Abstract
Due to specific bacterial microbiota, raw milk cheeses have appreciated sensory properties. However, they may pose a threat to consumer safety due to potential pathogens presence. This study evaluated the microbiological contamination of 98 raw milk cheeses from Beira Baixa, Portugal. Presence and enumeration of Coagulase Positive Staphylococci (CPS), Listeria monocytogenes, Salmonella spp., pathogenic Escherichia coli, and indicator microorganisms (non-pathogenic E. coli and Listeria spp.) was attained. E. coli antimicrobial resistance (AMR) was also evaluated. PCR and/or Whole genome sequencing (WGS) was used to characterize E. coli, Salmonella spp. and L. monocytogenes isolates. Sixteen cheeses (16.3%) were classified as Satisfactory, 59 (60.2%) as Borderline and 23 (23.5%) as Unsatisfactory/Potential Injurious to Health. L. monocytogenes, CPS > 104 cfu g−1, Extraintestinal pathogenic E. coli (ExPEC) and Salmonella spp. were detected in 4.1%, 6.1%, 3.1% and 1.0% of the samples, respectively. Listeria innocua (4.1%) and E. coli > 104 cfu g−1 (16.3%) were also detected. AMR E. coli was detected in 23/98 (23.5%) of the cheese samples, of which two were multidrug resistant. WGS identified genotypes already associated to human disease and Listeria spp. cluster analysis indicated that cheese contamination might be related with noncompliance with Good Hygiene Practices during cheese production.
Similar content being viewed by others
Introduction
Traditional cheeses are dairy products characteristic of a certain geographic region. These cheeses may be made from raw milk and the method of production is passed down from generation to generation. The microbiota of raw milk is complex, derived from many sources (e.g. microorganisms from teats, the farm environment, feedstuffs, as well as milking and processing equipment) and can have both positive and negative impacts on the cheese quality and shelf life and, consequently, on its economic potential. The diverse indigenous microbiota of raw milk cheeses provide fermentation, contribute to ripening and is responsible for the specific sensory properties of raw milk cheeses, namely a more intense and stronger flavor [1,2,3]. Also, some authors suggest a beneficial link between the consumption of raw milk microbes, and protection against the development of asthma and atopy, later in life [4, 5] and the reduction of the blood pressure in people with mild to moderate hypertension [6]. However, raw dairy products can be contaminated with pathogens and consequently may have health-related implications leading to severe illnesses [7]. In fact, estimates by the Center for Disease Control and Prevention, in the USA, based on data collected between 1993 and 2006, suggest that non pasteurized milk and milk products caused a disproportionate number of outbreaks and outbreak-associated illnesses relative to consumption of pasteurized products (≈150 × greater/unit of product consumed) [8]. Furthermore, Costard et al., made a study on raw milk and cheese data from 2009 to 2014 and estimated that consumption of these raw products causes 840 times more illnesses and 45 times more hospitalizations than pasteurized products [9].
Microbial contamination during cheese-making, ripening and storage may occur directly or through cross contamination events, during processing, in retail or in domestic environments [10]. Although the microbiological quality and safety of raw milk cheeses begin with milk, which may be the primary source of contamination [11], other sources of contamination can be present. Indeed, in the production environment, in food-contact surfaces and utensils that are not properly clean and sanitized some pathogenic microorganisms are able to adhere and persist forming biofilms [12]. The hands of the workers, and the unsafe handling and inadequate storage practices in domestic environments [13] are also potential sources of cheese contamination.
According to the European Food Safety Authority (EFSA) [14], between 2018 and 2022, milk and milk products caused 149 strong evidence outbreaks, with 1754 human cases, 298 hospitalizations and 22 deaths, on Europe [14]. Salmonella spp. was the leading causative agent being responsible for 27.52% of the outbreaks, Staphylococcus aureus toxins were associated with 15.44% and Shiga-toxin producing E. coli (STEC) was detected in 6.04% of these 149 outbreaks. L. monocytogenes, although only associated with 4.03% of the outbreaks, was responsible for a high number of deaths, 14 of the 22 reported [14].
Although raw milk cheeses are niche products on the global market, they constitute an important fraction of the cheese production in Portugal and are part of Portuguese cultural and gastronomic identity [15]. The protection and promotion of these products is essential as they help to reduce rural depopulation, develop existing resources and generate employment opportunities, among other benefits. Some of the Portuguese raw milk cheese brands (n = 11) have a registration of Protected Designation of Origin (PDO), which means that they have special characteristics related to the geographical place in which they were produced/ processed [16]. Beira Baixa is a historically important geographical area in terms of cheese production, and is one of the Portuguese demarcated regions for this artisanal activity. The geographical area of Beira Baixa cheeses production covers the municipalities of Castelo Branco district and part of the Santarém district, in the central region of Portugal. The type of rennet (of animal or of vegetal origin- Cynara Cardunculus) and the temperature, relative humidity and duration of the maturation period vary between brands. Beira Baixa cheeses with PDO label are “Castelo Branco”, “Amarelo da Beira Baixa” and “Picante da Beira Baixa”. “Castelo Branco” PDO labeled cheese is a semi-hard or semi-soft paste cured cheese with a yellowish color produced with ewe’s raw milk; “Amarelo da Beira Baixa” is a semi-hard or semi-soft paste cured cheese with a yellowish color produced with ewe’s or ewe’s and goat’s raw milk and “Picante da Beira Baixa” is a semi-hard to hard paste cured cheese with a greyish white color, produced with ewe’s and goat’s raw milk. Beira Baixa PDO labeled cheeses have a ripening period of at least 45 days.
The goal of this study was to contribute to the microbiological contamination assessment of cured raw milk cheeses produced in Beira Baixa region, Portugal. This assessment could potentially contribute to improve the implemented safety systems and consequently the quality of the cheeses in the studied region.
Materials and methods
Sampling
In this study, a total of 98 cured raw milk cheeses from Beira Baixa region, Portugal, were analyzed, corresponding to 32 different brands produced by 9 producers (Supplementary Table 1). All samples came from different batches, and were purchased in several retail establishments, nearby Lisbon area, from October 2022 to June 2023. Samples were kept under refrigeration conditions (2 °C to 4 °C) from purchase until analysis, which occurred within a maximum of 24 h after purchase and within their assigned shelf-life period.
Microbiological analysis
ISO 7218: 2007 general requirements and guidance for microbiological examinations [17] were followed for all microbiological analysis.
The 98 cheese samples were analyzed for the presence and enumeration of some common foodborne pathogens such as Coagulase Positive Staphylococci (CPS), Listeria monocytogenes, Salmonella spp. and pathogenic Escherichia coli, as well as of indicator microorganisms (non-pathogenic E. coli and Listeria spp. other than L. monocytogenes). Staphylococcal enterotoxins (SE) detection was also assessed for those samples with a CPS concentration ≥ 4.9 × 104 cfu/g.
Salmonella spp. detection and E. coli and Coagulase Positive Staphylococci (CPS) detection and enumeration
Sample preparation was performed as followed: 25 g of each cheese sample was homogenized, at 230 rpm for 1 min using a stomacher (Stomacher, 400 Circulator, London, UK), in a sterile bag with 225 mL of Buffered peptone water (BPW-Oxoid, Basingstoke, Hampshire, UK), as described on ISO 6579–1: 2017 protocol [18].
