Abstract
Acinetobacter baumannii infection presents a high mortality rate and few therapeutic options. This study aimed to evaluate clinical-microbiological characteristics and prognosis factors of patients diagnosed with A. baumanni. infections treated with oral doxycycline. A retrospective cohort of hospitalized patients with confirmed Acinetobacter spp. infection between 2018 and 2020 receives at least 3 days of oral doxycycline. Clinical and microbiological data were evaluated, including the outcome and molecular characterization of A. baumannii. Doxycycline minimal inhibitory concentrations were evaluated by the broth dilution method. One hundred patients were included with a median age of 51 years. The leading site of infection was pulmonary (n = 62), followed by the soft tissues and skin (n = 28). A. baumannii resistant to carbapenem was found on 94%. The gene blaOXA-23 and blaOXA-51 were amplified in all recovered isolates of A. baumannii (n = 44). Doxycycline MIC50 and MIC90 were 1 µg/mL and 2 µg/mL, respectively. Death rate at 14 days and 28 days of follow-up was 9% and 14%, respectively. The prognostic factors related to death at end of follow-up were age > 49 years [85.7% vs. 46%, CI 95% 6.9 (1.4–32.6), P = 0.015] and hemodialysis [28.6% vs. 7%, CI 95% 5.33 (1.2–22.1), P = 0.021]. Patients treated with doxycycline to A. baumannii presented a relatively low death rate, and risk factors related to death were age and hemodialysis. Further and larger studies should compare polymyxin to doxycycline to better understand the differences between these therapeutic options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter spp. are ubiquitous non-fermenting gram-negative bacilli. It was first described as Mima spp. during the early twentieth century [1]. The genus Acinetobacter was proposed during the 1960s, but it has been reported as an important human pathogen since the 1940s [2, 3]. Acinetobacter baumannii emerged as a pathogen related to health-associated infections (HAIs) [4,5,6]. Multidrug resistance of this pathogen has been demonstrated around the world, increasing the failure of treatment and mortality, mainly the resistance to carbapenem (carbapenem-resistant Acinetobacter baumannii—CRAB) [7, 8].
The treatment of CRAB is based on a few options, including polymyxin and tigecycline [9, 10]. According to the SENTRY surveillance program, polymyxin and minocycline are the most active therapeutic options [7]. Among tetracyclines, minocycline or doxycycline-based therapy presents a clinical success rate of 76% [11]. However, these options usually are used as adjunctive therapies once few data of monotherapy are available on literature [11]. The large volume of distribution (Vd) of tetracyclines may be an attractive pharmacokinetic property if used to non-severe infections. However, its use in critically-ill patients and/or blood stream infections is questionable.
Even before COVID-19, there were global efforts to decrease the antimicrobial resistance through antimicrobial stewardship programs which are based in sparing carbapenem, polymyxin, and tigecycline, considering the last therapeutic sources for the treatment of multidrug-resistant bacteria, including CRAB. After COVID-19 pandemic, antimicrobial resistance increased significantly due to the indiscriminate use of carbapenems and/or polymyxins to Acinetobacter spp. infections and further restricts therapeutic options. The use of tetracycline for A. baumannii can be an alternative to cephalosporins, carbapenems, and fluoroquinolones. This study aimed to evaluate clinical-microbiological characteristics and prognosis factors of patients diagnosed with Acinetobacter baumannii infections treated with oral doxycycline.
Methods
Settings
This was a retrospective cohort study of patients diagnosed with Acinetobacter baumannii infections treated with oral doxycycline. Data collection included 2 years (November 2017 to December 2020) from a University Hospital in the South of Brazil. Follow-up occurred until discharge or death. This study was approved by the Ethical committee from PUCPR (22,320,619.6.0000.0020).
Participants
Inclusion criteria for patients were as follows: (i) ≥ 18 years, (ii) admitted to hospital wards or ICU, (iii) confirmed infection due to doxycycline susceptible A. baumannii, and (iv) medical prescription of oral doxycycline for A. baumannii infection. Patients with a lack of clinical and microbiological information were excluded.
