Abstract
Background
Microbes threaten human health in space exploration. Studies have shown that Proteus mirabilis has been found in human space habitats. In addition, the biological characteristics of P. mirabilis in space have been studied unconditionally. The simulated microgravity environment provides a platform for understanding the changes in the biological characteristics of P. mirabilis.
Objective
This study intends to explore the effect of simulated microgravity on P. mirabilis, the formation of P. mirabilis biofilm, and its related mechanism.
Methods
The strange deformable rods were cultured continuously for 14 days under microgravity simulated in high-aspect rotating vessels (HARVs). The morphology, growth rate, metabolism, and biofilm formation of the strain were measured, and the phenotypic changes of P. mirabilis were evaluated. Transcriptome sequencing was used to detect differentially expressed genes under simulated microgravity and compared with phenotype.
Results
The growth rate, metabolic ability, and biofilm forming ability of P. mirabilis were lower than those of normal gravity culture under the condition of simulated microgravity. Further analysis showed that the decrease of growth rate, metabolic ability, and biofilm forming ability may be caused by the downregulation of related genes (pstS, sodB, and fumC).
Conclusion
The simulated microgravity condition enables us to explore the potential relationship between bacterial phenotype and molecular biology, thus opening up a suitable and constructive method for medical fields that have not been explored before. It provides a certain strategy for the treatment of P. mirabilis infectious diseases in space environment by exploring the microgravity of P. mirabilis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Manned space technology is developing rapidly. Astronauts and cabin equipment inevitably bring microbes, including bacteria, fungi, and viruses, into space in the process of manned spaceflight. The space environment is characterized by a series of prominent features more than 100 km above the earth’s surface, including strong ionizing radiation, high vacuum, ultra-low temperature, ultra-high temperature, and weightlessness [1,2,3]. Various factors in the space environment can induce genetic changes of microorganisms, and then affect the phenotypic characteristics of microorganisms, including morphology, growth rate, biofilm forming ability, virulence, and drug resistance, which may induce infectious diseases and affect the health of resident personnel. As a consequence, it is of great significance to study the phenotypic characteristics of microorganisms in space environment.
Proteus mirabilis belongs to Proteus and widely exists in soil, water, and feces [4]. In addition, P. mirabilis is highly pathogenic and is one of the main causes of persistent and refractory urinary tract infections. Studies have shown that P. mirabilis has been tested for in space and post-flight astronaut samples in human habitats [5, 6]. The occurrence of infectious diseases is more likely to occur due to the decline of human immunity in the space environment. As a consequence, how to prevent and treat the infection caused by P. mirabilis in the space environment is worthy of attention. However, studies on the biological characteristics of P. mirabilis in the space environment have not been carried out due to concerns about the cost, time, and safety of spacecraft flight.
Microgravity environment refers to the environment in which the apparent weight of the system is much smaller than its actual weight under the action of gravity. In fact, it is the most important environmental factor encountered in the process of manned spaceflight. Microgravity simulation experiment has become an important and necessary preparation for the development of manned spaceflight. The biological characteristics of microorganisms under simulated microgravity have been widely studied in recent years. Some results show that under the simulated microgravity conditions, microorganisms show the same biological characteristics as the space environment. For example, it was found that the virulence of Salmonella typhimurium increased under simulated microgravity, which was consistent with that of Salmonella typhimurium under space microgravity. Further studies at the molecular level also show that RNA-binding protein Hfq is involved in the process of simulating the effects of microgravity and space environment. A simulated microgravimeter was used to observe the changes in biological characteristics and internal mechanism of P. mirabilis under microgravity. It can provide a strategy for the prevention and treatment of infectious diseases caused by P. mirabilis in space environment in the future.
Materials and methods
Bacterial strains and culture conditions
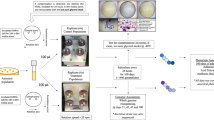
The whole study used P. mirabilis clinically separated from the urine of a male patient. The API20NE identification system (BioMèrieux, Craponne, France) and 16S rDNA gene sequencing were applied to identify the strains. The P. mirabilis strains grew aerobically in Luria-Bertani (LB) medium (with 0.5% agar) at 37 °C. The experimental (simulated microgravity, SMG) and the normal gravity (NG) control were set up in a high-aspect rotating vessel (HARV) bioreactor (Synthecon, Inc., Houston, TX, USA) and cultured continuously. Supplementary Figure 1 shows SMG culturing by rotating the bioreactor with the axis perpendicular to gravity, while the cultivation under NG is realized in case of its axis parallel to gravity [7]. P. mirabilis was inoculated in the HARV bioreactor with a dilution of 1:200. Each bioreactor was fully filled with fresh LB medium of ~55 ml, and the bubbles were removed carefully. After culturing in HARVs at 37 °C for 24 h at 25 rpm, the P. mirabilis cultures under SMG and NG were diluted and transferred into another HARVs fully filled with LB medium. P. mirabilis inoculation was cultured in the HARV bioreactors for 2 weeks. P. mirabilis cultured under SMG was called PMML strain, while that cultured under NG was called PMGL strain. The PMML and PMGL groups were serially diluted in phosphate-buffered saline (PBS), plated on LB agar, and counted. The cultures obtained were tested in various ways.
