.98 mg/kg), indicating a decrease in toxicity in the biochar-amended substrate. Significant weight loss was observed at the two highest imidacloprid treatments in the non-amended soil and only at the highest treatment in the biochar-amended substrate, further highlighting the beneficial effects of biochar. Catalase activity decreased significantly at the two highest concentrations of non-amended soil. Yet, in the amended soil, the activity remained high, especially in the highest concentration, where it was significantly higher than the controls. This indicated more severe oxidative stress in the absence of biochar. In all non-amended treatments, there was a significant acetylcholinesterase inhibition, while lower inhibition percentages were observed in the biochar-amended soil. In most endpoints, the addition of biochar alleviated the toxic effects of imidacloprid, which shows that biochar has the potential to be useful in soil remediation. However, there is still a need for field studies to identify the most effective application rate of biochar for land application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Neonicotinoids are neurotoxic insecticides designed to target the central nervous system of arthropods, acting as agonists to postsynaptic acetylcholine receptors, which are their molecular target sites (Goulson 2013; Jeschke and Nauen 2008). Unlike other groups of insecticides, it takes time for insects to develop resistance against neonicotinoids (Jeschke and Nauen 2008). They have been proven to be effective against a wide range of agricultural insects pests, and they are amongst the most used in the world (Jeschke and Nauen 2008; Jeschke et al. 2010). Examples of neonicotinoids include acetamiprid, clothianidin, thiacloprid, thiamethoxam, and imidacloprid.
According to Jeschke et al. (2010), imidacloprid was once the second most used pesticide in the world, having been registered in over 120 countries (Drobne et al. 2008). Imidacloprid has been mostly preferred because it provides long-term protection by persisting for years inside plant tissues and sometimes until plant maturity (Bonmatin et al. 2005; Oliver et al. 2010). Although designed to control agricultural pests such as the Russian wheat aphid (Diuraphis noxia)—according to the manufacturer—imidacloprid has been found to be deleterious to non-target organisms. Adverse effects include mortality, weight loss, sperm deformity, decrease in reproduction, nervous breakdown and more (Capowiez et al. 2005; Luo et al. 1999; Nyoka et al. 2018; Suchail et al. 2001; Zang et al. 2000). At a very low concentration (0.1 μg/L), imidacloprid was found to cause significant mortality in honeybees (Apis mellifera) after 10-day exposure (Suchail et al. 2001). Nyoka et al. (2018) documented a reproduction EC50 of 22 mg/kg after exposing the potworm Enchytraeus albidus to imidacloprid. In two laboratory studies conducted using Eisenia fetida, an LC50 of 2.3 mg/kg and evidence of sperm deformity were reported following a 14-day exposure to imidacloprid (Luo et al. 1999; Zang et al. 2000). When E. fetida was exposed to 24 different pesticides, imidacloprid was found to be the second most toxic to this species (Wang et al. 2012). When compared with thiacloprid, imidacloprid resulted in greater toxic effects to five invertebrates such as earthworms and enchytraeids (Silva et al. 2017). There is, therefore, a growing interest in moderating its toxicity, especially using cost-effective (and environmentally sound) approaches such as biochar amendment. Indeed rising concerns about the potential of imidacloprid to cause adverse effects to beneficial organisms have led the European Union to implement a ban on the outdoor use of this pesticide (Stokstad 2018). The USA has recently followed suit, banning 12 pesticides “that are like nicotine for bees” (Bendix 2019). Despite these regulatory efforts in developed countries, imidacloprid is still widely used in developing countries, such as South Africa, where deleterious effects on flying and crawling invertebrates (including beneficial ones) are expected to occur.
Biochar is one of the byproducts of pyrolysis, which occurs when biomass is heated under the limited presence or complete absence of oxygen in temperatures higher or equal to 250 °C. Biochar has received plenty of attention as a soil amendment, primarily for its ability to improve soil fertility, sequester carbon and provide other benefits to plants (Lehmann and Joseph 2015; Lehmann et al. 2011; Tan et al. 2015). It has been reported to improve adsorption–desorption and the degradation of pesticides in the soil (Khorram et al. 2016). According to Xu et al. (2012), the stability and absorbent properties of biochar make it a perfect remedy for polluted soils when used as a soil amendment, given that it can bind with toxicants, thus reducing their mobility. For example, Yu et al. (2009) revealed that the ability of biochar to sequester pesticide residues could result in reduced uptake of chlorpyrifos and carbofuran, two organic pesticides, by the spring onion (Allium cepa). Potential beneficial effects of biochar towards soil organisms have also been presented in a few studies. Khorram et al. (2016) investigated how biochar amendment affects the dissipation of fomesafen, and Wang et al. (2012) studied the influence of biochar amendment on chlorantraniliprole's bioavailability to earthworms. In these two studies, the bioavailability of both pesticides was reduced in the presence of biochar. More recently, Zhang et al. (2019) also found a decrease in the effects of the herbicide, mesotrione on E. fetida in biochar-amended soil. It remains unknown whether these advantageous effects of biochar, mostly documented on the whole organism (i.e., life-cycle parameters), also have a bearing on physiological (i.e., below organismal) processes. This is the knowledge gap that the present contribution intends to fill.