Antimicrobial stewardship program and microbiological tests
Since 2017, the antimicrobial stewardship program of the hospital included active surveillance of patients using intravenous antibiotics to evaluate the possibility of change to oral therapy (see details of the protocol for the oral switch in supplemental material – S1). Beyond the intravenous to oral switch approach, the carbapenem and polymyxin sparing approach were started, and alternative therapies were introduced. Considering the susceptible profile of A. baumanni in the institution published previously [12, 13], doxycycline was included in the routine susceptibility tests by disk diffusion [14]. All isolates were identified using MALDI-TOF Vitek® MS (BioMérièux, Marcy-l’Etoile, France) and susceptibility profile for other than doxycycline antibiotics by automated system MicroScan WalkAway (Beckman Coulter, Brea, CA).
Molecular analysis
In order to evaluate similarities between environmental and clinical isolates, consecutive CRAB isolates were stored − 80 °C to further molecular analysis for the blaOXA-23 gene and blaOXA51. Briefly, to determine oxacillinase-encoding genes, polymerase chain reaction (PCR) was performed for all isolates by using the following primers: blaOXA-23 (F-5′ GACACTAGGAGAAGCCATGAAG 3′; R-5′ CAGCATTACCGAAACCAATACG 3′; 6-FAM-CCAGTCTATCAGGAACTTGCGCGA-BHQ_1) and blaOXA-51 (F-5′ TGTCTAAGGAAGTGAAGCGTG 3′; R-5′ TGGATTGCACTTCATCTTGG 3′; CY5-XN-ACTTGGGTACCGATATCTGCATTGCC-BHQ-2) [15, 16].
Genetic similarity
Fifty isolates were inoculated in 300 μL of ultrapure water and 900 μL of 99% absolute alcohol followed by vortexing for 5 min and centrifugation at 17,005 g for 1 min. The size sample includes all isolates available and viable to test. The supernatant was discarded, and the mixture was vortexed for 5 min after the addition of 50 μL of 70% formic acid. Acetonitrile (50 μL) was added followed by vortexing for 5 min and centrifugation at 17,005 g for 1 min. Direct detection of the microorganism was performed using MALDI-TOF. Quality control of the readings was performed using the reference strain of E. coli ATCC® 8739. The detected pattern was analyzed for similarity using the taxonomy tool in the Saramis software (bioMérièux, Marcy-l'Étoile, France). Clones were considered those with > 75% similarities [17].
Variables and definitions
Confirmed infection due to A. baumannii was defined as fever ≥ 38 °C, leukocytosis ≥ 10.000 cells/mm [3], clinical symptoms on each site of infection, and positive culture from the suspected site of infection according to Infectious Disease Society of America recommended cut-offs values [18].
Patients were evaluated on end-of-therapy and end-of-hospitalization. Demographic and clinical characteristics, comorbidities, intensive care unit (ICU) support, and clinical and microbiological outcomes were evaluated. Additionally, acute physiology and chronic health evaluation II (APACHE II) was assessed on day of suspected infection.
Clinical cure was defined as an absence of fever, improvement in clinical symptoms and laboratory exams, and absence of need for antimicrobial therapy according to the medical decision. Clinical failure was defined as death or changing on antimicrobial therapy choice due to clinical worsening. The microbiological cure was defined as a negative culture from the site of infection after antimicrobial therapy. Microbiological failure was defined as persistent positive cultures after antimicrobial therapy.
Statistical analysis
Continuous variables were expressed as median values and interquartile range (IQR) and analyzed by Student t-test or Mann Whitney U test. Categorical variables were expressed as absolute frequencies and proportions and analyzed by Chi-square or Fisher test. Variables with P < 0.2 were selected to binary logistic regression. SPSS (IBM, Chicago, IL, USA) was used for statistical analysis. Statistically significant values were considered if P < 0.05.
Results
Sample size
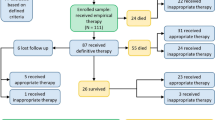
A hundred and sixteen patients with Acinetobacter spp. infections were found between November 2017 and December 2020. From these, six were non-A. baumannii and ten were excluded due to lack of sufficient clinical information for analysis.