For detection and enumeration of E. coli and CPS, the AFNOR validated TEMPO® EC (BIO12/13–02/05) and TEMPO® STA (BIO12/28–04/10) automated most probable number (MPN) system (bioMérieux, Marcyl l’Etoile, France) were used, respectively, according to the manufacturer’s instructions. For these analyses, 1 mL of decimal dilutions 10–1 and 10–3 of the primary mixture, in tryptone salt diluent (Biokar Diagnostics, Pantin, France), were used. The remaining mixture was incubated at 37 °C ± 1 °C for 18 h ± 2 h (non-selective pre-enrichment) and used for the detection of Salmonella spp. and for plating out of E. coli. For the detection of Salmonella spp., 0.1 mL of the incubated non-selective pre-enrichment was transferred into 10 mL of Salmonella Xpress 2 (SX2) broth (bioMérieux), incubated at 41.5 °C ± 1 ºC, for 24 h ± 2 h and VIDAS® Easy SLM (bioMérieux) AFNOR validated method (BIO-12/16–09/05) was used, according to the manufacturer’s instructions. For those samples positive for Salmonella spp., in VIDAS, a drop of the SX2 enrichment, was streaked on IRIS Salmonella agar (BIOKAR Diagnostics) and another one on xylose lysine deoxycholate (XLD, bioMérieux) agar and incubated at 37 °C ± 1 ºC, for 24 h ± 3 h.
For E. coli plating out, a loopful of the incubated non-selective pre-enrichment was streaked on the surface of Chromogenic Coliform Agar (CCA, Biokar Diagnostics) plates, and incubated at 37 °C during 24 h ± 2 h. Hemolytic activity of presumptive E. coli colonies was tested by sub-culture on Columbia Agar + 5% Sheep Blood (COS; bioMérieux) and incubation at 37 °C during 24 h ± 2 h. The presumptive E. coli and Salmonella spp. isolates were confirmed on VITEK®2 compact system (bioMérieux) and all positive isolates stored at -80 °C in broth with 20% glycerol.
Detection and enumeration of Listeria spp.
For L. monocytogenes detection, 25 g of each cheese sample was added to 225 ml of half Fraser broth (bioMérieux), as the primary enrichment culture, in a stomacher bag and were homogenized in a stomacher for 1 min and incubated for 24 ± 1 h at 30 °C. One hundred microliters of the primary enrichment culture, was then added to 10 mL of Fraser broth (bioMérieux), as a second enrichment culture, and incubated at 37 °C for 24 h ± 2 h [19]. After incubation, 0.5 mL of the second enrichment culture was tested using the AFNOR validated VIDAS® LMO2 automated method (BIO12/11–03/04), according to the manufacturer’s instructions.
L. monocytogenes enumeration was performed according to ISO/11290–2 horizontal method [20]. Briefly, 10 g of each VIDAS® LMO2 positive sample was added to 90 mL of BPW and homogenised (Initial Suspension). L. monocytogenes presumptive colonies were counted after the spread of 1 mL of the Initial Suspension on the surface of Microinstant® Listeria Agar (Ottaviani e Agosti) (Biokar Diagnostics) plates and incubation at 37 °C for 48 h ± 2 h. L. monocytogenes presumptive colonies (blue colored surrounded by an opaque halo) as well as Listeria spp., other than L. monocytogenes (blue colonies without an opaque halo) were isolated on Columbia Agar + 5% Sheep Blood (COS; bioMérieux) at 37 °C for 24 h ± 2 h, where hemolytic activity was assessed. Confirmation of the identification of the isolates was attained on VITEK®2 compact system (bioMérieux), following the manufacturer’s instructions. All positive isolates were stored in Tryptone Soy Broth (TSB; Biokar Diagnostics) with 20% glycerol, at -80 °C.
Detection of Staphylococcal Enterotoxins (SE)
All cheese samples presenting Coagulase Positive Staphylococci levels ≥ 4.9 × 104 cfu/g were tested for the presence of staphylococcal enterotoxins (SE), as described on ISO 19020:2017 [21]. In summary, the first step consisted in an extraction where toxin diffusion was attained by adding 25 g of each tested cheese sample to 40 mL of distilled water at 37 °C ± 1 °C and, after homogenization, the mixture was shaken for 30–60 min at room temperature in an VXR basic Vibrax orbital shaker (Ika®,Staufen, Germany). The pH was adjusted, the mixture centrifuged and then the resulting supernatant was concentrated by dialysis with a 6000–8000 Da molecular cut-off membrane (Spectrum Laboratories,Rancho Dominguez, CA, USA) against 30% (w/v) of polyethylene glycol 20,000 (Merck,Darmstadt, Germany), overnight, at 4 °C. Finally, immunoenzimatic detection was performed using the automated method VIDAS® Staph enterotoxin II (SET 2) (bioMérieux).
Microbiological results interpretation and statistical analysis
Microbiological results were interpreted according to the criteria showed on Table 1. The used criteria was based on the National Institute of Health Doutor Ricardo Jorge (INSA) guidelines for the interpretation of microbiological assays [22], the Commission Regulation (EC) Nº 2073/2005 [23], the Health Protection Agency- Guidelines for assessing the Microbiological Safety of Ready-to-eat Foods Placed on the market [24] and on the Luxembourg Microbiological criteria applicable to foodstuffs [25].
A cheese brand obtained a Satisfactory microbiological quality classification when all the samples of that brand were classified as Satisfactory for all the tested parameters. Borderline classification was attained when at least one of the tested samples was classified as Borderline, in at least one of the tested parameters, and none of them was classified as Unsatisfactory, in any of the tested parameters. Unsatisfactory/Potential Injurious to Health (U/PIH) classification was attributed when at least one sample was classified as Unsatisfactory/Potentially Injurious to Health, in at least one of the tested parameters.
Fisher- Freeman- Halton’s exact test of independence, with a 99.0% degree of confidence, was performed, using IBM SPSS Statistics 27.0.1 software, to determine if there was a significant relationship between the independent variables “microbiological safety classification” and the type of milk used in cheese production (“presence of cow’s milk”, “presence of ewe’s milk”, “presence of goat’s milk”) and between “microbiological safety classification” and registration of “Protected Designation of Origin (PDO)”.
Antimicrobial susceptibility testing of E. coli and Salmonella spp. isolates, Salmonella spp. serotyping and pathogenic E. coli identification
Since the use of antimicrobial agents in animal farming is considered as one of the most critical factors that contribute to the emergence and dissemination of antibiotic resistant bacteria, and because E. coli is considered a potential indicator of antimicrobial resistance (AMR), E. coli isolates (pathogenic and non-pathogenic) AMR was evaluated.
E. coli antimicrobial susceptibility testing (AST) was performed on 91 strains, isolated from 91 cheese samples, using a panel of 17 antimicrobials (Amoxicillin-Clavulanic Acid, Ampicillin, Azithromycin, Cefepime, Cefotaxime, Cefoxitin, Ceftazidime, Ceftriaxone, Chloramphenicol, Ciprofloxacin, Gentamicin, Meropenem, Nalidixic Acid, Sulfamethoxazole, Tetracycline, Tigecycline, Trimethoprim), following the Kirby-Bauer method and the European Committee on Antimicrobial Susceptibility Testing recommendations (EUCAST) [26].
For Salmonella spp. isolate, 19 antimicrobials were tested (Amikacin, Amoxicillin-Clavulanic Acid, Ampicillin, Azithromycin, Cefepime, Cefotaxime, Cefoxitin, Ceftazidime, Ceftriaxone, Chloramphenicol, Gentamicin, Kanamycin, Meropenem, Nalidixic Acid, Pefloxacin, Sulfamethoxazole, Tetracycline, Tigecycline, Trimethoprim), following the same method and recommendations [26].
An isolate was considered as multidrug resistant (MDR) when presenting resistance to three or more antimicrobial classes [27].
Salmonella spp. was serotyped by the slide agglutination method for O and H antigens (SSI, Copenhagen, Denmark), according to the Kauffmann-White-Le Minor scheme [28].
The pathogenicity of the E. coli isolates was assessed by the detection of intestinal pathotype- specific virulence genes (eae, aggR, aaiC, aatA, elt, esth, estp, ipaH, stx1 and stx2) as previously described [29].
