Abstract
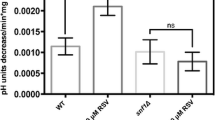
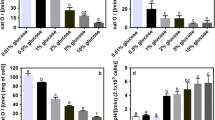
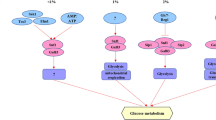
Cancer is a leading cause of death worldwide, reporting nearly 10 million deaths in 2020. One of the hallmarks of cancer cells is their capability to evade growth suppressors and sustain proliferative signaling resulting in uncontrolled growth. The AMPK pathway, a catabolic via to economize ATP, has been associated with cancer. AMPK activation is related to cancer progression in advanced stages, while its activation by metformin or phenformin is associated with cancer chemoprevention. Thus, the role of the AMPK pathway in cancer growth modulation is not clear. Saccharomyces cerevisiae might be a useful model to elucidate AMPK participation in growth regulation since it shares a highly conserved AMPK pathway. Therefore, this work is aimed at evaluating the role of the AMPK pathway on S. cerevisiae growth under different nutritional conditions. Herein, we provide evidence that the SNF1 gene is necessary to maintain S. cerevisiae growth with glucose as a sole carbon source at every concentration tested. Resveratrol supplementation inhibited the exponential growth of snf1∆ strain at low glucose levels and decreased it at high glucose levels. SNF1 gene deletion impaired exponential growth in a carbohydrate concentration-dependent manner independently of nitrogen source or concentration. Interestingly, deletion of genes encoding for upstream kinases (SAK1, ELM1, and TOS3) also had a glucose dose-dependent effect upon exponential growth. Furthermore, gene deletion of regulatory subunits of the AMPK complex impacted exponential growth in a glucose-dependent manner. Altogether, these results suggest that the SNF1 pathway affects the exponential growth of S. cerevisiae in a glucose-dependent manner.





Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Wang Z, Wang N, Liu P, Xie X (2016) AMPK and cancer. Exp Suppl 107:203–226. https://doi.org/10.1007/978-3-319-43589-3_9
Zhang Y, Meng Q, Sun Q, Xu ZX, Zhou H, Wang Y (2021) LKB1 deficiency-induced metabolic reprogramming in tumorigenesis and non-neoplastic diseases. Mol Metab. 44:101131. https://doi.org/10.1016/j.molmet.2020.101131
Hadad SM, Baker L, Quinlan PR, Robertson KE, Bray SE, Thomson G et al (2009) Histological evaluation of AMPK signalling in primary breast cancer. BMC Cancer 9:307. https://doi.org/10.1186/1471-2407-9-307
Cai H, Scott E, Kholghi A, Andreadi C, Rufini A, Karmokar A et al (2015) Cancer chemoprevention: evidence of a nonlinear dose response for the protective effects of resveratrol in humans and mice. Science translational medicine. 7:298ra117. https://doi.org/10.1126/scitranslmed.aaa7619
Morales DR, Morris AD (2015) Metformin in cancer treatment and prevention. Annu Rev Med 66:17–29. https://doi.org/10.1146/annurev-med-062613-093128
Hedbacker K, Carlson M (2008) SNF1/AMPK pathways in yeast. Front Biosci 13:2408–2420. https://doi.org/10.2741/2854
Kaspar von Meyenburg H (1969) Energetics of the budding cycle of Saccharomyces cerevisiae during glucose limited aerobic growth. Arch Mikrobiol 66:289–303. https://doi.org/10.1007/bf00414585
Vaupel P, Multhoff G (2021) Revisiting the Warburg effect: historical dogma versus current understanding. J Physiol 599:1745–1757. https://doi.org/10.1113/jp278810
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674. https://doi.org/10.1016/j.cell.2011.02.013
Dimmer KS, Fritz S, Fuchs F, Messerschmitt M, Weinbach N, Neupert W et al (2002) Genetic basis of mitochondrial function and morphology in Saccharomyces cerevisiae. Mol Biol Cell 13:847–853. https://doi.org/10.1091/mbc.01-12-0588
Martinez-Ortiz C, Carrillo-Garmendia A, Correa-Romero BF, Canizal-Garcia M, Gonzalez-Hernandez JC, Regalado-Gonzalez C et al (2019) SNF1 controls the glycolytic flux and mitochondrial respiration. Yeast 36:487–494. https://doi.org/10.1002/yea.3399
Olivares-Marin IK, Madrigal-Perez LA, Canizal-Garcia M, Garcia-Almendarez BE, Gonzalez-Hernandez JC, Regalado-Gonzalez C (2018) Interactions between carbon and nitrogen sources depend on RIM15 and determine fermentative or respiratory growth in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 102:4535–4548. https://doi.org/10.1007/s00253-018-8951-3
Babicki S, Arndt D, Marcu A, Liang Y, Grant JR, Maciejewski A et al (2016) Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res 8:W147-153. https://doi.org/10.1093/nar/gkw419
Hagman A, Piškur J (2015) A study on the fundamental mechanism and the evolutionary driving forces behind aerobic fermentation in yeast. PloS one. 10:e0116942. https://doi.org/10.1371/journal.pone.0116942
Verduyn C, Zomerdijk TPL, van Dijken JP, Scheffers WA (1984) Continuous measurement of ethanol production by aerobic yeast suspensions with an enzyme electrode. Appl Microbiol Biotechnol 19:181–185. https://doi.org/10.1007/BF00256451
Wu H, Chen L, Zhu F, Han X, Sun L, Chen K. (2019). The cytotoxicity effect of resveratrol: cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins (Basel). 11. https://doi.org/10.3390/toxins11120731.
