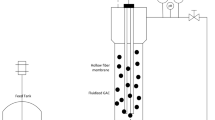
Abstract
Microfouling can have significant economic impacts for hydroelectric power plants. However, knowledge concerning the composition and metabolism of microbial biofilm in cooling systems remains scarce. We examined the metagenome present in a cooling system, comprising a filter (F) and heat exchanger (HE), in the Nova Ponte hydroelectric power plant in Brazil, to identify bacteria and pathways that could be targeted to monitor and control biofilm formation. Our data revealed that the microfouling sample from heat exchanger 1 (HEM1), with porous consistency, presented enriched bacterial members not frequently described as biofilm formers in cooling systems, besides it has been shown to be an autoinducer repression pathway. Furthermore, the microfouling sample from heat exchanger 2 (HEM2), with gelatinous consistency, seemed to be an established biofilm, containing enriched bacterial groups such as Desulfotomaculum and Crenothrix and autoinducers, with biotechnological relevance in industrial biofilms. The results demonstrate that biofilm composition will vary depending on different abiotic conditions and the antifouling strategy used, including type of compound, concentration, and frequency of use. Therefore, all these variables must be evaluated when a power plant is affected by microbial slime in the cooling system. Our findings could help to define strategies for efficient and ecofriendly measures to contain microfouling in power plants.





Similar content being viewed by others
References
Venugopalan VP, Rajagopal S, Jenner HA (2011) Operational and environmental issues relating to industrial cooling water systems: an overview. In: Rajagopal S, Jenner H, Venugopalan V (eds) Operational and Environmental Consequences of Large Industrial Cooling Water Systems. Springer, Boston MA, pp 1–12. https://doi.org/10.1007/978-1-4614-1698-2_1
Bognolo G, John G, Evans J, Karsa D (1992) Surfactants and the environment—some recent developments Industrial applications of surfactants (III). The Royal Society of Chemistry Cambridge:23–28. https://doi.org/10.1080/08927014.2011.618636
Turnpenny AW, Bruijs MC, Wolter C, Edwards N (2011) Regulatory aspects of choice and operation of large-scale cooling systems in Europe Operational and environmental consequences of large industrial cooling water systems, pp 421–454. https://doi.org/10.1007/978-1-4614-1698-2_20
Di Pippo F, Di Gregorio L, Congestri R, Tandoi V, Rossetti S (2018) Biofilm growth and control in cooling water industrial systems. FEMS Microbiol Ecol 94:fiy044. https://doi.org/10.1093/femsec/fiy044
Reis MP, de Paula RS, Reis ALM, Souza CC, Junior RBO, Ferreira JA, Mota HR, de Carvalho MD, Jorge EC, Cardoso AV (2021) Microbial composition of a hydropower cooling water system reveals thermophilic bacteria with a possible role in primary biofilm formation. Biofouling 37(2):246–256. https://doi.org/10.1080/08927014.2021.1897790
Bridier A, Briandet R, Thomas V, Dubois-Brissonnet F (2011) Resistance of bacterial biofilms to disinfectants: a review. Biofouling 27(9):1017–1032. https://doi.org/10.1080/08927014.2011.626899
Flemming H-C, Wingender J (2010) The biofilm matrix. Nat Rev Microbiol 8(9):623–633. https://doi.org/10.1038/nrmicro2415
Cloete TE, Jacobs L, Brözel VS (1998) The chemical control of biofouling in industrial water systems. https://doi.org/10.1023/A:1008216209206