The detection of at least one of the pathotype-specific genes in each isolate allowed the classification of potentially pathogenic (STEC, Shiga Toxin-producing E. coli; EAEC, Enteroaggregative E. coli; EPEC, Enteropathogenic E. coli; ETEC, Enterotoxigenic E. coli; EIEC, Enteroinvasive E. coli). Furthermore, E. coli pathogenicity was also inferred by Whole Genome Sequencing (WGS), since some of the likely non-pathogenic E. coli isolates were MDR and/or hemolytic, and were classified as Extraintestinal pathogenic E. coli (ExPEC). In these cases, the presence of two or more typical virulence genes allowed the ExPEC classification [30].
Listeria spp., Salmonella spp. and E. coli whole-genome sequencing, in Silico typing and screening of E. coli virulence/AMR genes
Extraction of genomic DNA from all MDR and hemolytic E. coli as well as from all Listeria innocua, L. monocytogenes and Salmonella spp. isolates was achieved using the ISOLATE II Genomic DNA Kit (Bioline, London, England, UK). Extracted DNA was quantified, with the dsDNA HS Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), in the Qubit fluorometer (Invitrogen, Waltham, MA, USA), according to the manufacturer’s instructions. NexteraXT library preparation protocol (Illumina, San Diego, CA, USA) was used for DNA preparation for sequencing. Cluster generation and sequencing (2 × 150 bp) were performed on either a MiSeq, a NextSeq 550 or NextSeq 2000 instrument (Illumina).
Regarding Listeria spp., we performed read quality control, trimming and de novo genome assembly with the INNUca pipeline v4.2.2 (https://github.com/B-UMMI/INNUca) [31], using default parameters. In brief, FastQC v0.11.5 (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) and Trimmomatic v0.38 [32] were used for reads quality control and improvement, and de novo assembly was perfomed with SPAdes v3.14 [33]. Bowtie2 v2.2.9 [34] and Pilon v1.23 [35] were applied for final assembly curation. Kraken2 v2.0.7 [36] was used for the screening of species confirmation/contamination and mlst v2.18.1 (https://github.com/tseemann/mlst) for Sequence Type (ST) determination.
FastQ files of each E. coli and Salmonella spp. isolates were uploaded on Enterobase QAssembly pipeline, v3.61 (https://enterobase.warwick.ac.uk/species/ecoli/upload_reads and https://enterobase.warwick.ac.uk/species/senterica/upload_reads, respectively) where trimming was achieved with Sickle v1.33, de novo genome assembly with SPADES v3.9.0, assembly polish with BWA 0.7.12-r1039 and species confirmation with Kraken.
E. coli and Salmonella spp. assemblies were uploaded on the Center for Genomic Epidemiology web services (http://www.genomicepidemiology.org/services/) to determine the presence of E. coli virulence genes (VirulenceFinder 2.0), Salmonella spp. in silico serotyping (SeqSero 1.2), E. coli in silico serotyping (SerotypeFinder 2.0), antimicrobial resistance genes (ResFinder 4.1), and in silico Multilocus Sequence Typing (MLST) (MLST 2.0).
Sequencing reads were deposited on the European Nucleotide Archive (ENA) under the bioprojects PRJEB31216 (Listeria spp.), PRJEB54735 (E. coli) and PRJEB32515 (Salmonella spp.), as well as on EFSA WGS portal. Supplementary Table 1 presents the accession numbers for each isolate.
Core-Genome clustering analysis of Listeria spp.
For L. monocytogenes, allele-calling was performed over the INNUca polished genome assemblies with chewBBACA v2.8.5 [37] using the core-genome Multi Locus Sequence Typing (cgMLST) 1,748-loci Pasteur schema [38] available at Chewie-NS website (https://chewbbaca.online/, downloaded on June 23rd, 2022) [39]. The cgMLST clustering analysis was performed with ReporTree v.2.0.3 (https://github.com/insapathogenomics/ReporTree) [40] using GrapeTree (MSTreeV2 method) [41], with clusters of closely related isolates being determined and characterized at a distance thresholds of 1, 4, 7 and 15 allelic differences (ADs). A threshold of seven ADs can provide a proxy to the identification of genetic clusters with potential epidemiological concordance (i.e., “outbreaks”) [42].
For L. innocua, in the absence of a cgMLST schema, a core-genome alignment of the INNUca polished assemblies was constructed with Parsnp v.1.7.4 implemented on Harvest suite [43], using the default parameters, with exception of parameter –C, which was adjusted to 2000 in order to maximize the resolution. The core-genome SNP-based clustering analysis was performed with ReporTree v.2.0.3 (https://github.com/insapathogenomics/ReporTree) [40] using GrapeTree (MSTreeV2 method) [41], with clusters of closely related isolates being determined and characterized at a SNP thresholds of 1, 4, 7 and 15 SNPs. This core-genome SNP-based clustering analysis relied on a core-genome alignment (comprising 95% of the L. innocua genome size) involving a total 33,081 variant sites. Interactive phylogenetic tree visualization was conducted with GrapeTree [41].
Results
Microbiological safety of the samples
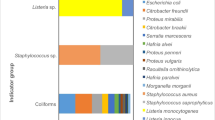
From a total of 98 tested cheeses, 16 (16.3%) were classified as Satisfactory, 59 (60.2%) as Borderline and 23 (25.5%) as Unsatisfactory/ Potential Injurious to Health (Fig. 1, Supplementary Table 1). L. monocytogenes was detected in 4/98 (4.1%) of the samples, three of which in a level > 100 cfu/g. L. innocua was also detected in 4/98 (4.1%) of the samples. In 16 samples, E. coli was present in levels > 104 cfu/g (16.3%) and in 3/98 (3.1%) Extraintestinal pathogenic E. coli (ExPEC) was identified. Salmonella spp. was detected in one sample (1.0%) and CPS > 104 cfu/g was detected in 6/98 (6.1%) of the samples (Fig. 1, Supplementary Table 1). Staphylococcal enterotoxins (SE) were not detected in any of the analyzed samples.
Microbiological results of the 98 tested cheeses, by producer (N = 9; 1–9) and brand (N = 32; 1A-9A). In the three first columns of the heatmap- like figure, the code Blue for Yes and Grey for No was used to inform about the existence of a Protected Designation of Origin (PDO) for each sample, the isolation of an E. coli with antimicrobial resistance (AMR) to at least one of the tested antimicrobials, and the detection of an hemolytic (Hemo) E. coli. The Green for Satisfactory; Yellow for Borderline and Red for Unsatisfactory/ Potential Injurious to Health code was used to characterize the Microbiological Safety classification of each of the 98 samples regarding the tested parameters (E. coli, Coagulase Positive Staphylococci (CPS), pathogenic (pathog) E. coli; Salmonella spp. (Salm) and Listeria spp. (List). In the last two columns of the heatmap, the global Microbiological Safety classification of each sample and brand are presented
The classification Unsatisfactory/Potential Injurious to Health (U/PIH) on 13 samples, was exclusively due to the enumeration of E. coli > 104 cfu/g (13/23; 56.5%). The other 10 samples were classified as U/PIH due to the presence of CPS > 104 cfu/g (2/23; 8.7%); L. monocytogenes > 102 cfu/g (1/23; 4.4%); ExPEC (2/23; 8.7%); E. coli and CPS > 104 cfu/g and L. monocytogenes > 102 cfu/g (2/23; 8.7%); CPS > 104 cfu/g and ExPEC (1/23; 4.4%); E. coli and CPS > 104 cfu/g (1/23; 4.4%); and finally, one cheese (1/23; 4.4%) was classified as U/PIH because Salmonella spp. was detected in 25 g (Fig. 1, Supplementary Table 1).
When focusing on brands’ microbiological safety, from the 32 analyzed brands, 15 (15/32; 46.9%) were categorized as U/PIH, 16 (16/32; 50.0%) as Borderline and only one (1/32; 3.1%) as Satisfactory (Fig. 1). With the exception of producers 3 and 9, for which only one brand was analyzed (classified as Borderline), all the other producers presented always some brands classified as U/PIH (Fig. 1).