Despite the beneficial attributes of biochar, some authors have highlighted the potential adverse effects associated with biochar soil amendment, notably its ability to reduce the efficacy of soil pest control endeavors because of its high absorption capacity (Graber et al. 2011a, b). Nevertheless, most studies on the beneficial effects of biochar have seldom provided supporting data at the biomarker level; the present study sought to provide new information on the impact of biochar amendment on the toxicity of imidacloprid to the survival, reproduction, biomass change as well as biomarker responses of the earthworm E. fetida.
2 Materials and methods
2.1 Study organism
Eisenia fetida (Oligochaeta) was readily available from a laboratory population housed at our institution. This population was maintained on a soil substrate and fed weekly with cow dung. Prior to the exposure, selected adult worms were washed with deionized water and placed on clean filter paper overnight, where they were allowed to empty their guts.
2.2 Chemical
Aphicide Plus® (manufactured by Efekto, a South African-based company specializing in the production of pesticides) containing 20 g of imidacloprid per liter, was used in the present study. According to the manufacturer’s instructions, an aqueous solution of 400 mg of imidacloprid per liter is recommended for field applications. However, this concentration (w/w, i.e., 400 mg/kg of dry soil) could not be used in the present study because imidacloprid has been reported to have a median lethal concentration (LC50) ranging from 2 to 4 mg/kg of dry soil in earthworms such Eisenia fetida, Aporrectodea nocturna and Allolobophora icterica (Luo et al. 1999; Zang et al. 2000; Capowiez et al. 2005). For the purpose of the present study, therefore, Aphicide Plus® was used to make the following nominal concentration series of imidacloprid, following the method of Nyoka et al. (2018): 0 (negative control) 0.75, 1.5, 2.25, and 3 mg/kg of dry soil.
2.3 Soil preparation and biochar amendment
The exposure substrate was the OECD artificial soil, which is a mix of 10% of sphagnum peat, 20% kaolin clay and 70% sand, as portrayed by the Organization for Economic Cooperation and Development (OECD 2004). The biochar used in this study was purchased from Carbon Fertilizer (C Fert™) in Johannesburg, South Africa. It was made from pyrolysis of pine tree biomass at 400–450 °C. The pyrolysis time was 12 h for 100 kg of the feed. To prepare biochar-amended OECD soil, biochar pellets were ground to a fine powder and passed through a 2 mm sieve. The amended substrates were prepared by replacing 10% dry weight of OECD soil with biochar. In 1-L Consol jars, 50 g of biochar were mixed with 450 g dry weight of OECD soil to create a 500 g exposure substrate. The moisture of all exposure substrates was maintained between 40 and 60% soil water holding capacity, as recommended by the OECD (OECD 2004). The aqueous solutions of imidacloprid were thoroughly mixed with the soils before the introduction of the worms.
2.4 Bioassays
Ten adult Eisenia fetida were weighed individually and exposed to the imidacloprid concentration range mentioned above in 500 g of biochar-amended and non-amended OECD soil. The exposure was performed in triplicate in 1-L Consol glass jars with perforated lids. The treatments were incubated at 20 ± 2 °C for 28 days in a Kelvinator incubator (K385 FF) in the laboratory. Once a week, exposure jars were weighed to estimate the amount of water lost through evaporation. In each exposure group, water loss was replenished with deionized water, and the worms were fed with 5 g of finely grounded urine-free cow dung on a weekly basis. Earthworms were weighed individually before the exposure and at the end of the exposure period on day 28. After that, all biological samples were stored at −80 °C until biomarker analyses could be performed.
2.4.1 Survival
The survival rates of earthworms in all the exposure treatments were determined at the end of the exposure period. The exposure substrates were transferred to white plastic trays where live earthworms were confirmed by hand sorting. The earthworms unresponsive to tactile stimulus were counted as dead.
2.4.2 Reproduction
Reproduction was estimated by counting the number of cocoons produced during the experiments. After hand sorting the live and dead earthworms, the exposure substrates were sieved through 2 mm sieves to facilitate the cocoon count. After that, the harvested cocoons were transferred to Petri dishes containing clean distilled water to allow them to hatch (Venter and Reinecke 1988). The Petri dishes were monitored daily for an additional 28 days. The hatchlings were recorded and removed from the Petri dishes as they emerged. During this period, it was ensured that the cocoons remained immersed in water to prevent desiccation.
2.4.3 Biochemical responses
Two earthworms from each treatment were randomly selected for biomarker analyses. For biomarker analyses, the earthworms were defrosted at 4 °C and the tail-end sections of the worms were cut-off to prepare the homogenates.