Clinical characteristics
One hundred patients were included, 81 (81%) were male, and median age was 51 years [33–62]. Site of infection were pulmonary (n = 62), soft tissues and skin (SST) (n = 28), osteomyelitis (n = 6), urinary tract infection (n = 2), and intra-abdominal (n = 2). Twenty-three patients (23%) were admitted to ICU (APACHE = 14 [10,11,12,13,14,15,16,17,18,19,20,21,22,23]), and six patients (6%) with vasoactive drugs prescription. The duration of hospitalization was 49 days [31–87], while the length of stay before and after culture were 21 days [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32] and 23 days [11–46]. Median doxycycline time of treatment was eight days [6,7,8,9,10,11,12]. Clinical-microbiological characteristics are on Table 1.
Microbiologic characteristics
Carbapenem-resistant A. baumannii was found on 94 (94%) using an automated antimicrobial susceptibility test. Resistance to amikacin, cefepime, and quinolones was found in 91%, 96%, and 97%, respectively. All A. baumannii isolated were susceptible to doxycycline using disk diffusion method. Fourteen clinical isolates were recovered to evaluate (i) doxycycline MIC by broth dilution and (ii) molecular analysis. Doxycycline minimal inhibitory concentration varied from 0.25 to 2 µg/mL (MIC50 = 1 µg/mL and MIC90 = 2 µg/mL). The gene blaOXA-23 and blaOXA-51 were amplified in all isolates of carbapenem-resistant Acinetobacter baumannii.
From the general microbiological isolates (environmental and clinical), results were the same that from the fourteen clinical isolates (MIC50-90 1–2 mg/L and gene blaOXA-23/blaOXA-51 amplified in all strains) (Supplementary Material – Table 1). The hierarchical clustering of the MALDI-TOF peak profiles identified three different A. baumannii clusters, with an average genomic similarity rate of 75%. Cluster I had a cluster of 47 isolates, cluster II comprised 2 isolates, and cluster III had only one bacterial isolate (Supplementary Material – Fig. 1).
Concomitant pathogens
In 31 patients, A. baumanni was identified together with other pathogens. Gram-negative bacilli and gram-positive cocci concomitants with Acinetobacter spp. infection account for 20 (60.6%) and 13 (39.3%) isolates, respectively (Table 2). Thirty-eight (38%) patients received other antibiotic therapy with a gram-negative bacilli spectrum along with doxycycline. Considering the susceptibility pattern, three patients (3%) received double antibiotic coverage to Acinetobacter spp. along with doxycycline: cefepime (n = 1), amikacin (n = 1), and polymyxin (n = 1).
Outcomes and prognosis factor
Death at 14 days of follow-up occurred in 9 patients (9%). In univariate analysis, death was related to age [63 years (50–74) vs.49 years (32–61), P = 0.029], vasoactive drug (22.2% vs. 4.4%, P = 0.03), and doxycycline days of treatment [5 days (3–7.5) vs. 8 days (6–13), P = 0.001) (Table 3). In binary logistic regression, death at 14 days of follow-up was associated with age > 49 years [89% vs. 51%, CI 95% 8.5 (1.02–71.13), P = 0.047] (Table 3). Combination therapy was not associated with better outcomes.
Death at 28 days of follow-up occurred in 14 patients (14%). In univariate analysis, death was related to age [63.5 years (50–70) vs.47.5 years (32–60), P = 0.002], hemodialysis (28.6% vs 7%, P = 0.013), and doxycycline days of treatment [6 days (3–8) vs. 8 (5–13), P = 0.014] (Table 4). In binary logistic regression age > 49 years [85.7% vs. 46%, CI 95% 6.9 (1.4–32.6), P = 0.015] and hemodialysis [28.6% vs. 7%, CI 95% 5.33 (1.2–22.1), P = 0.021] (Table 4). Combination therapy was not associated with better outcomes.