When looking at the obtained results considering the type of milk used in cheese production, it was possible to find a statistical significant association between microbiological safety classification and the presence of cow’s milk (Fisher-Freeman-Halton = 11.785, p = 0.001) (Table 2). There was a clear decrease in the number of Satisfactory samples and an increase of Unsatisfactory/Potentially Injurious to Health samples, when cheese samples contained cow’s milk (Table 2). This association occurred also for microbiological safety classification regarding Coagulase Positive Staphylococci and E. coli enumeration and the presence of cow’s milk in the cheese samples (Table 2). There was no statistical significant association between microbiological safety classification and the presence of ewe’s or goat’s milk, nor statistical significant association between microbiological safety classification and registration of Protected Designation of Origin (Table 2). There was also no statistical significant association between microbiological safety classification of the cheese samples and the number of types of milk used (Fisher-Freeman-Halton = 10.250, p = 0.025) (data not shown).
Genotypic and phenotypic characterization of E. coli, Salmonella spp. and Listeria spp. isolates
E. coli isolates were recovered from 91 out of the 98 samples. The remaining seven samples either did not contain E. coli or it was not possible to isolate E. coli, due to high levels of contamination with other microorganisms.
Antimicrobial resistant (AMR) E. coli was detected in 23/98 (23.5%) of the cheese samples, of which two (2/23; 8.7%) were multidrug resistant (MDR) (Table 3).
None of the 91 E. coli isolates presented any of the surveyed intestinal pathotype-specific virulence genes (eae, aggR, aaiC, aatA, elt, esth, estp, ipaH, stx1 and stx2). WGS of the MDR (two) and hemolytic (one, that was antimicrobial susceptible) isolates, confirmed that they are all ExPEC.
The ExPEC hemolytic isolate belongs to ST14755 and to serotype O175:H16 (totally susceptible), while the two MDR ExPEC isolates belong to ST362; O4:H6 (resistant to ampicillin, tetracycline and sulfamethoxazole) and to ST155; OND:H11 (resistant to ampicillin, tetracycline, chloramphenicol and sulfamethoxazole).
Salmonella spp. strain, isolated from one of the cheeses, belongs to serotype Duisburg, and ST4046. Although containing one gene and one point mutation associated with antimicrobial resistance (aac(6')-Iaa; parC:p.T57S), this isolate was phenotypically susceptible to all the tested antimicrobials.
The four Listeria monocytogenes isolates belong to the following STs: ST1 (isolated from one of the 6B samples), ST5 (isolated from two samples of brand 1B) and ST7 (isolated from one of the 9A samples). The Listeria innocua isolates belong to ST492 (two of the 2G and one of the 2F samples) and ST603 (isolated from one of the 1C samples).
Core-genome clustering analysis of Listeria spp. isolates
Despite the low number of isolates (four per species), core-genome clustering analysis of L. monocytogenes and L. innocua isolates was performed in order to assess the genetic relatedness among them and its potential correlation with cheese producer/brands (Fig. 2).
Core-genome clustering analysis of Listeria spp. isolates. (A) For L. monocytogenes (four isolates), the Minimum Spanning Tree (MST) was constructed based on the cgMLST 1748-loci Pasteur schema [38]. Each circle (node) contains the brand code and represents a unique allelic profile, with numbers on the connecting lines representing allelic distances (AD) between nodes. Straight and dotted lines reflect nodes linked with ADs below and above a threshold of seven ADs, which can provide a proxy to the identification of genetic clusters with potential epidemiological concordance [42]. (B) For L. innocua (four isolates), the MST was constructed based on a core-genome SNP-based alignment (comprising 95% of the L. innocua genome size) involving a total 33,081 variant sites. Each circle (node) contains the brand code designation and represents a unique SNP profile, with numbers on the connecting lines representing SNP distances between nodes. Straight and dotted lines reflect nodes linked with a SNP distance below and above a threshold of 15 SNPs. For both panels, data visualization was adapted from GrapeTree dashboard [41], with the node colors reflecting the producer and the surrounding shadows indicating the traditional seven-loci MLST classification
L. monocytogenes cgMLST revealed one cluster of closely related isolates (allelic distance = 4) comprising two ST5 isolates from brand 1B (producer 1). Similarly, L. innocua core-genome SNP-based alignment showed one cluster of ST492 isolates interconnected by ≤ 9 SNPs (enrolling 3 isolates, 2 from brand 2G and one from brand 2F), all also linked to the same producer (producer 2) (Fig. 2).
Discussion
Cheeses may have physicochemical characteristics and shelf life favorable to the growth or survival of pathogenic bacteria [44, 45]. For instance, a pooled prevalence of Listeria monocytogenes (3.6%- sheep; 12.8%- goat), Salmonella spp. (5.9%- goat), Staphylococcus aureus (16.0%- goat) and STEC (2.8%- sheep; 4.3%- goat) in sheep and goat milk cheeses has been reported in a recent meta-analysis approach [46], which clearly demonstrates that these microorganisms are frequently detected in this type of foodstuffs. In particular, the production of cheeses free from L. monocytogenes may be a challenge for producers, since this pathogen is ubiquitous in nature, can grow at refrigeration temperatures, and forms biofilms, being difficult to eradicate from food contact surfaces in the production environment. In fact, many outbreaks associated with the consumption of cheeses have been caused by L. monocytogenes [47,48,49,50,51].
In this study, we have detected L. monocytogenes in four samples (4/98 = 4.1%) (3 with concentrations > 102 cfu/g), and further found the presence of the indicator microorganism L. innocua in another four (4/98 = 4.1%). These results emphasize the need for monitoring good hygiene and manufacturing practices, as well as raw milk microbiological quality, in the context of Regulation (EC) N°852/2004 on the hygiene of foodstuffs [52], in the sector of Portuguese artisanal cheese industry.
The three identified Listeria monocytogenes sequence types (ST1, ST5, ST7) were already isolated, by others, in foodstuffs and associated to human disease [38, 53, 54]. All the Listeria monocytogenes detected STs were also already associated to Dairy Processing Facilities [55,56,57], with ST5 being described as a particularly persistent ST that might harbor specific efflux pump systems and heavy metal resistance genes that may possibly provide a higher tolerance to disinfectants [55].
The cgMLST analysis of L. monocytogenes isolates and SNP-analysis of L. innocua isolates suggest that, in this study, cheese contamination is potentially related with non-compliance with Good Hygiene Practices at producer level, since the two observed clusters (one for L. monocytogenes and one for L. innocua) are producer specific.
S. aureus is a common cause of bovine mastitis and is frequently detected in raw milk [58, 59]. In fact, in this study, we have found a statistically significant association between the microbiological safety classification of the samples regarding CPS enumeration and the use of cow’s milk in cheese production (Fisher-Freeman-Halton = 10.624, p = 0.004) (Table 2). Furthermore, enterotoxin-producing S. aureus exhibits a high osmotolerance, growing or surviving in cheeses with aw levels as low as 0.86 (equivalent to about 20% NaCl), provided that all other conditions are optimal [60]. Thus, this bacterium may be a safety problem in raw milk cheeses, being already described as implicated in several outbreaks linked with milk and milk products [61]. In fact, in the present study, the detection of coagulase-positive Staphylococci > 104 cfu/g in 6.1% (6/98) of the samples highlights for the importance of implementation of more efficient hygiene control measures during the process of cheese production and adequate selection of the raw materials. High counts of Staphylococci are also the main nonconformities found in Brazilian artisanal cheeses [62].
Salmonella spp. and pathogenic E. coli (in particular STEC) are also pathogens/hazards of concern when talking about cheese, being already detected in several outbreaks linked to the consumption of this category of foodstuffs [63,64,65,66,67,68,69].
In this research, Salmonella spp. was detected in one sample (1/98 = 1.0%). Serotype Duisburg, identified in the Salmonella spp. isolate, was recently associated to a foodborne outbreak that occurred in the United States of America in 2021, involving the consumption of cashew brie, a vegan alternative to Brie cheese [70].