Catalase (CAT) activity was measured as described in Cohen et al. (1970). The experimental mixtures and standard conditions were as follows: phosphate buffer, 0.01 M pH 7.4, 93 µL H2O2 and 10 µL homogenates of the earthworm, 19 µL H2SO4, 130 µL KMnO4. The procedure was carried out at 4 °C. The absorbance variation was measured at 492 nm on a spectrophotometer (Beckman, DU 7400, USA), and readings were taken within 30–60 s. Protein concentration was determined by Bradford (1976), where standard solutions were prepared using bovine serum albumin (BSA) and 1:4 diluted Bio-Rad Protein Assay Dye Reagent Concentrate (BIO-RAD, Hercules, California, USA) was added. Thereafter, the absorbance was measured using a nanodrop machine (Jenway Genova Nano 3 in 1 Micro-volume Spectrophotometer). For CAT activity, a standard solution of phosphate buffer, H2SO4 and KMnO4 was used, and the following calculations were performed to determine CAT activity (in µmol H2O2/min/mg protein):
Here, k represents the first-order reaction rate constant, S0 equals to an average of standard absorbance reading, S3 equals to standard minus average absorbance of sample, and t is the time taken to measure the reaction.
Acetylcholinesterase (AChE) activity was determined according to the protocol described by Ellman et al. (1961). To measure AChE activity, samples were homogenized in ice-cold phosphate buffer containing 0.02 M potassium dihydrogen phosphate, pH 7.5. Homogenates were then centrifuged at 9500g (4 °C) for a period of 10 min. Potassium phosphate buffer (0.09 M, pH 7.4) was used as a blank. The absorbance was assessed spectrophotometrically at 412 nm. AChE activity was determined by calculating the average absorbance of the readings at each time interval from time 0 to time 6 min. The linear graph for each sample was drawn and expressed as the change in absorbance over time. Then, the gradient was calculated for each sample curve and divided by six minutes. AChE activity was calculated as follows: (Absorbance/min/mg protein) = (Abs/min) ÷ mg protein. Thereafter, the inhibition percentage was computed using the control’s AChE activity as the standard activity. Protein content was determined, as mentioned in the previous section.
2.4.4 Statistics
Descriptive statistics were performed on the raw data using the statistical package ToxRat® Professional version 2.10.05 (Toxicity Response Analysis and Testing; ToxRat solutions GmbH, Alsdorf, Germany) and Graphpad Prism (Graphpad Prism version 5.00 for Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com). ToxRat® was also used to calculate effective concentrations (ECs) and lethal concentrations (LCs) whenever possible. Normally distributed data with homogeneous variances were analyzed using One-way ANOVA, with Bonferroni post-test. Normally distributed data with non-homogeneous variances were analyzed using the parametric Bonferroni t-test for non-homogeneous variances. Non-parametric data were analyzed using the Kruskal–Wallis ANOVA followed by Dunns’ test. The level of significance was set to be p < 0.05. Weight losses or gains were estimated by subtracting day 0 weights from day 28 weights. Positive values indicated weight gain, while negative values were an indication of weight loss.
3 Results and discussion
3.1 Survival
Significantly higher mortality (~ 67%), when compared to the respective control, was observed at the highest imidacloprid concentration in the non-biochar-amended soil (p < 0.05). In the biochar-amended soil, there were no significant differences in mortality between the control and treatments (Fig. 1). The comparison of survival rates between non-amended and biochar-amended soils reveals that mortality was significantly different only between the highest treatments, with significantly more mortality occurring in the non-amended soil. The median lethal concentration (LC50) for imidacloprid in E. fetida was found to be 2.69 (2.50–2.98) mg/kg in the soil without biochar. The same could not be computed in the soil amended with biochar due to low mortality (i.e., LC50 > 3 mg/kg).
These results indicate that the addition of biochar improved the survival rate of Eisenia fetida. Malińska et al. (2016), in testing the effect of pre-composed sewage sludge, observed no mortality when Eisenia fetida was exposed in biochar-amended sewage sludge, as opposed to non-amended sewage sludge. Moreover, in the absence of a toxicant, Liesch et al. (2010) revealed that amending the soil with less than 67.5 mg/ha of biochar does not affect survival in any way. These authors observed no difference in mortality of Eisenia fetida in soil amended with pine chip biochar at application rates of 0, 22.5, 45 and 67.5 mg/ha. Evidently, the presence of 10% biochar has no lethal implications to E. fetida as it was observed in the biochar-amended control of the present study.
The LC50 calculated in the present study is at least 148-fold below the recommended concentration for Aphicide Plus® field use, which is 400 mg imidacloprid/kg dry soil. This finding suggests that the use of this insecticide at its current recommended concentration for field application poses a severe threat to beneficial organisms like earthworms.
Different LC50s for imidacloprid have been documented for different species. In accordance with the present study, Luo et al. (1999) and Zang et al. (2000) both reported an LC50 of 2.3 mg/kg from their laboratory studies conducted using Eisenia fetida, exposed to imidacloprid for 14 days. In a different study, Kreutzweiser et al. (2008) reported an LC50 of 25 mg/kg for E. fetida after 35-day exposure to imidacloprid, while Mostert et al. (2002) using earthworms of the Pheretima group exposed in OECD soil found an LC50 of 155 mg/kg after 24 h, 5 mg/kg after 48 h and 3 mg/kg after seven days. These variations in LC50 could be explained by differences in the species, exposure duration and the form or origin of imidacloprid. A not determined LC50 in the biochar-amended soil in the present study indicates that within the concentration range investigated, 10% biochar amendment was sufficient to reduce the lethality of imidacloprid to Eisenia fetida significantly.