Phenotypic analysis
Scanning electron microscopy (SEM)
PMML and PMGL strains were cultured in LB medium, washed with sterile phosphate-buffered saline (PBS; pH 7.4), and fixed with 4% glutaraldehyde overnight. The samples were washed again with PBS, dehydrated in increasing grades of ethanol, and then critical pointdried. After coating the specimens with gold-palladium, they were observed using an FEI Quanta 200 scanning electron microscope (USA).
Growth rate assay
The Bioscreen C system and BioLink software (Lab Systems) were applied to monitor P. mirabilis growth. The overnight growing of P. mirabilis strains was made in LB medium at 37 °C. Twenty microliters of the overnight culture at a concentration of 106 CFU/ml was cultivated in Bioscreen C 96-well microtiter plates (Lab Systems). The plates containing fresh LB growth medium of 350 μl per well were incubated under continuous shaking at the maximum amplitude for 24 h at 37 °C. The OD630 was measured every 2 h. P. mirabilis growth experiments were performed in triplicate.
Carbon source utilization and chemical sensitivity assay
The biochemical features of two strains were tested by using the Biolog GENIII MicroPlate (Biolog, CA) and 94 phenotypic tests, including utilization of 71 carbon sources and assays of 23 chemical sensitivity. The overnight growing of P. mirabilis strains on agar plates was made at 37 °C. The centrifugal tube containing two strains was washed with PBS and inoculated into the IF-A inoculum (Biolog, CA). A turbidimeter was used to adjust the concentration of P. mirabilis suspension to 108 CFU/ml, and the suspension of 100 μl was inoculated into each well of the 96-well plate. After 24-h incubation at 37 °C, an automated Biolog microplate reader at 630 nm was used to measure the absorbance. The experiment was carried out in triplicate.
Biofilm assay
Crystal violet staining (a)
After being diluted (1:100) in 5-ml LB medium, the PMML and PMGL cultures were moved to glass tubes and incubated at 37 °C for 24 h at 200 rpm. The planktonic bacteria were removed. Soon afterwards, deionized water was used to wash each tube three or four times. Then, 0.1% of crystal violet dye was used to stain the glass tubes for 15 min at 37 °C. The experiment was carried out in triplicate.
Crystal violet staining (b)
After adding approximately 200 μl of the P. mirabilis suspension culture (1–5 × 107 CFU/ml) to 96-well microculture plates, the mixture was incubated at 37 °C for 24 h. PBS was used to wash the wells two times. In total, 200 μl of 0.1% crystal violet was employed to stain the cells (Sigma, St. Louis, MO, USA) for 30 min and washed with PBS. Next, the microculture plates were dried, and the dissolution of the stained biomass samples in 95% ethanol was made. The Thermo Multiskan Ascent Instrument (Thermo, USA) was applied to determine OD570 for each well. The experiments were carried out in triplicate.
Analysis of biofilm formation ability via confocal laser scanning microscopy (CLSM)
The two strains were cultured on LB medium overnight and inoculated in 35-mm confocal dishes (Solarbio, Beijing, China). The plates were incubated at 37 °C for 24 h and stained with a Filmtracer LIVE/DEAD Biofilm Viability Kit (Invitrogen, Carlsbad, CA, USA) in accordance with the manufacturer’s instructions. Three image stacks were obtained from each sample randomly by CLSM using a Leica TCS SP8 microscope with a Lecia TCS SP8 CSU imaging system. All the experiments were carried out in triplicate.
Transcriptomic sequencing and comparison
Sequencing and filtering
P. mirabilis cells were collected after 5-min centrifugation at 8000 × g at 4 °C. The RNeasy Protect Bacteria Mini Kit (Qiagen, Germany) was used to extract total RNA from the two strains according to the manufacturer’s instructions. The 10-min centrifugation of P. mirabilis was made at 10,000 × g at 4 °C. Chloroform added with the supernatant was mixed for 15 s. After being transferred into a tube filled with isopropanol, the upper aqueous phase was centrifuged at 13,600 × g for 20 min at 4 °C. After removing the supernatant, 3-min centrifugation of the mixture of precipitate and ethanol was made at 12,000 × g and 4 °C. Soon afterwards, the supernatant was discarded, and the 20-s centrifugation of the sample was made at 12,000 × g and 4 °C. After removing the residual liquid by air-drying, the dissolution of RNA pellet in RNase-free water was made. The purity of the samples is tested by NanoDrop™. Divalent cations were used to fragment the purified mRNAs into small pieces (~200 bp). The first strand of cDNA using reverse transcriptase and random primers was generated by applying RNA fragments. Following this, DNA polymerase I and RNase H were used to create the second strand of cDNA. The enrichment and quantification of cDNA fragments were made by using PCR amplification and Qubit 2.0, respectively. Lastly, the BGISEQ-500 was used to construct and sequence cDNA libraries. The SOAPnuke software (version 1.5.2) was applied to filter raw reads, and adapter reads and poly N reads were removed from the raw data to acquire clean reads. Clean reads were located to the reference genome by Bowtie2-2.2.3 [8]. Gene expression was quantified by HTSeq v0.6.1, and the calculation of the fragments per kilobase of transcript per million mapped reads (FPKM) of each gene was made according to the gene length. Lastly, the mapping of read counts to the genes was made. The DESeq R package (1.18.0) was employed to make differential gene expression analysis of the two strains. Through DESeq analysis, it is considered that genes yielding a p value of < 0.05 are differentially expressed. The KEGGseq R package was applied to analyze Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment of differentially expressed genes (DEGs). It was considered that DEGs with a p value of < 0.05 are statistically significant. KOBAS software was used to assess the statistical enrichment of DEGs in KEGG pathways.