Although no STEC isolates were found in the analyzed cheeses, we have detected the presence of Extraintestinal pathogenic E. coli (ExPEC) in three samples (3/98 = 3.1%), in accordance with our previous study performed on Alentejo raw milk cheeses [71]. ExPEC is recognized as the most common gram-negative pathogen in humans, causes most urinary tract infections (UTIs) in young healthy women, is the leading cause of bacteremia in adults, and is the second most common cause of neonatal meningitis [72]. This pathotype was also already detected in raw milk cheeses from various Latin America countries [73,74,75], and may potentially be transmitted to humans via food [76]. We have further found the presence of non-pathogenic E. coli > 104 cfu/g in 16.3% of the samples (16/98), highlighting for potential non-conformities during processing along cheese production chain. ExPEC isolates were characterized as belonging to STs 14755, 155, and 362. E. coli ST155 and ST362 are highly widespread in nature, and have been isolated from wildlife, livestock, water, foodstuffs and humans, among other sources (Enterobase, https://enterobase.warwick.ac.uk/species/index/ecoli). E. coli ST155 has been described as a potential foodborne pathogen [77, 78]. E. coli ST 362 is known as a biofilm-producer [79] and was the dominant ST found in the calf ESBL-producing E. coli isolates from a dairy farm in Germany [80]. The ST14755, a novel sequence-type, was detected in a susceptible ExPEC hemolytic isolate, and belongs to serotype O175:H16.
Drug resistant microorganisms have become a threat to public health, with acute and chronic infections increasingly failing to respond to antimicrobial drugs. It is estimated that 700,000 people die because of resistant infections every year, and that by the year of 2050, 10 million deaths/ year can occur. This will result in a cumulative global economic loss of USD 100 trillion, due to the antimicrobial resistance effect [81]. It is well known that the unrestrained use of antibiotics to control disease in farm animals and in humans increases the number and frequency of antibiotic resistant bacterial isolates [82, 83]. In fact, in this study, 23.5% of the tested cheese samples presented E. coli isolates resistant to antimicrobials, with two of these strains being MDR. Antimicrobial resistance gene content of unprocessed animal products may potentially play a role in the acquisition of antimicrobial resistance of human pathogens [84].
The link of the above-mentioned pathogens with foodborne outbreaks related to cheese consumption, as well as the association between enhanced virulence and bacteria stress responses to cheese manufacturing, cheese matrix itself and storage conditions [85], highlights for the importance of the development of new instruments to control the presence of these bacteria during the process and, consequently, in the ready-to-eat end-product. Microbiological safety assessment of raw milk cheeses placed on the market is one of these instruments and may be essential to identify critical points along cheese production and to implement timely corrective measures, that may allow artisan cheesemakers to continue to produce their appreciated artisanal cheeses but with improved safety standards.
The results of this study clearly demonstrate that raw milk cheeses from Beira Baixa region, Portugal, may contain pathogenic and/or indicator microorganisms above the stipulated guideline limits, being some resistant to antimicrobials. It is essential not only to monitor the hygiene at the primary production (farm level), but also to implement corrective measures during cheese processing, to ensure control at the established critical points. In this study, we have found a statistical significant association between the microbiological safety classification of the samples and the use of cow’s milk in cheese production (Fisher-Freeman-Halton = 11.785, p = 0.001) (Table 2). This association also occurs when considering the microbiological safety classification of the samples regarding CPS and E. coli enumeration (Table 2), the two parameters that mostly contributed for the U/PIH classification of the samples. This result may point out for a potential safety critical point in Beira Baixa cheese production related with the hygiene at the cow’s milk production farms, and is a good example of how the microbiological safety assessment of the final product may be important to identify potential critical points along production.
The implementation and maintenance of training for the application of good manufacturing practices, and the constant monitoring of the quality of the production process in the Portuguese artisanal cheese industry, is of critical importance. To highlight also the importance of controlling the quality of the milk and to test environmental surfaces like food contact surfaces (e. g. cheese-making equipment, utensils, shelves for maturation/ripening) or non-food contact surfaces for preventing cross contamination with microbiological hazard thus improving the quality and safety of the final product.
Data availability
All supporting data and protocols have been provided within the article or through supplementary data files.
References
Casalta E, Sorba JM, Aigle M, Ogier JC (2009) Diversity and dynamics of the microbial community during the manufacture of Calenzana, an artisanal Corsican cheese. Int J Food Microb 133:243–251. https://doi.org/10.1016/j.ijfoodmicro.2009.05.022
Chambers D, Esteve E, Retiveau AR (2010) Effect of milk pasteurization on flavor properties of seven commercially available French cheese types. J Sens Stud 25:494–511. https://doi.org/10.1111/j.1745-459X.2010.00282.x
Masoud W, Vogensen FK, Lillevang S, Abu Al-Soud W, Sørensen SJ, Jakobsen M (2012) The fate of indigenous microbiota, starter cultures, Escherichia coli, Listeria innocua and Staphylococcus aureus in Danish raw milk and cheeses determined by pyrosequencing and quantitative real time (qRT)- PCR. Int J Food Microbiol 153(1–2):192–202. https://doi.org/10.1016/j.ijfoodmicro.2011.11.014
Debarry J, Garn H, Hanuszkiewicz A, Dickgreber N, Blumer N, von Mutius E, Bufe A, Gatermann S, Renz H, Holst O, Heine H (2007) Acinetobacter lwoffii and Lactococcus lactis strains isolated from farm cowsheds possess strong allergy-protective properties. J Allergy Clin Immunol 119:1514–1521. https://doi.org/10.1016/j.jaci.2007.03.023
Ege MJ, Mayer M, Schwaiger K, Mattes J, Pershagen G, van Hage M, Scheynius A, Bauer J, von Mutius E (2012) Environmental bacteria and childhood asthma. Allergy 67(12):1565–1571. https://doi.org/10.1111/all.12028
Crippa G, Zabzuni D, Bravi E, Piva G, De Noni I, Bighi E, Rossi F (2018) Randomized, Double Blind Placebo-Controlled Pilot Study of the Antihypertensive Effects of Grana Padano D.O.P. Cheese Consumption in Mild—Moderate Hypertensive Subjects. Eur Rev Med Pharmacol Sci 22:7573–7581. https://doi.org/10.26355/eurrev_201811_16299
Oliver SP, Jayarao BM, Almeida RA (2005) Foodborne pathogens in milk and the dairy farm environment: food safety and public health implications. Foodborne Pathog Dis 2(2):115–129. https://doi.org/10.1089/fpd.2005.2.115
Langer AJ, Ayers T, Grass J, Lynch M, Angulo FJ, Mahon BE (2012) Nonpasteurized dairy products, disease outbreaks, and state laws—United States, 1993–2006. Emerg Infect Dis 18(3):385–391. https://doi.org/10.3201/eid1803.111370
Costard S, Espejo L, Groenendaal H, Zagmutt FJ (2017) Outbreak-related disease burden associated with consumption of unpasteurized cow’s milk and cheese, United States, 2009–2014. Emerg Infect Dis 23:957–964. https://doi.org/10.3201/eid2306.151603
Possas A, Bonilla-Luque OM, Valero A (2021) From Cheese-Making to Consumption: Exploring the Microbial Safety of Cheeses through Predictive Microbiology Models. Foods 10(2):355. https://doi.org/10.3390/foods10020355
André MCDPB, Campos MRH, Borges LJ, Kipnis A, Pimenta FC, Serafini ÁB (2008) Comparison of Staphylococcus aureus isolates from food handlers, raw bovine milk and Minas Frescal cheese by antibiogram and pulsed-field gel electrophoresis following SmaI digestion. Food Control 19:200–207. https://doi.org/10.1016/j.foodcont.2007.03.010
Schön K, Schornsteiner E, Dzieciol M, Wagner M, Müller M, Schmitz-Esser S (2016) Microbial communities in dairy processing environment floor-drains are dominated by product-associated bacteria and yeasts. Food Control 70:210–215. https://doi.org/10.1016/j.foodcont.2016.05.057
Kousta M, Mataragas M, Skandamis P, Drosinos EH (2010) Prevalence and sources of cheese contamination with pathogens at farm and processing levels. Food Control 21(6):805–815. https://doi.org/10.1016/j.foodcont.2009.11.015
Official European Union website, EFSA Foodborne outbreaks – dashboard. https://www.efsa.europa.eu/en/microstrategy/FBO-dashboard. Accessed 27 March 20234
Dias J (2022) The use of cheese from Alentejo in Portuguese gastronomy: A travel through history. Int J Gastron Food Sci 29:100579. https://doi.org/10.1016/j.ijgfs.2022.100579
European Commission. Agriculture and rural development. Geographical indications and quality schemes explained. https://agriculture.ec.europa.eu/farming/geographical-indications-and-quality-schemes/geographical-indications-and-quality-schemes-explained_en. Accessed 27 March 2024
ISO 7218:2007, Microbiology of food and animal feeding stuffs — General requirements and guidance for microbiological examinations.