3.2 Reproduction
Figure 2 depicts that in the non-amended soil, cocoon production (fecundity) was significantly lower at 1.5 mg/kg (p ≤ 0.001) when compared to the respective control. Cocoon production was completely inhibited in the 2.25 and 3 mg/kg treatments. Regardless of biochar amendment, cocoon production was significantly reduced in all the treatments compared to the control (p ≤ 0.05). Nevertheless, there was no total inhibition of E. fetida reproduction in the biochar-amended substrates at the highest test concentration. When comparing non-amended and biochar-amended treatments, the beneficial effect of biochar on cocoon production was visible even in the controls, where it was significantly higher in the biochar-amended soil. This suggests that biochar increases the reproduction of E. fetida, and this beneficial effect could be because of the nutritious effect of biochar (organic carbon content) (Lehmann and Joseph 2015) as well as its sorption (Tang et al. 2013) of natural toxic compounds found in the soil. More cocoons were produced in the biochar-amended soil, though significant variation in the treatments was only seen at 2.25 mg/kg.
Moreover, the EC50s for fecundity were found to be 1.10 (1.09–1.11) mg/kg for non-amended substrates and 1.52 (0.63–3.66) mg/kg in amended substrates showing a slight decrease in toxicity in the biochar-amended soil. Nonetheless, this indicates that amending the soil with biochar minimizes the harmful effects of imidacloprid on earthworms' reproductive success. This result agrees with the findings of Malińska et al. (2016), who observed higher cocoon production in Eisenia fetida after one month of exposure to the sewage sludge amended with biochar as opposed to the non-amended alternative.
In the present study, once the cocoons were allowed to hatch after an additional incubation time of 28 days in distilled water, the number of juveniles (fertility) from the substrate without biochar was significantly lower than that in the respective control from 1.5 mg/kg upward (p ≤ 0.05). However, in the biochar-amended substrate, the number of juveniles produced in the respective control was significantly higher than that in all the imidacloprid treatments (Fig. 3, p ≤ 0.001). The comparison between amended and non-amended treatments also showed greater hatching rates in the biochar-amended substrate. These rates were statistically greater in the control and 0.75 mg/kg imidacloprid treatment (p < 0.05).
An EC50 = 0.92 mg/kg (0.56–1.49) in the non-biochar-amended soil was computed although it was slightly lower than the EC50 = 0.98 mg/kg (0.47–2.10) calculated in the biochar-amended soil for fertility. Due to the overlapping confidence intervals, these values indicate that overall, the presence of biochar did not significantly alleviate the toxic effects of imidacloprid for this particular endpoint. Our findings, nevertheless, suggest that fertility (cocoon hatching) is more sensitive than fecundity (cocoon production).
Literature evidence reveals that as little as 0.5 mg/kg of imidacloprid has the potential to cause sperm deformities in earthworms and affect reproduction (Luo et al. 1999). Reproduction appeared as the most sensitive endpoint in the present study when compared to survival, and this corroborates the findings of Wang et al. (2015). For a similar duration as that of the current study, Wang et al. (2015) reported a significant reduction caused by imidacloprid in cocoon production and hatchability of E. fetida at 0.5 and 1 mg/kg, respectively.
3.3 Biomass
Figure 4 depicts that a statistically significant loss in biomass was only observed at the two highest treatments, 2.25 and 3 mg/kg (p ≤ 0.001) in non-amended OECD artificial soil. A pairwise comparison of total biomass between homologous treatments indicated that significant differences were only present within the two highest treatments (2.25 and 3 mg/kg), where weight loss was significantly higher in the non-amended soil. The half-maximal effective concentration for biomass (EC50) in non-amended soil groups was found to be 2.19 (1.71–2.77) mg/kg. In contrast, the reduction in biomass was not substantial enough to help generate an EC50 in the presence of biochar.
During the exposure period, it was observed that food was usually uneaten in the experimental treatments with higher imidacloprid concentration. Similar to our results, Dittbrenner et al. (2011) observed biomass loss in both E. fetida and L. terrestris after exposure to imidacloprid. Kreutzweiser et al. (2008) reported similar results after exposure of E. fetida and Dendrobaena octaedra to imidacloprid for 35 days. Reduction in body weight is more than just loss in biomass. It could affect other essential parameters, like reproduction and survival. It is an ecologically relevant and valuable endpoint to consider when various factors are studied (Capowiez et al. 2005). The beneficial effect of biochar on earthworm biomass has been reported elsewhere. Malińska et al. (2016) reported an increase in the total weight of Eisenia fetida after exposure to the sewage sludge amended with 28% willow woodchips biochar.