Discussion
In this retrospective cohort, patients with A. baumannii infections were treated with oral doxycycline (n = 100); the therapy was employed in different scenarios, including mild to moderate infections. Twenty-three patients (23%) were in ICU when A. baumannii infection occurred, six of them were in weaning order of vasoactive drugs. Thirty-eight patients (38%) received double antimicrobial therapy with GNB spectrum. However, neither ICU was related to worse prognosis nor combination therapy was related to better outcomes. Factors associated with death at 28 days of follow-up were age > 49 years and hemodialysis.
The last data regarding A. baumannii susceptibility based on the SENTRY surveillance program demonstrates polymyxin and tetracyclines as the most effective antimicrobial options [7]. However, the variability between world regions is also demonstrated. In Latin America, 90% of A. baumannii strains were susceptible to tetracyclines, while in Europe, rates dropped to 70%. Additionally, rates lower than 30% were also reported in Asia [19]. Therefore, doxycycline as an empirical treatment to A. baumannii must be based on careful susceptibility profile analysis. Considering it, we have previously described the doxycycline susceptibility profile of CRAB in our hospital of 80% in patients with healthcare-associated bacteremia and meningitis [13].
The pharmacokinetics of doxycycline confers interesting properties for the treatment of mild-to-moderate infections in the lungs, skin and soft tissues, bone, and central nervous system. Doxycycline may be prescribed as 100 mg PO q12h or 200 mg q24h. Once the volume of distribution is 0.7L/kg, and half-life varies between 18 and 24, 200 mg q24h or even q12h regimens may benefit severe patients due to earlier steady-state achievement [20]. Doxycycline peak serum concentrations vary according to the dose regimen. Single 100 mg PO may achieve 1.7–2 mg/L while 200 mg PO reaches 5–6 mg/L [21]. Bactericidal pharmacodynamic target is demonstrated to be 8–16 times the minimal inhibitory concentration (MIC), although 2–4 times the MIC may present a bacteriostatic effect [22].
Doxycycline MIC ranged from 0.25 to 2 mg/L with a MIC50 and MIC90 of 1 and 2 mg/L, respectively. This susceptibility profile is very different from other studies with CRAB from different regions of the world. Several studies with CRAB present higher resistance, including MIC50/90 of 32/64 mg/L in Iran and in a multicentric study in Europe [23, 24]. Doxycycline pharmacokinetic parameters, patient clinical presentation, and pathogen MIC must be analyzed to avoid potential therapy failure due to insufficient tissue levels. Doxycycline MIC50 was 1 µg/mL, suggesting that doxycycline has bacteriostatic activity in patients with Acinetobacter baumannii. This hypothesis could be confirmed by in vitro tests (time kill-curve). Twelve isolates presented MIC lower than 1 mg/L, a good susceptibility profile for possible bactericidal activity of doxycycline.
Our molecular findings are in accordance with previous studies demonstrating that carbapenem resistance in A. baumannii is strongly associated with the blaOXA-23 gene [25]. Carbapenems MICs are also demonstrated to be higher when blaOXA-23 or blaOXA-51 genes are associated with insertion sequences such as ISAba1. Once blaOXA-23 is also associated with A. baumannii hospital outbreaks due to widespread capacity, infectious disease specialists could see tetracyclines as an option to spare polymyxin or tigecycline overuse during outbreaks also. The clonality analysis showed that the largest proportion of A. baumannii belongs to cluster I, and only 3 isolates from different clusters. Other species of Acinetobacter spp. were not included in this analysis. The genetic similarity was evaluated using MALDI-TOF as tool for investigation of A. baumannii outbreaks [26]. The advantage of MALDI-TOF is the low cost and speed.
Differently from extensively drug-resistant Enterobacteriaceae, combined therapy to CRAB did not demonstrate better survival rates [28]. In our study, from 100 patients, 38 received another antibiotic. However, considering the A. baumannii susceptibility profile, all of them received only doxycycline as active drug. Additional combinations described in our study were used with the intention to expand the antimicrobial spectrum, once 31 (31%) patients with A. baumannii infection presented another pathogen isolated from the same site. Traditional therapeutic options to CRAB treatment, such as tetracyclines, polymyxin, and tigecycline, were evaluated in previous studies with full results variability. A common conclusion of a meta-analysis is that combined therapy to non-fermenting GNB infections is not superior to monotherapy [29, 30]; nevertheless, divergences among therapeutic options are reported.