ISO 6579–1:2017 Microbiology of the food chain- Horizontal method for the detection, enumeration and serotyping of Salmonella
ISO 11290–1:2017, Microbiology of food chain — Horizontal methos for the detection and enumeration of Listeria monocytogenes and of Listeria spp. — Part 1: Detection method
ISO 11290–2:2017, Microbiology of food chain — Horizontal methos for the detection and enumeration of Listeria monocytogenes and of Listeria spp. — Part 2: Enumeration method
ISO 19020:2017, Microbiology of food chain — Horizontal methos for the immunoenzymatic detection of staphylococcal enterotoxins in foodstuffs
Instituto Nacional de Saúde Doutor Ricardo Jorge. Interpretação de resultados de ensaios microbiológicos em alimentos prontos para consumo e em superfícies do ambiente de preparação e distribuição alimentar: valores-guia. Lisboa: INSA IP, 2019. http://repositorio.insa.pt/bitstream/10400.18/5610/3/INSA_Interpreta%c3%a7%c3%a3o%20de%20resultados%20de%20ensaios%20microbiol%c3%b3gicos_Valores-guia_2019.pdf. Accessed 27 March 2024.
Commission Regulation (EC) Nº 2073/2005 of 15 November 2005 on microbiological criteria for foodstuffs. Official Journal of the European Union: L338/1-L338/26. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32005R2073. Accessed 27 March 2024.
HPA. (2009). Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods Placed on the Market. Health Protection Agency, London. https://assets.publishing.service.gov.uk/media/5a7efde0e5274a2e8ab497a4/Guidelines_for_assessing_the_microbiological_safety_of_ready-to-eat_foods_on_the_market.pdf . Accessed 27 March 2024.
Luxembourg Ministère de la Santé, Critères microbiologiques applicables aux denrées alimentaires- Lignes directrices pour l’interprétation. F-054 Rev05; 2018. https://securite-alimentaire.public.lu/dam-assets/fr/professionnel/Denrees-alimentaires/Qualite-microbiologique/recueil_criteres_microbiologiques/F-054-05.pdf .Accessed 27 March 2024.
EUCAST The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. http://www.eucast.org. Accessed 27 March 2024.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B et al (2011) Multidrugresistant, extensively drugresistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3):268–281. https://doi.org/10.1111/j.1469-0691.2011.03570.x
Grimont PAD, Weill FX (2007) Antigenic formulae of the Salmonella serovars. In: WHO collaborating centre for reference and research on Salmonella, 9th edn. WHO Collaborating Centre for reference and research on Salmonella: Institute Pasteur, France, pp. 1–166.
Pista A, Silveira L, Ribeiro S, Fontes M, Castro R, Coelho A, Furtado R, Lopes T, Maia C, Mixão V, et. al. (2022) Pathogenic Escherichia coli, Salmonella spp. and Campylobacter spp. in Two Natural Conservation Centers of Wildlife in Portugal: Genotypic and Phenotypic Characterization. Microorganisms 10(11):2132. doi: https://doi.org/10.3390/microorganisms10112132
Sora VM, Meroni G, Martino PA, Soggiu A, Bonizzi L, Zecconi A (2021) Extraintestinal Pathogenic Escherichia coli: Virulence Factors and Antibiotic Resistance. Pathogens 10(11):1355. https://doi.org/10.3390/pathogens10111355
Llarena A-K, Ribeiro-Gonçalves BF, Silva DN, Halkilahti J, Machado MP, Da Silva MS, Jaakkonen A, Isidro J, Hämäläinen C, Joenperä J et al (2018) INNUENDO: A Cross-sectoral Platform for the Integration of Genomics in the Surveillance of Food-borne Pathogens. EFSA Support Publ 15:1498E. https://doi.org/10.2903/sp.efsa.2018.EN-1498
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. https://doi.org/10.1093/bioinformatics/btu170
Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A (2020) Using SPAdes De Novo Assembler. Curr Protoc Bioinform 70(1):e102. https://doi.org/10.1002/cpbi.102
Langmead B (2010) Aligning Short Sequencing Reads with Bowtie. Curr Protoc Bioinform 32:11.7.1–11.7.14. https://doi.org/10.1002/0471250953.bi1107s32
Walker BJ, Abeel T, Shea T, Priest M, Abouelliel A, Sakthikumar S, Cuomo CA, Zeng Q, Wortman J, Young SK et al (2014) Pilon: An Integrated Tool for Comprehensive Microbial Variant Detection and Genome Assembly Improvement. PLoS ONE 9(11):e112963. https://doi.org/10.1371/journal.pone.0112963
Wood DE, Salzberg SL (2014) Kraken: Ultrafast Metagenomic Sequence Classification Using Exact Alignments. Genome Biol 15(3):R46. https://doi.org/10.1186/gb-2014-15-3-r46
Silva M, Machado MP, Silva DN, Rossi M, Moran-Gilad J, Santos S, Ramirez M, Carriço JA (2018) chewBBACA: A Complete Suite for Gene-by-Gene Schema Creation and Strain Identification. Microb Genom 4(3):e000166. https://doi.org/10.1099/mgen.0.000166
Moura A, Criscuolo A, Pouseele H, Maury MM, Leclercq A, Tarr C, Björkman JT, Dallman T, Reimer A, Enouf V et al (2016) Whole genome-based population biology and epidemiological surveillance of Listeria monocytogenes. Nat Microbiol 2:16185. https://doi.org/10.1038/nmicrobiol.2016.185
Mamede R, Vila-Cerqueira P, Silva M, Carriço JA, Ramirez M (2020) Chewie Nomenclature Server (chewie-NS): A Deployable Nomenclature Server for Easy Sharing of Core and Whole Genome MLST Schemas. Nucleic Acids Res 49:D660–D666. https://doi.org/10.1093/nar/gkaa889
Mixão V, Pinto M, Sobral D, Di Pasquale A, Gomes JP, Borges V (2023) ReporTree: a surveillance-oriented tool to strengthen the linkage between pathogen genetic clusters and epidemiological data. Genome Med 15(1):43. https://doi.org/10.1186/s13073-023-01196-1
Zhou Z, Alikhan N-F, Sergeant MJ, Luhmann N, Vaz C, Francisco AP, Carriço JA, Achtman M (2018) GrapeTree: Visualization of Core Genomic Relationships among 100,000 Bacterial Pathogens. Genome Res 28(9):1395–1404. https://doi.org/10.1101/gr.232397.117
VanWalle I, Björkman JT, Cormican M, Dallman T, Mossong J, Moura A, Pietzka A, Ruppitsch W, Takkinen J (2018) European Listeria WGS typing group. Retrospective validation of whole genome sequencing-enhanced surveillance of listeriosis in Europe, 2010 to 2015. Eurosurveillance 23(33):1700798. https://doi.org/10.2807/1560-7917.ES.2018.23.33.1700798
Treangen TJ, Ondov BD, Koren S, Phillippy AM (2014) The Harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol 15(11):524. https://doi.org/10.1186/s13059-014-0524-x