3.4 Catalase
Under oxidative stress, reactive oxygen species (ROS) are expressed in earthworms (Chen et al. 2018). In dealing with these ROS, catalase is deployed as one of the first lines of defence to scavenge excess ROS (Zhang et al. 2013). There was no significant difference (p > 0.05) in the present study in CAT activity between the biochar-amended treatments 0.75 and 1.5 mg/kg and their respective control. In the higher concentrations of 2.25 and 3 mg/kg imidacloprid, CAT activity was significantly higher than that in the control, in the amended substrates (p < 0.05).
For the substrates without biochar amendment, significant differences were observed in CAT activities. There was a significant increase (p ≤ 0.05) in CAT activity at 1.5 mg/kg followed by a significant (p ≤ 0.05) decrease in CAT activities for 2.25 and 3 mg/kg imidacloprid treatments (Fig. 5). Catalase activity may increase when earthworms are subjected to mild stress, but generally, it decreases under severe stress (Liu et al. 2014). This explains the trend in CAT activity observed in non-biochar-amended substrates. The observed decrease/inhibition in CAT activities as the concentration of imidacloprid rises could imply that the organisms were under more severe stress in the non-amended than the biochar-amended substrates. The initial increase in CAT activity in lower imidacloprid concentrations has been documented before. Zhang et al. (2013) reported an increase in CAT activity in E. fetida from 0.2 to 2 mg/kg of imidacloprid, with the highest concentration of 4 mg/kg of imidacloprid causing similar CAT activities as in the control.
In the presence of biochar, a significant increase in catalase activity was only observed at the highest treatment, 3 mg/kg (Fig. 5), thus implying that earthworms were experiencing comparatively less stress than in the non-amended substrates. In all the treatments below 3 mg/kg, CAT activity was not statistically different from that of the control, indicating the relative absence of oxidative stress in these treatments. Zhang et al. (2019) also observed no change in the activity of three antioxidant enzymes (dismutase, catalase, peroxidase) of E. fetida when treatments of the herbicide mesotrione were amended with ≤ 10% wheat straw-derived biochar. In a substrate amended with a biochar amount twice as high as ours (200 g/kg , ~ 20%) and without any other chemical, Li et al. (2011) observed no significant increase in malondialdehyde (oxidative stress biomarker) in Eisenia fetida. However, some types of biochar (depending on the concentration) may induce oxidative stress in earthworms. For example, there was a noticeable increase in total antioxidant capacity of Lumbricus terrestris in soils treated with 1 and 5% biochar, indicating the presence of oxidative stress (Sanchez-Hernandez et al. 2019).
Overall, when biochar-amended and non-amended treatments were compared (Fig. 5), it was observed that there were significant differences in CAT activities from 1.5 mg/kg and higher concentrations with greater activity recorded in the two highest treatments of the amended substrates. These results suggest that amendment with biochar could effectively decrease the levels of oxidative stress experienced by earthworms after exposure to imidacloprid. It is possible that the addition of biochar prevented greater contact between the earthworms and the toxicant by reducing the imidacloprid concentration in the pore water available to the earthworms (Jin et al. 2016).
3.5 Acetylcholinesterase
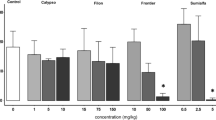
In the non-biochar-amended soil, there was a slight increase in AChE inhibition as the concentration of imidacloprid increased; however, there was no significant difference (p > 0.05) in AChE inhibition between all treatments (Fig. 6). In biochar-amended soil, there was also a steady increase in AChE inhibition as the imidacloprid concentration increased. Moreover, in these amended treatments, there was a significant difference in inhibition rate between the lowest treatment 0.7 mg/kg and two higher treatments, 2.25 and 3 mg/kg, with the highest inhibition rate occurring at the treatment with the highest concentration of imidacloprid (Fig. 6; p ≥ 0.05).
Inhibition of AChE activity of Eisenia fetida after 28 days of exposure to imidacloprid in non-amended- and biochar-amended OECD artificial soils. The control treatment was used as a reference point to create inhibition percentages. Different letters above the bars indicate the statistical difference between the groups (p < 0.05). Error bars represent standard deviation
AChE is considered to be the main cholinesterase in earthworms, and it plays a critical role in the functioning of the nervous system. Observed inhibition implies that imidacloprid was toxic to earthworm. As a neurotoxic pesticide, imidacloprid is originally intended to disrupt the nervous system of target organism by binding to and activating nicotinic acetylcholine receptors (Matsuda et al. 2001). This is possibly the case for earthworms. This mode of action leads to the accumulation of acetylcholine, and there is a need for eliminating the excess acetylcholine. As others have previously indicated, our results suggest that imidacloprid affects AChE activity (Tomizawa and Casida 2003).
As it is the case in the present study with imidacloprid, other authors have previously documented the deleterious effects of chlorpyrifos, another neurotoxic agent, after assessing its effects on AChE in E. fetida (Rao et al. 2003; Reinecke and Reinecke (2007). The decline of AChE activity in the present study is attributed to the fact that imidacloprid is an agonistic that binds to AChE receptors. In the overall comparison, there was a decrease in AChE activity in non-biochar-amended substrate compared to biochar-amended substrate, as witnessed by the consistently high AChE inhibition rates in the former group (Fig. 6). The reduced inhibition observed in the biochar-amended substrates suggests that biochar can reduce this pesticide's neurotoxic effects on earthworms, thus supporting our findings at the higher biological level (life-cycle parameters) in the present study.