Di Gregorio L, Tandoi V, Congestri R, Rossetti S, Di Pippo F (2017) Unravelling the core microbiome of biofilms in cooling tower systems. Biofouling 33(10):793-806. https://doi.org/10.1080/08927014.2017.1367386
Wolska KI, Grudniak AM, Rudnicka Z, Markowska K (2016) Genetic control of bacterial biofilms. J Appl Genet 57:225–238. https://doi.org/10.1007/s13353-015-0309-2
He L-Y, He L-K, Liu Y-S, Zhang M, Zhao J-L, Zhang Q-Q, Ying G-G (2019) Microbial diversity and antibiotic resistome in swine farm environments. Sci Total Environ 685:197–207. https://doi.org/10.1016/j.scitotenv.2019.05.369
Bonaglia S, Broman E, Brindefalk B, Hedlund E, Hjorth T, Rolff C, Nascimento FJ, Udekwu K, Gunnarsson JS (2020) Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 248:126023. https://doi.org/10.1016/j.chemosphere.2020.126023
Meyer F, Paarmann D, D'Souza M, Olson R, Glass EM, Kubal M, Paczian T, Rodriguez A, Stevens R, Wilke A (2008) The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics 9(1):1–8. https://doi.org/10.1186/1471-2105-9-386
Mitra S, Rupek P, Richter DC, Urich T, Gilbert JA, Meyer F, Wilke A, Huson DH (2011) Functional analysis of metagenomes and metatranscriptomes using SEED and KEGG. BMC Bioinformatics 12(1):1–8. https://doi.org/10.1186/1471-2105-12-S1-S21
Costa PS, Reis MP, Ávila MP, Leite LR, de Araújo FM, Salim AC, Oliveira G, Barbosa F, Chartone-Souza E, Nascimento AM (2015) Metagenome of a microbial community inhabiting a metal-rich tropical stream sediment. PLoS One 10(3):e0119465. https://doi.org/10.1371/journal.pone.0119465
Fisher WD (1958) On grouping for maximum homogeneity. J Am Stat Assoc 53(284):789–798. https://doi.org/10.1080/01621459.1958.10501479
Parks DH, Beiko RG (2010) Identifying biologically relevant differences between metagenomic communities. Bioinformatics 26(6):715–721. https://doi.org/10.1093/bioinformatics/btq041
da Silva TD, Giordani RB, Macedo AJ (2013) Biofilmes bacterianos patogênicos: aspectos gerais, importância clínica e estratégias de combate. Revista Liberato 14(22):213–236
Monroe D (2007) Looking for chinks in the armor of bacterial biofilms. PLoS Biol 5(11):e307. https://doi.org/10.1371/journal.pbio.0050307
Liu Y, Zhang W, Sileika T, Warta R, Cianciotto NP, Packman A (2009) Role of bacterial adhesion in the microbial ecology of biofilms in cooling tower systems. Biofouling 25(3):241–253. https://doi.org/10.1080/08927010802713414
Melo LF, Flemming H-C. 2010. 18 Mechanistic aspects of heat exchanger and membrane biofouling and prevention.
Adarsh V, Mishra M, Chowdhury S, Sudarshan M, Thakur A, Chaudhuri SR (2007) Studies on metal microbe interaction of three bacterial isolates from East Calcutta Wetland. J Biol Sci 7:80–88. https://doi.org/10.3844/ojbsci.2007.80.88
Silver S, Phung LT (1996) Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol 50(1):753–789. https://doi.org/10.1146/annurev.micro.50.1.753
Wang T, Flint S, Palmer J (2019) Magnesium and calcium ions: roles in bacterial cell attachment and biofilm structure maturation. Biofouling 35(9):959–974. https://doi.org/10.1080/08927014.2019.1674811
Wang J, Liu M, Xiao H, Wu W, Xie M, Sun M, Zhu C, Li P (2013) Bacterial community structure in cooling water and biofilm in an industrial recirculating cooling water system. Water Sci Technol 68(4):940–947. https://doi.org/10.2166/wst.2013.334
Parnasa R, Nagar E, Sendersky E, Reich Z, Simkovsky R, Golden S, Schwarz R (2016) Small secreted proteins enable biofilm development in the cyanobacterium Synechococcus elongatus. Sci Rep 6(1):32209. https://doi.org/10.1038/srep32209