Kim K, Lee H, Gwak E, Yon Y (2014) Kinetic behavior of Escherichia coli on various cheeses under constant and dynamic temperature. Asian-Australas J Anim Sci 27(7):1013–1018. https://doi.org/10.5713/ajas.2013.13579
Lahou E, Uyttendaele M (2017) Growth potential of Listeria monocytogenes in soft, semi-soft and semi-hard artisanal cheeses after post-processing contamination in deli retail establishments. Food Control 76:13–23. https://doi.org/10.1016/j.foodcont.2016.12.033
Gonzales-Barro U, Gonçalves-Tenório A, Rodrigues A, Cadavez V (2017) Foodborne pathogens in raw milk and cheese of sheep and goat origin: a meta-analysis approach. Curr Opin Food Sci 18:7–13. https://doi.org/10.1016/j.cofs.2017.10.002
Magalhães R, Almeida G, Ferreira V, Santos I, Silva J, Mendes MM, Pita J, Mariano G, Mâncio I, Sousa MM et al (2015) Cheese-related listeriosis outbreak, Portugal, March 2009 to February 2012. Eurosurveill J 20(17):21104. https://doi.org/10.2807/1560-7917.es2015.20.17.21104
de Castro V, Escudero JM, Rodriguez JL, Muniozguren N, Uribarri J, Saez D, Vazquez J (2012) Listeriosis outbreak caused by Latin-style fresh cheese, Bizkaia, Spain, August 2012. Eurosurveillance 17(42):20298
Amato E, Filipello V, Gori M, Lomonaco S, Losio MN, Parisi A, Huedo P, Knabel SJ, Pontello M (2017) Identification of a major Listeria monocytogenes outbreak clone linked to soft cheese in Northern Italy—2009–2011. BMC Infect Dis 17:1–7. https://doi.org/10.1186/s12879-017-2441-6
Fretz R, Pichler J, Sagel U, Much P, Ruppitsch W, Pietzka AT, Stöger A, Huhulescu S, Heuberger S, Appl G et al (2010) Update: Multinational listeriosis outbreak due to “quargel”, a sour milk curd cheese, caused by two different L. monocytogenes serotype 1/2a strains, 2009–2010. Eurosurveillance 15(16):19543
Koch J, Dworak R, Prager R, Becker B, Brockmann S, Wicke A, Wichmann- Schauer H, Hof H, Werber D, Stark K (2010) Large listeriosis outbreak linked to cheese made from pasteurized milk, Germany, 2006–2007. Foodborne Pathog Dis 7(12):1581–1584. https://doi.org/10.1089/fpd.2010.0631
The European Parliament and the Council of the European Union. Regulation (EC) No 852/2004 of the European Parliament and of the Council of 29 April 2004 on the hygiene of foodstuffs (2004). Official Journal of the European Communities L139/1
Painset A, Björkman JT, Kiil K, Guillier L, Mariet JF, Félix B, Amar C, Rotariu O, Roussel S, Perez-Reche F et al (2019) LiSEQ—Whole-genome sequencing of a cross-sectional survey of Listeria monocytogenes in ready-to-eat foods and human clinical cases in Europe. Microb Genom 5(2):e000257. https://doi.org/10.1099/mgen.0.000257
Vallejo P, Cilla G, López-Olaizola M, Vicente D, Marimón JM (2022) Epidemiology and Clinical Features of Listeriosis in Gipuzkoa, Spain, 2010–2020. Front Microbiol 13:894334. https://doi.org/10.3389/fmicb.2022.894334
Muhterem-Uyar M, Ciolacu L, Wagner KH, Wagner M, Schmitz-Esser S, Stessl B (2018) New Aspects on Listeria monocytogenes ST5-ECVI Predominance in a Heavily Contaminated Cheese Processing Environment. Front Microbiol 9:64. https://doi.org/10.3389/fmicb.2018.00064
Andritsos ND, Mataragas M (2023) Characterization and Antibiotic Resistance of Listeria monocytogenes Strains Isolated from Greek Myzithra Soft Whey Cheese and Related Food Processing Surfaces over Two-and-a-Half Years of Safety Monitoring in a Cheese Processing Facility. Foods 12(6):1200. https://doi.org/10.3390/foods12061200
Kaszoni-Rückerl I, Mustedanagic A, Muri-Klinger S, Brugger K, Wagner KH, Wagner M, Stessl B (2020) Predominance of Distinct Listeria innocua and Listeria monocytogenes in Recurrent Contamination Events at Dairy Processing Facilities. Microorganisms 8(2):234. https://doi.org/10.3390/microorganisms8020234
Castro RD, Pedroso SHSP, Sandes SHC, Silva GO, Luiz KCM, Dias RS, Filho RAT, Figueiredo HCP, Santos SG, Nunes AC et al (2020) Virulence factors and antimicrobial resistance of Staphylococcus aureus isolated from the production process of Minas artisanal cheese from the region of Campo das Vertentes, Brazil. J Dairy Sci 103:2098–2110. https://doi.org/10.3168/jds.2019-17138
Fusco V, Chieffi D, Fanelli F, Logrieco AF, Cho GS, Kabisch J, Böhnlein C, Franz CMAP (2020) Microbial quality and safety of milk and milk products in the 21st century. Compr Rev Food Sci Food Saf 19:2013–2049. https://doi.org/10.1111/1541-4337.12568
Qi Y, Miller KJ (2000) Effect of low water activity on staphylococcal enterotoxin A and B biosynthesis. J Food Prot 63(4):473–478. https://doi.org/10.4315/0362-028x-63.4.473
De Buyser ML, Dufour B, Maire M, Lafarge V (2001) Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int J Food Microbiol 67(1–2):1–17. https://doi.org/10.1016/s0168-1605(01)00443-3
Pineda APA, Campos GZ, Pimentel-Filho NJ, Franco BDGM, Pinto UM (2021) Brazilian Artisanal Cheeses: Diversity, Microbiological Safety, and Challenges for the Sector. Front Microbiol 12:666922. https://doi.org/10.3389/fmicb.2021.666922
Robinson E, Travanut M, Fabre L, Larréché S, Ramelli L, Pascal L, Guinard A, Vincent N, Calba C, Meurice L et al (2020) Outbreak of Salmonella Newport associated with internationally distributed raw goats’ milk cheese, France, 2018. Epidemiol Infect 148:e180. https://doi.org/10.1017/S0950268820000904
Plumb ID, Schwensohn CA, Gieraltowski L, Tecle S, Schneider ZD, Freiman J, Cote A, Noveroske D, Kolsin J, Brandenburg J et. al. (2019) Outbreak of Salmonella Newport Infections with Decreased Susceptibility to Azithromycin Linked to Beef Obtained in the United States and Soft Cheese Obtained in Mexico—United States, 2018–2019. Morb Mortal Wkly Rep 68(33):713–717. https://doi.org/10.15585/mmwr.mm6833a1
Vignaud ML, Cherchame E, Marault M, Chaing E, Le Hello S, Michel V, Jourdan-Da Silva N, Lailler R, Brisabois A, Cadel-Six S (2017) MLVA for Salmonella enterica subsp. enterica serovar Dublin: Development of a method suitable for inter-laboratory surveillance and application in the context of a raw milk cheese outbreak in France in 2012. Front Microbiol 8:295. https://doi.org/10.3389/fmicb.2017.00295.