3.6 Overall effects of biochar on imidacloprid
The alleviating effects of biochar on the toxicity of imidacloprid to E. fetida can be explained by considering the various properties of biochar. When biochar is produced through the process of pyrolysis, it is conferred specific physical and chemical properties, including improved surface area and porosity (pore size distribution) which permit the sorption of chemicals (Yu et al. 2011; Tang et al. 2013). Increased sorption rate in the soil is possibly a consequence of increased organic carbon content from the addition of biochar (Lehmann and Joseph 2015). Weak binding strength between the pesticide and biochar also weakens desorption (reverse of sorption) of the pesticide in soil solution (Zhang et al. 2010; Tatarková et al. 2013). Biochar’s surface adsorption and absorption are essential factors that contribute to reducing the toxicological effects of chemicals on organisms (Khorram et al. 2016). However, degradation is also one of the crucial processes that significantly influence the efficacy of toxicant’s molecules (Muter et al. 2014). It has also been demonstrated that amending soil with biochar can lower the leaching and dissipation of MCPA herbicide due to biochar's strong binding capacity (Tatarková et al. 2013). Therefore, reduced toxic effects of imidacloprid to Eisenia fetida in biochar-amended soils could be attributed to the mechanisms mentioned above.
In the present study, the alleviating effects of biochar on the toxicity of imidacloprid were recorded across the endpoints investigated. Focusing on life-cycle parameters, for instance, the different LC50s and EC50s showed that fertility (or hatchling success) was the most sensitive endpoint with an EC50 of 0.92 mg/kg in non-biochar-amended soil. In the presence of biochar, this value increased slightly to 0.98 mg/kg (indicating a slight decrease in toxicity), although the change was not statistically significant (based on overlapping 95% confidence intervals, see Sect. 3.2). Inversely, the least sensitive endpoint was survival, with an LC50 of 2.69 mg/kg in non-biochar-amended soil. Biochar amendment caused a significant increase of this index, which could not be calculated in amended soil, indicating a substantial decrease in imidacloprid toxicity (see Sect. 3.2).
Our results are in agreement with those of other studies. For instance, biochar was reported to reduce chlorantraniliprole’s bioavailability and intake by two earthworm species (Wang et al. 2012). Additionally, Khorram et al. (2016) reported reduced uptake of the pesticide fomesafen by earthworms in soils amended with 0.5%, 1% and 2% of biochar. Wang et al. (2015) also observed reduced atrazine bioaccumulation in the earthworms Metaphire guillelmi and E. fetida in biochar-amended soils.
The fact that biochar could not totally suppress the toxic effects of imidacloprid points to some limitations inherent with its use. Pyrolysis temperature could help to explain some of these limitations. The biochar used in the current study was produced at 400–450 °C pyrolysis temperature with an average pore size of about 100 μm. It has been reported that biochar produced below 850 °C has limited sorption capacity because of the small surface area, which restricts the absorption of contaminants, whereas biochar produced at higher temperature tends to have an increased surface area (Yu et al. 2009; Lehmann et al. 2011; Tang et al. 2013). To alleviate chemical toxicity, the optimal biochar feed or pyrolysis conditions still need to be investigated.
4 Conclusion
The present study reveals the significant alleviating properties of biochar on the toxicity of imidacloprid to E. fetida, using a combination of life-cycle parameters and biomarker responses. Our findings show that amending soil with biochar significantly protects the experimental organism at both life-cycle parameters (i.e., survival, reproduction and biomass) and biomarker levels (i.e., catalase and acetylcholine esterase). These novel results show that although biochar can be useful in reducing the potential risks of chemical pollutions to soil invertebrates, its efficiency ultimately depends on some of its inherent properties and the pollution levels found in the environment. In the present case, the threshold from which biochar could no longer prevent significant deleterious effects lied in between 1.5 and 2.25 mg of imidacloprid per kg of soil. In contrast, for field applications of Aphicide Plus® (the imidacloprid formula used in this study), the manufacturer recommends the topical application of an aqueous solution containing 400 mg of imidacloprid per liter (i.e., 400 mg/kg soil, w/w). This is way over the 1.5–2.25 mg/kg threshold mentioned above using 10% biochar amendment. Although we are unsure of the manner in which higher amendment percentages will influence imidacloprid toxicity, it seems based on our findings that enforcing the controlled use of this pesticide (through partial bans), like many developing countries have done (Bendix 2019), would be the safest way to prevent widespread environmental effects of imidacloprid and similar pesticides.
Availability of data and material
Data and calculations tools are available on request from the corresponding author (nyokawinnie@gmail.com).