Han H, Zhang T, Jin Z, Guo H, Wei X, Liu Y et al (2017) Blood glucose concentration and risk of liver cancer: systematic review and meta-analysis of prospective studies. Oncotarget. 8:50164–50173. https://doi.org/10.18632/oncotarget.16816
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104:19345–19350. https://doi.org/10.1073/pnas.0709747104
Schiliro C, Firestein BL. (2021). Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells. 10. https://doi.org/10.3390/cells10051056.
Pineda CT, Ramanathan S, FonTacer K, Weon JL, Potts MB, Ou YH et al (2015) Degradation of AMPK by a cancer-specific ubiquitin ligase. Cell 160:715–728. https://doi.org/10.1016/j.cell.2015.01.034
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z et al (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 17:113–124. https://doi.org/10.1016/j.cmet.2012.12.001
Matsumoto S, Iwakawa R, Takahashi K, Kohno T, Nakanishi Y, Matsuno Y et al (2007) Prevalence and specificity of LKB1 genetic alterations in lung cancers. Oncogene 26:5911–5918. https://doi.org/10.1038/sj.onc.1210418
Wingo SN, Gallardo TD, Akbay EA, Liang MC, Contreras CM, Boren T et al (2009) Somatic LKB1 mutations promote cervical cancer progression. PloS one 4:e5137. https://doi.org/10.1371/journal.pone.0005137
Olivares-Marin IK, González-Hernández JC, Regalado-Gonzalez C, Madrigal-Perez LA (2018) Saccharomyces cerevisiae exponential growth kinetics in batch culture to analyze respiratory and fermentative metabolism. J Visual Exp JoVE. https://doi.org/10.3791/58192
Vara-Ciruelos D, Dandapani M, Russell FM, Grzes KM, Atrih A, Foretz M et al (2019) Phenformin, but not metformin, delays development of T cell acute lymphoblastic leukemia/lymphoma via cell-autonomous AMPK activation. Cell Rep 27:690-698.e694. https://doi.org/10.1016/j.celrep.2019.03.067
González A, Hall MN, Lin SC, Hardie DG (2020) AMPK and TOR: The yin and yang of cellular nutrient sensing and growth control. Cell Metab 31:472–492. https://doi.org/10.1016/j.cmet.2020.01.015
Carlson M, Osmond BC, Botstein D (1981) Mutants of yeast defective in sucrose utilization. Genetics 98:25–40. https://doi.org/10.1093/genetics/98.1.25
Carrillo-Garmendia A, Martinez-Ortiz C, Martinez-Garfias JG, Suarez-Sandoval SE, González-Hernández JC, Nava GM et al (2022) Snf1p/Hxk2p/Mig1p pathway regulates hexose transporters transcript levels, affecting the exponential growth and mitochondrial respiration of Saccharomyces cerevisiae. Fungal Gene Biol : FG & B. 161:103701. https://doi.org/10.1016/j.fgb.2022.103701
Hsu JW, Chen KJ, Lee FJ (2015) Snf1/AMP-activated protein kinase activates Arf3p to promote invasive yeast growth via a non-canonical GEF domain. Nat Commun 6:7840. https://doi.org/10.1038/ncomms8840
Behroozaghdam M, Dehghani M, Zabolian A, Kamali D, Javanshir S, HasaniSadi F et al (2022) Resveratrol in breast cancer treatment: from cellular effects to molecular mechanisms of action. Cell Mole Life Sci : CMLS 79:539. https://doi.org/10.1007/s00018-022-04551-4
Wu XY, Zhai J, Huan XK, Xu WW, Tian J, Farhood B (2022) A systematic review of the therapeutic potential of resveratrol during colorectal cancer chemotherapy. Mini Rev Med Chem. https://doi.org/10.2174/1389557522666220907145153