Oral therapy in severe patients is a questionable approach. A recent study with critically-ill patients demonstrated that oral switch could be safe in patients with adequate enteral feeding after improving the initial signs of sepsis [31]. The authors included doxycycline in the intravenous-to-oral antibiotic switch therapy. However, the number of patients and their outcome was not detailed. In our study, polymyxins and tigecycline would be alternatives to doxycycline; however, these drugs should be considered last treatment resources if we evaluate toxicities, environmental selective pressure, and costs. Doxycycline is a less expensive option and as safe as classical ones available. Death rate at 14 and 28 days of follow-up were 9% and 14%, respectively. This finding may support the safety of doxycycline in the treatment of A. baumannii. Similar to our results, tetracycline-based therapy presented a clinical failure of 23% [11]. In this cohort, the factors associated with death at end of follow-up were age and hemodialysis. Considering that doxycycline is not cleared by dialysis filters nor urine output, and that age is commonly demonstrated as a risk factor to death in patients with hospital acquired infections, our related findings might not be attributed to the oral doxycycline therapy, but a common risk factor to death in stable patients diagnosed with A. baumannii infection.
This is a retrospective cohort and may present bias considering the analysis of the authors’ point of view. A control group with other therapy, as polymyxin, could be included as a comparator; however, another bias would be implied, different severity between doxycycline and polymyxin group. These drugs present different pharmacokinetics, route of administration, and the stewardship program of the institution claim to avoid the use of polymyxin due to the risk of polymyxin-resistance [32], costs [33], and high nephrotoxicity [34]. The combined therapy in some patients could be a risk of misinterpretation of the outcome due to synergic, additive, or antagonistic effects.
Conclusion
Patients treated with doxycycline to A. baumannii presented a relatively low death rate, and risk factors related to death were age and hemodialysis. Further and larger studies should compare polymyxin to doxycycline to better understand the differences between these therapeutic options. From this retrospective observational study, doxycycline appears to be a possible option to CRAB infections.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582
Baumann P, Doudoroff M, Stanier RY (1968) A study of the Moraxella group. II. Oxidative-negative species (genus Acinetobacter). J Bacteriol 95:1520–1541
De Bord GG (1948) Mima polymorpha in meningitis. J Bacteriol 55:764–765
Buxton AE, Anderson RL, Werdegar D, Atlas E (1978) Nosocomial respiratory tract infection and colonization with Acinetobacter calcoaceticus. Epidemiologic characteristics. Am J Med 65:507–513
Castle M, Tenney JH, Weinstein MP, Eickhoff TC (1978) Outbreak of a multiply resistant Acinetobacter in a surgical intensive care unit: epidemiology and control. Heart Lung 7:641–644
Abrutyn E, Goodhart GL, Roos K, Anderson R, Buxton A (1978) Acinetobacter calcoaceticus outbreak associated with peritoneal dialysis. Am J Epidemiol 107:328–335
Gales AC, Seifert H, Gur D, Castanheira M, Jones RN, Sader HS (2019) Antimicrobial susceptibility of Acinetobacter calcoaceticus-Acinetobacter baumannii complex and Stenotrophomonas maltophilia clinical isolates: results from the SENTRY antimicrobial surveillance program (1997–2016). Open Forum Infect Dis 6:S34–S46
Oh DH, Kim YC, Kim EJ, Jung IY, Jeong SJ, Kim SY, Park MS, Kim A, Lee JG, Paik HC (2019) Multidrug-resistant Acinetobacter baumannii infection in lung transplant recipients: risk factors and prognosis. Infect Dis (Lond) 51:493–501