References
Bendix A (2019) The US Environmental Protection Agency (EPA) recently banned 12 products containing neonicotinoid, a pesticide that functions similarly to nicotine. United States
Bonmatin JM, Marchand PA, Charvet R, Moineau I, Bengsch ER, Colin ME (2005) Quantification of imidacloprid uptake in maize crops. J Agric Food Chem 53:5336–5341
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Capowiez Y, Rault M, Costagliola G, Mazzia C (2005) Lethal and sublethal effects of imidacloprid on two earthworm species (Aporrectodea nocturna and Allolobophora icterica). Biol Fertil Soils 41:135–143
Chen J, Saleem M, Wang C, Liang W, Zhang Q (2018) Individual and combined effects of herbicide tribenuron-methyl and fungicide tebuconazole on soil earthworm Eisenia fetida. Sci Rep 8:2967
Cohen G, Dembiec D, Marcus J (1970) Measurement of catalase activity in tissue extracts. Anal Biochem 34:30–38
Dittbrenner N, Schmitt H, Capowiez Y, Triebskorn R (2011) Sensitivity of Eisenia fetida in comparison to Aporrectodea caliginosa and Lumbricus terrestris after imidacloprid exposure. Body mass change and histopathology. J Soils Sediments 11:1000
Drobne D, Blažič M, Van Gestel CAM, Lešer V, Zidar P, Jemec A et al (2008) Toxicity of imidacloprid to the terrestrial isopod Porcellio scaber (Isopoda, Crustacea). Chemosphere 71:1326–1334
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Goulson D (2013) An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol 50:977–987
Graber ER, Tsechansky L, Gerstl Z, Lew B (2011a) High surface area biochar negatively impacts herbicide efficacy. Plant Soil 353:95–106
Graber ER, Tsechansky L, Khanukov J, Oka Y (2011b) Sorption, volatilization and efficacy of the fumigant 1,3-dichloropropene in a biochar-amended soil. Soil Sci Soc Am J 75(4):1365–1373
Jeschke P, Nauen R (2008) Neonicotinoids—from zero to hero in insecticide chemistry. Pest Manag Sci 64:1084–1098
Jeschke P, Nauen R, Schindler M, Elbert A (2010) Overview of the status and global strategy for neonicotinoids. J Agric Food Chem 59:2897–2908
Jin J, Kang M, Sun K, Pan Z, Wu F, Xing B (2016) Properties of biochar-amended soils and their sorption of imidacloprid, isoproturon, and atrazine. Sci Total Environ 550:504–513
Khorram MS, Zhang Q, Lin D, Zheng Y, Fang H, Yu Y (2016) Biochar: a review of its impact on pesticide behavior in soil environments and its potential applications. J Environ Sci 44:269–279
Kreutzweiser DP, Good KP, Chartrand DT, Scarr TA, Holmes SB, Thompson DG (2008) Effects on litter-dwelling earthworms and microbial decomposition of soil-applied imidacloprid for control of wood-boring insects. Pest Manag Sci 64:112–118
Lehmann J, Joseph S (2015) Biochar for environmental management: science, technology and implementation. Routledge, Philadelphia
Lehmann J, Rillig MC, Thies J, Masiello CA, Hockaday WC, Crowley D (2011) Biochar effects on soil biota—a review. Soil Biol Biochem 43:1812–1836
Li D, Hockaday WC, Masiello CA, Alvarez PJJ (2011) Earthworm avoidance of biochar can be mitigated by wetting. Soil Biol Biochem 43:1732–1737
Liesch AM, Weyers SL, Gaskin JW, Das KC (2010) Impact of two different biochars on earthworm growth and survival. Ann Environ Sci 4:1–9
Liu T, Zhu L, Han Y, Wang J, Wang J, Zhao Y (2014) The cytotoxic and genotoxic effects of metalaxy-M on earthworms (Eisenia fetida). Environ Toxicol Chem 33:2344–2350
Luo Y, Zang Y, Zhong Y, Kong Z (1999) Toxicological study of two novel pesticides on earthworm Eisenia foetida. Chemosphere 39:2347–2356
Malińska K, Zabochnicka-Świątek M, Cáceres R, Marfà O (2016) The effect of precomposted sewage sludge mixture amended with biochar on the growth and reproduction of Eisenia fetida during laboratory vermicomposting. Ecol Eng 90:35–41
Matsuda K, Buckingham SD, Kleier D, Rauh JJ, Grauso M, Sattelle DB (2001) Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22:573–580
Mostert MA, Schoeman AS, Merwe MVD (2002) The relative toxicities of insecticides to earthworms of the Pheretima group (Oligochaeta). Pest Manag Sci 58(5):446–450
Muter O, Berzins A, Strikauska S, Pugajeva I, Bartkevics V, Dobele G et al (2014) The effects of woodchip-and straw-derived biochars on the persistence of the herbicide 4-chloro-2-methylphenoxyacetic acid (MCPA) in soils. Ecotoxicol Environ Saf 109:93–100
Nyoka NW-K, Kanyile SN, Bredenhand E, Prinsloo GJ, Otomo PV (2018) Biochar alleviates the toxicity of imidacloprid and silver nanoparticles (AgNPs) to Enchytraeus albidus (Oligochaeta). Environ Sci Pollut Res 25:10937–10945