Olivares-Marin IK, Gonzalez-Hernandez JC, Madrigal-Perez LA (2019) Resveratrol cytotoxicity is energy-dependent. J food Biochem 43:e13008. https://doi.org/10.1111/jfbc.13008
Ramos-Gomez M, Olivares-Marin IK, Canizal-Garcia M, Gonzalez-Hernandez JC, Nava GM, Madrigal-Perez LA (2017) Resveratrol induces mitochondrial dysfunction and decreases chronological life span of Saccharomyces cerevisiae in a glucose-dependent manner. J Bioenerg Biomembr 49:241–251. https://doi.org/10.1007/s10863-017-9709-9
Madrigal-Perez LA, Canizal-Garcia M, Gonzalez-Hernandez JC, Reynoso-Camacho R, Nava GM, Ramos-Gomez M (2016) Energy-dependent effects of resveratrol in Saccharomyces cerevisiae. Yeast 33:227–234. https://doi.org/10.1002/yea.3158
Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, Fogarty S et al (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11:554–565. https://doi.org/10.1016/j.cmet.2010.04.001
Kong W, Zhu H, Zheng S, Yin G, Yu P, Shan Y et al (2022) Larotrectinib induces autophagic cell death through AMPK/mTOR signalling in colon cancer. J Cell Mol Med. https://doi.org/10.1111/jcmm.17530
Bost F, Decoux-Poullot AG, Tanti JF, Clavel S (2016) Energy disruptors: rising stars in anticancer therapy? Oncogenesis 5:e188. https://doi.org/10.1038/oncsis.2015.46
Nicastro R, Tripodi F, Guzzi C, Reghellin V, Khoomrung S, Capusoni C et al (2015) Enhanced amino acid utilization sustains growth of cells lacking Snf1/AMPK. Biochem Biophys Acta 1853:1615–1625. https://doi.org/10.1016/j.bbamcr.2015.03.014
Orlova M, Ozcetin H, Barrett L, Kuchin S (2010) Roles of the Snf1-activating kinases during nitrogen limitation and pseudohyphal differentiation in Saccharomyces cerevisiae. Eukaryot Cell 9:208–214. https://doi.org/10.1128/ec.00216-09
Bonanno L, Zulato E, Pavan A, Attili I, Pasello G, Conte P, et al. (2019). LKB1 and tumor metabolism: the interplay of immune and angiogenic microenvironment in lung cancer. International journal of molecular sciences. 20. https://doi.org/10.3390/ijms20081874
Wang L, Yang X, Jiang HY, Song ZM, Lin X, Hu XP et al (2022) Protein kinases Elm1 and Sak1 of Saccharomyces cerevisiae exerted different functions under high-glucose and heat shock stresses. Appl Microbiol Biotechnol 106:2029–2042. https://doi.org/10.1007/s00253-022-11840-2
Yang X, Meng L, Lin X, Jiang HY, Hu XP, Li CF (2021) Role of Elm1, Tos3, and Sak1 protein kinases in the maltose metabolism of baker’s yeast. Front Microbiol 12:665261. https://doi.org/10.3389/fmicb.2021.665261
Moore JK, Chudalayandi P, Heil-Chapdelaine RA, Cooper JA (2010) The spindle position checkpoint is coordinated by the Elm1 kinase. J Cell Biol 191:493–503. https://doi.org/10.1083/jcb.201006092
Kang H, Tsygankov D, Lew DJ (2016) Sensing a bud in the yeast morphogenesis checkpoint: a role for Elm1. Mol Biol Cell 27:1764–1775. https://doi.org/10.1091/mbc.E16-01-0014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Responsible Editor: Rosane Freitas Schwan.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Correa-Romero, B.F., Olivares-Marin, I.K., Regalado-Gonzalez, C. et al. The role of the SNF1 signaling pathway in the growth of Saccharomyces cerevisiae in different carbon and nitrogen sources. Braz J Microbiol 54, 1083–1091 (2023). https://doi.org/10.1007/s42770-023-00954-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-023-00954-y