Ung A, Baidjoe AY, Van Cauteren D, Fawal N, Fabre L, Guerrisi C, Danis K, Morand A, Donguy MP, Lucas E et al (2019) Disentangling a complex nationwide Salmonella Dublin outbreak associated with raw-milk cheese consumption, France, 2015 to 2016. Eurosurveillance 24(3):1700703. https://doi.org/10.2807/1560-7917.ES.2019.24.3.1700703
Jones G, Lefèvre S, Donguy MP, Nisavanh A, Terpant G, Fougère E, Vaissière E, Guinard A, Mailles A, de Valk H et al (2019) Soutbreak of shiga toxin-producing Escherichia coli (STEC) O26 paediatric haemolytic uraemic syndrome (HUS) cases associated with the consumption of soft raw cow’s milk cheeses, France, march to May 2019. Eurosurveillance 24(22):1900305. https://doi.org/10.2807/1560-7917.ES.2019.24.22.1900305
Currie A, Galanis E, Chacon PA, Murray R, Wilcott L, Kirkby P, Honish L, Franklin K, Farber J, Parker R et al (2013) (2018) Outbreak of Escherichia coli O157:H7 Infections Linked to Aged Raw Milk Gouda Cheese, Canada. J Food Prot 81(2):325–331. https://doi.org/10.4315/0362-028X.JFP-17-283
McCollum JT, Williams NJ, Beam SW, Cosgrove S, Ettestad PJ, Ghosh TS, Kimura AC, Nguyen L, Stroika SG, Vogt RL et al (2012) Multistate outbreak of Escherichia coli O157:H7 infections associated with in-store sampling of an aged raw-milk Gouda cheese, 2010. J Food Prot 75(10):1759–1765. https://doi.org/10.4315/0362-028X.JFP-12-136
Lewis K, Vasser M, Garman K, Higa J, Needham M, Irving D J, Cavallo S, Marks DS, Kirchner M, Madad A et. al. (2023) Notes from the Field: Multistate, Multiserotype Outbreak of Salmonella Infections Linked to Cashew Brie - United States, 2021. MMWR. Morb Mortal Wkly Rep 72(21):589–590. https://doi.org/10.15585/mmwr.mm7221a4
Praça J, Furtado R, Coelho A, Correia CB, Borges V, Gomes JP, Pista A, Batista R (2023) Listeria monocytogenes, Escherichia coli and Coagulase Positive Staphylococci in Cured Raw Milk Cheese from Alentejo Region. Portugal Microorganisms 11(2):322. https://doi.org/10.3390/microorganisms11020322
Poolman JT, Wacker M (2016) Extraintestinal pathogenic Escherichia coli, a common human pathogen: challenges for vaccine development and progress in the field. J Infect Dis 213(1):6–13. https://doi.org/10.1093/infdis/jiv429
Campos de ACLP, Puño-Sarmineto JJ, Medeiros LP, Gazal LES, Maluta RP, Navarro A, Kobayashi RKT, Fagan EP, Nakazato G (2018) Virulence genes and antimicrobial resistance in Escherichia coli from cheese made from unpasteurized milk in Brazil. Foodborne Pathog Dis 15:94–100. https://doi.org/10.1089/fpd.2017.2345
Guzman-Hernandez R, Contreras-Rodriguez A, Hernandez-Velez R, Perez-Martinez I, Lopez-Merino A, Zaidi MB, Estrada-Garcia T (2016) Mexican unpasteurized fresh cheeses are contaminated with Salmonella, non-O157 Shiga toxin producing Escherichia coli and potential uropathogenic E. coli strains: A public health risk. Int Food Microb 237:10–16. https://doi.org/10.1016/j.ijfoodmicro.2016.08.018
Ribeiro LF, Barbose MM, Pinto FR, Maluta RP, Oliveira MC, de Souza V, de Medeiros MI, Borges LA, do Amaral LA, Fairbrother JM (2016) Antimicrobial resistance and virulence factors of Escherichia coli in cheese made from unpasteurized milk in three cities in Brazil. Foodborne Pathog Dis 13(9), 469–476. https://doi.org/10.1089/fpd.2015.2106
Wasiński B. Extra-intestinal pathogenic Escherichia coli–threat connected with food-borne infections (2019) Ann Agric Environ Med 26(4):532–537. https://doi.org/10.26444/aaem/111724.
Maluta RP, Logue CM, Casas MR, Meng T, Guastalli EA, Rojas TC, Montelli AC, Sadatsune T, de Carvalho RM, Nolan LK et al (2014) Overlapped sequence types (STs) and serogroups of avian pathogenic (APEC) and human extra-intestinal pathogenic (ExPEC) Escherichia coli isolated in Brazil. PLoS ONE 9(8):e105016. https://doi.org/10.1371/journal.pone.0105016
Aworh MK, Kwaga JKP, Hendriksen RS, Okolocha EC, Thakur S (2021) Genetic relatedness of multidrug resistant Escherichia coli isolated from humans, chickens and poultry environments. Antimicrob Resist Infect Control 10(1):58. https://doi.org/10.1186/s13756-021-00930-x
Zhuge X, Zhou Z, Jiang M, Wang Z, Sun Y, Tang F, Xue F, Ren J, Dai J (2021) Chicken-source Escherichia coli within phylogroup F shares virulence genotypes and is closely related to extraintestinal pathogenic E. coli causing human infections. Transbound Emerg Dis 68(2):880–895. https://doi.org/10.1111/tbed.13755
Homeier-Bachmann T, Kleist JF, Schütz AK, Bachmann L (2022) Distribution of ESBL/AmpC-Escherichia coli on a Dairy Farm. Antibiotics (Basel) 11(7):940. https://doi.org/10.3390/antibiotics11070940
O’Neill J. Tackling drug-resistant infections globally: final report and recommendations The Review on antimicrobial resistance. May 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf. Accessed 27 March 2024
Kamaruzzaman EA, Abdul Aziz S, Bitrus AA, Zakaria Z, Hassan L (2020) Occurrence and characteristics of extended-spectrum β-Lactamase-producing Escherichia coli from dairy cattle, milk, and farm environments in Peninsular Malaysia. Pathogens 9(12):1007. https://doi.org/10.3390/pathogens9121007
Brown K, Mugoh M, Call DR, Omulo S (2020) Antibiotic residues and antibiotic-resistant bacteria detected in milk marketed for human consumption in Kibera. Nairobi PLoS One 15(5):e0233413. https://doi.org/10.1371/journal.pone.0233413
Tóth AG, Csabai I, Krikó E, Tőzsér D, Maróti G, Patai AV, Makrai L, Szita G, Solymosi N (2020) Antimicrobial resistance genes in raw milk for human consumption. Sci Rep 10(1):7464. https://doi.org/10.1038/s41598-020-63675-4
Dos Santos Rosario AIL, da Silva MY, Castro VS, da Silva MCA, Conte-Junior CA, da Costa MP (2021) Everybody loves cheese: crosslink between persistence and virulence of Shiga-toxin Escherichia coli. Crit Rev Food Sci Nutr 61(11):1877–1899. https://doi.org/10.1080/10408398.2020.1767033
Acknowledgements
The authors express their gratitude to the Technology and Innovation Unit of the National Institute of Health Doutor Ricardo Jorge for performing the Next Generation Sequencing runs.
Funding
Open access funding provided by FCT|FCCN (b-on). This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, R.F. and R.B.; methodology, R.M., R.F., A.C., E.S., V.B. and A.P.; validation, R.F., C.B.C., V.B., J.P.G., A.P. and R.B.; formal analysis, V.B., J.P.G, A.P. and R.B; writing—original draft preparation, R.B.; writing—review and editing,, R.M., R.F, A.C., E.S., C.B.C., V.B., J.P.G, A.P and R.B. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare no conflict of interests.
Additional information
Communicated by Responsible Editor: Luis Augusto Nero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mendonça, R., Furtado, R., Coelho, A. et al. Raw milk cheeses from Beira Baixa, Portugal—A contributive study for the microbiological hygiene and safety assessment. Braz J Microbiol (2024). https://doi.org/10.1007/s42770-024-01332-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42770-024-01332-y