OECD (2004) Organization for economic cooperation and development, earthworm reproduction test. Guideline for Testing Chemicals no. 222, Paris, France
Oliver JB, Fare DC, Youssef N, Scholl SS, Reding ME, Ranger CM et al (2010) Evaluation of a single application of neonicotinoid and multi-application contact insecticides for flatheaded borer management in field grown red maple cultivars. J Environ Hortic 28:135–149
Rao JV, Surya Pavan Y, Madhavendra SS (2003) Toxic effects of chlorpyrifos on morphology and acetylcholinesterase activity in the earthworm, Eisenia foetida. Ecotoxicol Environ Saf 54:296–301
Reinecke SA, Reinecke AJ (2007) The impact of organophosphate pesticides in orchards on earthworms in the Western Cape, South Africa. Ecotoxicol Environ Saf 66:244–251
Sanchez-Hernandez JC, Ríos JM, Attademo AM, Malcevschi A, Cares XA (2019) Assessing biochar impact on earthworms: implications for soil quality promotion. J Hazard Mater 366:582–591
Silva CdL, Brennan N, Brouwer JM, Commandeur D, Verweij RA, van Gestel CAM (2017) Comparative toxicity of imidacloprid and thiacloprid to different species of soil invertebrates. Ecotoxicology 26:555–564
Stokstad E (2018) European Union expands ban of three neonicotinoid pesticides. Science, online first acess. 23 October 2019. https://www.sciencemag.org/news/2018/04/european-union-expands-ban-three-neonicotinoid-pesticides
Suchail S, Guez D, Belzunces LP (2001) Discrepancy between acute and chronic toxicity induced by imidacloprid and its metabolites in Apis mellifera. Environ Toxicol Chem 20:2482–2486
Tan X, Liu Y, Zeng G, Wang X, Hu X, Gu Y et al (2015) Application of biochar for the removal of pollutants from aqueous solutions. Chemosphere 125:70–85
Tang J, Zhu W, Kookana R, Katayama A (2013) Characteristics of biochar and its application in remediation of contaminated soil. J Biosci Bioeng 116:653–659
Tatarková V, Hiller E, Vaculík M (2013) Impact of wheat straw biochar addition to soil on the sorption, leaching, dissipation of the herbicide (4-chloro-2-methylphenoxy) acetic acid and the growth of sunflower (Helianthus annuus L.). Ecotoxicol Environmental Saf 92:215–221
Tomizawa M, Casida JE (2003) Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Rev Entomol 48:339–364
Venter JM, Reinecke AJ (1988) The life-cycle of the compost worm Eisenia fetida (Oligochaeta). S Afr J Zool 23:161–165
Wang Y, Cang T, Zhao X, Yu R, Chen L, Wu C et al (2012) Comparative acute toxicity of twenty-four insecticides to earthworm, Eisenia fetida. Ecotoxicol Environmental Saf 79:122–128
Wang K, Pang S, Mu X, Qi S, Li D, Cui F et al (2015) Biological response of earthworm, Eisenia fetida, to five neonicotinoid insecticides. Chemosphere 132:120–126
Xu G, Lv Y, Sun J, Shao H, Wei L (2012) Recent advances in biochar applications in agricultural soils: benefits and environmental implications. Clean (Weinh) 40:1093–1098
Yu X-Y, Ying G-G, Kookana RS (2009) Reduced plant uptake of pesticides with biochar additions to soil. Chemosphere 76:665–671
Yu X-Y, Mu C-L, Gu C, Liu C, Liu X-J (2011) Impact of woodchip biochar amendment on the sorption and dissipation of pesticide acetamiprid in agricultural soils. Chemosphere 85:1284–1289
Zang Y, Zhong Y, Luo Y, Kong ZM (2000) Genotoxicity of two novel pesticides for the earthworm, Eisenia fetida. Environ Pollut 108:271–278
Zhang HH, Lin KD, Wang HL, Gan J (2010) Effect of Pinus radiata derived biochars on soil sorption and desorption of phenanthrene. Environ Pollut 158(9):2821–2825
Zhang Q, Zhu L, Wang J, Xie H, Wang J, Han Y et al (2013) Oxidative stress and lipid peroxidation in the earthworm Eisenia fetida induced by low doses of fomesafen. Environ Sci Pollut Res 20:201–208
Zhang Q, Saleem M, Wang C (2019) Effects of biochar on the earthworm (Eisenia foetida) in soil contaminated with and/or without pesticide mesotrione. Sci Total Environ 671:52–58
Funding
The financial assistance of the National Research Foundation (NRF) of South Africa (grant number SFH160619172260) to Ngitheni Nyoka is hereby acknowledged. Opinions expressed and conclusions arrived at are those of the authors and are not necessary to be attributed to the NRF.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nyoka, N.WK., Ogbeide, O. & Otomo, P.V. Reproduction and biomarker responses of Eisenia fetida after exposure to imidacloprid in biochar-amended soil. Biochar 3, 615–624 (2021). https://doi.org/10.1007/s42773-021-00103-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42773-021-00103-4