Liang CA, Lin YC, Lu PL, Chen HC, Chang HL, Sheu CC (2018) Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect 24:908 e1-908 e7
Tucker H, Wible M, Gandhi A, Quintana A (2017) Efficacy of intravenous tigecycline in patients with Acinetobacter complex infections: results from 14 Phase III and Phase IV clinical trials. Infect Drug Resist 10:401–417
Falagas ME, Vardakas KZ, Kapaskelis A, Triarides NA, Roussos NS (2015) Tetracyclines for multidrug-resistant Acinetobacter baumannii infections. Int J Antimicrob Agents 45:455–460
Cieslinski JM, Arend L, Tuon FF, Silva EP, Ekermann RG, Dalla-Costa LM, Higgins PG, Seifert H, Pilonetto M (2013) Molecular epidemiology characterization of OXA-23 carbapenemase-producing Acinetobacter baumannii isolated from 8 Brazilian hospitals using repetitive sequence-based PCR. Diagn Microbiol Infect Dis 77:337–340
Schuertz KF, Tuon FF, Palmeiro JK, Conte D, Telles JPM, Trevisoli LE, Dalla-Costa LM (2018) Bacteremia and meningitis caused by OXA-23-producing Acinetobacter baumannii - molecular characterization and susceptibility testing for alternative antibiotics. Braz J Microbiol 49 Suppl 1(Suppl 1):199–204. https://doi.org/10.1016/j.bjm.2018.04.002
CLSI (2017) Performance standards for antimicrobial susceptibility testing. 27th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute
Huang XZ, Cash DM, Chahine MA, Nikolich MP, Craft DW (2012) Development and validation of a multiplex TaqMan real-time PCR for rapid detection of genes encoding four types of class D carbapenemase in Acinetobacter baumannii. J Med Microbiol 61:1532–1537
Woodford N, Ellington MJ, Coelho JM, Turton JF, Ward ME, Brown S, Amyes SG, Livermore DM (2006) Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents 27:351–353
Mencacci A, Monari C, Leli C, Merlini L, De Carolis E, Vella A, Cacioni M, Buzi S, Nardelli E, Bistoni F, Sanguinetti M, Vecchiarelli A (2013) Typing of nosocomial outbreaks of Acinetobacter baumannii by use of matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol 51:603–606
Miller JM, Binnicker MJ, Campbell S, Carroll KC, Chapin KC, Gilligan PH, Gonzalez MD, Jerris RC, Kehl SC, Patel R, Pritt BS, Richter SS, Robinson-Dunn B, Schwartzman JD, Snyder JW, Telford S 3rd, Theel ES, Thomson RB Jr, Weinstein MP, Yao JD (2018) A guide to utilization of the microbiology laboratory for diagnosis of infectious diseases: 2018 update by the Infectious Diseases Society of America and the American Society for Microbiology. Clin Infect Dis 67:e1–e94
Sohail M, Rashid A, Aslam B, Waseem M, Shahid M, Akram M, Khurshid M, Rasool MH (2016) Antimicrobial susceptibility of Acinetobacter clinical isolates and emerging antibiogram trends for nosocomial infection management. Rev Soc Bras Med Trop 49:300–304
Cunha BA, Baron J, Cunha CB (2018) Similarities and differences between doxycycline and minocycline: clinical and antimicrobial stewardship considerations. Eur J Clin Microbiol Infect Dis 37:15–20
Agwuh KN, MacGowan A (2006) Pharmacokinetics and pharmacodynamics of the tetracyclines including glycylcyclines. J Antimicrob Chemother 58:256–265
Cunha BA, Domenico P, Cunha CB (2000) Pharmacodynamics of doxycycline. Clin Microbiol Infect 6:270–273
Ranjbar R, Farahani A (2019) Study of genetic diversity, biofilm formation, and detection of Carbapenemase, MBL, ESBL, and tetracycline resistance genes in multidrug-resistant Acinetobacter baumannii isolated from burn wound infections in Iran. Antimicrob Resist Infect Control 8:172
Seifert H, Stefanik D, Sutcliffe JA, Higgins PG (2018) In-vitro activity of the novel fluorocycline eravacycline against carbapenem non-susceptible Acinetobacter baumannii. Int J Antimicrob Agents 51:62–64