Tan L-R, Xia P-F, Sun X-F, Guo N, Song C, Li Q, Wang S-G (2016) Ecological insights into low-level antibiotics interfered biofilms of Synechococcus elongatus. RSC Adv 6(81):78132–78135. https://doi.org/10.1039/C6RA15025J
Ruiz MM (2014) Análise da biodiversidade microbiana em ambiente aquático com despejo contínuo de efluentes de hidrocarbonetos [thesis]. Universidade Federal do Amazonas, Manaus (AM) https://tede.ufam.edu.br/handle/tede/4304
Park H-D, Noguera DR (2008) Nitrospira community composition in nitrifying reactors operated with two different dissolved oxygen levels. J Microbiol Biotechnol 18(8):1470–1474. https://doi.org/10.4014/jmb.0804.313
Li T, Guo F, Lin Y, Li Y, Wu G (2019) Metagenomic analysis of quorum sensing systems in activated sludge and membrane biofilm of a full-scale membrane bioreactor. Journal of Water Process Engineering 32:100952. https://doi.org/10.1016/j.jwpe.2019.100952
Luo J, Zhu Y, Song A, Wang L, Shen C, Gui Z, Zhang Q, Cao J (2019) Efficient short-chain fatty acids recovery from anaerobic fermentation of wine vinasse and waste activated sludge and the underlying mechanisms. Biochem Eng J 145:18–26. https://doi.org/10.1016/j.bej.2019.02.010
Tao W, Zhang X-X, Zhao F, Huang K, Ma H, Wang Z, Ye L, Ren H (2016) High levels of antibiotic resistance genes and their correlations with bacterial community and mobile genetic elements in pharmaceutical wastewater treatment bioreactors. PLoS One 11(6):e0156854. https://doi.org/10.1371/journal.pone.0156854
Balamurugan P, Hiren Joshi M, Rao T (2011) Microbial fouling community analysis of the cooling water system of a nuclear test reactor with emphasis on sulphate reducing bacteria. Biofouling 27(9):967–978. https://doi.org/10.1080/08927014.2011.618636
Çetin D, Bilgiç S, Dönmez G (2007) Biocorrosion of low alloy steel by Desulfotomaculum sp. and effect of biocides on corrosion control. ISIJ Int 47(7):1023–1028. https://doi.org/10.2355/isijinternational.47.1023
Chan C, Emerson D, Luther G III (2016) The role of microaerophilic Fe-oxidizing micro-organisms in producing banded iron formations. Geobiology 14(5):509–528. https://doi.org/10.1111/gbi.12192
Mulder EG, Deinema MH (1981) The sheathed bacteria. The Prokaryotes: a Handbook on Habitats, Isolation, and Identification of Bacteria, pp 425–440. https://doi.org/10.1007/978-3-662-13187-9_27
Ghiorse W (1984) Biology of iron-and manganese-depositing bacteria. Annu Rev Microbiol 38(1):515–550. https://doi.org/10.1146/annurev.mi.38.100184.002503
Kappler A, Emerson D, Gralnick J, Roden E, Muehe E (2015) Geomicrobiology of iron. Chapter 17. In: Ehrlich’s Geomicrobiology. Taylor & Francis Ltd
Collin F, Karkare S, Maxwell A (2011) Exploiting bacterial DNA gyrase as a drug target: current state and perspectives. Appl Microbiol Biotechnol 92:479–497. https://doi.org/10.1007/s00253-011-3557-z
Flemming H-C, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S (2016) Biofilms: an emergent form of bacterial life. Nat Rev Microbiol 14(9):563–575. https://doi.org/10.1038/nrmicro.2016.94
Boyd CD, O'Toole GA (2012) Second messenger regulation of biofilm formation: breakthroughs in understanding c-di-GMP effector systems. Annu Rev Cell Dev Biol 28:439–462. https://doi.org/10.1146/annurev-cellbio-101011-155705
Fazli M, Almblad H, Rybtke ML, Givskov M, Eberl L, Tolker-Nielsen T (2014) Regulation of biofilm formation in P seudomonas and B urkholderia species. Environ Microbiol 16(7):1961–1981. https://doi.org/10.1111/1462-2920.12448
Liang Z-X (2015) The expanding roles of c-di-GMP in the biosynthesis of exopolysaccharides and secondary metabolites. Nat Prod Rep 32(5):663–683. https://doi.org/10.1039/C4NP00086B
Jenal U, Reinders A, Lori C (2017) Cyclic di-GMP: second messenger extraordinaire. Nat Rev Microbiol 15(5):271–284. https://doi.org/10.1038/nrmicro.2016.190
Burmølle M, Ren D, Bjarnsholt T, Sørensen SJ (2014) Interactions in multispecies biofilms: do they actually matter? Trends Microbiol 22(2):84–91. https://doi.org/10.1016/j.tim.2013.12.004
Papenfort K, Bassler BL (2016) Quorum sensing signal–response systems in Gram-negative bacteria. Nat Rev Microbiol 14(9):576–588. https://doi.org/10.1038/nrmicro.2016.89
Zhao J, Quan C, Jin L, Chen M (2018) Production, detection and application perspectives of quorum sensing autoinducer-2 in bacteria. J Biotechnol 268:53–60. https://doi.org/10.1016/j.jbiotec.2018.01.009
Di Cagno R, De Angelis M, Calasso M, Gobbetti M (2011) Proteomics of the bacterial cross-talk by quorum sensing. J Proteome 74(1):19–34. https://doi.org/10.1016/j.jprot.2010.09.003
Taga ME, Semmelhack JL, Bassler BL (2001) The LuxS-dependent autoinducer AI-2 controls the expression of an ABC transporter that functions in AI-2 uptake in Salmonella typhimurium. Mol Microbiol 42(3):777–793. https://doi.org/10.1046/j.1365-2958.2001.02669.x
Taga ME, Miller ST, Bassler BL (2003) Lsr-mediated transport and processing of AI-2 in Salmonella typhimurium. Mol Microbiol 50(4):1411–1427. https://doi.org/10.1046/j.1365-2958.2003.03781.x
Ma H, Wang X, Zhang Y, Hu H, Ren H, Geng J, Ding L (2018) The diversity, distribution and function of N-acyl-homoserine lactone (AHL) in industrial anaerobic granular sludge. Bioresour Technol 247:116–124. https://doi.org/10.1016/j.biortech.2017.09.043
Funding
This work was supported by the Companhia Energética de Minas Gerais (CEMIG) R&Ds ANEEL GT-343 and GT-0604.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Responsible Editor: Solange I. Mussatto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Figure S1. Microbial slime in the heat exchangers of the Nova Ponte hydroelectric power plant. (A) HEM1 sample. (B) HEM2 sample. (TIF 2778 kb)
ESM 2:
Figure S2. An integrated view of nitrogen metabolism of the cooling system components of the Nova Ponte hydroelectric power plant. Gray squares represent the presence of an enzyme sequence in the (A) HEM1 metagenome, (B) HEM2 metagenome, (C) FS metagenome. (TIF 383 kb)
ESM 3:
Figure S3. An integrated view of methane metabolism of the cooling system components of the Nova Ponte hydroelectric power plant. Gray squares represent the presence of an enzyme sequence in the (A) HEM1 metagenome, (B) HEM2 metagenome, (C) FS metagenome. (TIF 201 kb)
ESM 4:
Figure S4. An integrated view of sulfur metabolism of the cooling system components of the Nova Ponte hydroelectric power plant. Gray squares represent the presence of an enzyme sequence in the (A) HEM1 metagenome, (B) HEM2 metagenome, (C) FS metagenome. (TIF 367 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reis, M.d., de Paula, R.S., e Souza, C.C. et al. Linking microbial slime community structure with abiotic factors and antifouling strategy in hydroelectric cooling systems. Braz J Microbiol 54, 1547–1557 (2023). https://doi.org/10.1007/s42770-023-01020-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01020-3