Chagas TP, Carvalho KR, de Oliveira Santos IC, Carvalho-Assef AP, Asensi MD (2014) Characterization of carbapenem-resistant Acinetobacter baumannii in Brazil (2008–2011): countrywide spread of OXA-23-producing clones (CC15 and CC79). Diagn Microbiol Infect Dis 79:468–472
Bianco A, Quirino A, Giordano M, Marano V, Rizzo C, Liberto MC, Foca A, Pavia M (2016) Control of carbapenem-resistant Acinetobacter baumannii outbreak in an intensive care unit of a teaching hospital in Southern Italy. BMC Infect Dis 16:747
Rim JH, Lee Y, Hong SK, Park Y, Kim M, D’Souza R, Park ES, Yong D, Lee K (2015) Insufficient discriminatory power of matrix-assisted laser desorption ionization time-of-flight mass spectrometry dendrograms to determine the clonality of multi-drug-resistant Acinetobacter baumannii isolates from an intensive care unit. Biomed Res Int 2015:535027
Tuon FF, Rocha JL, Merlini AB (2015) Combined therapy for multi-drug-resistant Acinetobacter baumannii infection–is there evidence outside the laboratory? J Med Microbiol 64:951–959
Paul M, Daikos GL, Durante-Mangoni E, Yahav D, Carmeli Y, Benattar YD, Skiada A, Andini R, Eliakim-Raz N, Nutman A, Zusman O, Antoniadou A, Pafundi PC, Adler A, Dickstein Y, Pavleas I, Zampino R, Daitch V, Bitterman R, Zayyad H, Koppel F, Levi I, Babich T, Friberg LE, Mouton JW, Theuretzbacher U, Leibovici L (2018) Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 18:391–400
Zusman O, Altunin S, Koppel F, DishonBenattar Y, Gedik H, Paul M (2017) Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother 72:29–39
Gasparetto J, Tuon FF, Dos Santos OD, Zequinao T, Pipolo GR, Ribeiro GV, Beninca PD, Cruz JAW, Moraes TP (2019) Intravenous-to-oral antibiotic switch therapy: a cross-sectional study in critical care units. BMC Infect Dis 19:650
Freire MP, Van Der Heijden IM, do Prado GV, Cavalcante LS, Boszczowski I, Bonazzi PR, Rossi F, Guimaraes T, D’Albuquerque LA, Costa SF, Abdala E (2014) Polymyxin use as a risk factor for colonization or infection with polymyxin-resistant Acinetobacter baumannii after liver transplantation. Transpl Infect Dis 16:369–78
Tuon FF, Rocha JL, Gasparetto J (2019) Polymyxin B and colistin-the economic burden of nephrotoxicity against multidrug resistant bacteria. J Med Econ 22:158–162
Tuon FF, Rigatto MH, Lopes CK, Kamei LK, Rocha JL, Zavascki AP (2014) Risk factors for acute kidney injury in patients treated with polymyxin B or colistin methanesulfonate sodium. Int J Antimicrob Agents 43:349–352
Acknowledgements
The authors thank Nathalia C. Scandolara Cardoso for starting the study and Victoria Stadler Tasca Ribeiro for the support.
Author information
Authors and Affiliations
Contributions
Felipe Francisco Tuon was responsible for the organization and coordination of the study and responsible for the data analysis.
Carolina Hikari Yamada was the chief investigator and wrote the draft of this manuscript.
Ana Paula de Andrade performed microbiological analysis.
Lavinia Nery Villa Stangler Arend performed microbiological analysis.
Dayana dos Santos Oliveira performed data analysis.
João Paulo Telles wrote the draft of this manuscript and performed statistical analysis.
All authors contributed to the writing of the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Ethical committee from PUCPR (22320619.6.0000.0020).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Luis Augusto Nero
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tuon, F.F., Yamada, C.H., de Andrade, A.P. et al. Oral doxycycline to carbapenem-resistant Acinetobacter baumannii infection as a polymyxin-sparing strategy: results from a retrospective cohort. Braz J Microbiol 54, 1795–1802 (2023). https://doi.org/10.1007/s42770-023-01015-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-01015-0