Statistical analysis of gene expression values and DEGs
After mapping the clean reads to the reference strain by applying HISAT (version 2.0.4) and Bowtie2 (version 2.2.5) [9], the gene expression level with RSEM [8] was calculated. Cluster software (version 3.0) was applied to make cluster analysis of gene expression, and Java TreeView (3.0) [10] was used to visualize the results of cluster analysis.
Functional annotation and enrichment analysis
The genes from the reference sequences in the KEGG database were selected using the BLAST software to compare and annotate gene function. The gene expression profiles of different sample groups were analyzed using cluster analysis. The transcriptome data was analyzed by identifying DEGs according to the KEGG pathway. Gene enrichment analysis was performed using KEGG functional annotation. A p value of ≤ 0.05 indicated significant enrichment of DEGs.
Quantitative real-time PCR (qPCR)
Total RNA and random hexamer primers with SuperscriptIII reverse transcriptase (Invitrogen) using qPCR were employed to synthesize cDNA. The StepOnePLUS PCR system (Thermo, USA) with cDNA as the template was used to carry out the experiment in duplicate for each RNA sample. The related fold change of the target genes in the test and controlled RNA samples was determined. The 16S rRNA gene was regarded as an internal reference. The primer sequences used in this study are shown in Table 1.
Statistical analysis
The quantitative experiments were performed in triplicate and the data were represented as the mean ± standard deviation (S.D.). A statistical comparison of the data was conducted using two-tailed Student’s t-test. Statistical significance between the two groups was defined as p ≤ 0.05.
Results
Phenotypic characteristics
Electron microscopy findings
Scanning electron microscopy was performed to observe single-cell morphology in the PMML and PMGL strains. The results showed that the PMML had no changes in intercellular mucus and had smooth cell walls than the PMGL strains (Fig. 1).
Experimental setup of the high-aspect rotating vessel bioreactors. P. mirabilis cells in the HARV bioreactor are grown under the simulated microgravity (SMG) condition with its axis of rotation perpendicular to gravity or grown under the normal gravity (NG) with its axis of rotation vertical to gravity. The bioreactors are filled with LB medium and the bubbles are removed
Growth rate assay
A slight difference was observed between the two strains in terms of growth rate with time (Fig. 2, Table 2). When compared with PMGL, the PMML strain exhibited a decreased growth rate, especially after 14 h (p = 0.0095).
Chemical sensitivity and carbon source utilization assays
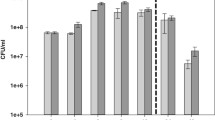
The chemical sensitivity assay showed decreased utilization of α-d-lactose and d-mannose (p = 0.0226 and p = 0.046, respectively) by PMML compared to that by PMGL (Fig. 3). It is speculated that P. mirabilis adapts to a new environment by changing the characteristics of its metabolism, as seen in the simulated microgravity environment. However, the mechanisms involved in this metabolic change are still unclear. Carbon source utilization assays showed that there were no significant differences between other metabolites and stress response pores. However, the Biolog experiment of this study was completed on ground, and not in an actual space environment and, therefore, requires further exploration.
Biofilm assay
The biofilm forming ability of P. mirabilis
The biofilm forming ability of P. mirabilis is considered a significant phenotype. This ability was analyzed in the PMML and PMGL cultures after a 2-week cultivation. The biofilms formed on the glass tubes were monitored by crystal violet staining (a). OD570 was measured after crystal violet staining (b). The adhered pellicles were further quantified using the OD570 values. PMGL showed a higher biofilm forming ability than PMML (p = 0.0022) (Fig. 4).
Chemical sensitivity assays of the two strains. The PMML and PMGL strains were incubated in a Biolog GENIII MicroPlate at 37 °C for 24 h in LB medium and tested for carbon source utilization and chemical sensitivity. The effect of simulated microgravity (SMG) on the expression of α-d-lactose (a) and d-mannose (b) is shown. Asterisk indicates significant difference at p < 0.05
Analysis of biofilm formation ability of P. mirabilis strain using CLSM
Biofilms of PMML and PMGL strains were cultured in 35-mm confocal dishes. Cells were stained with a Filmtracer LIVE/DEAD Biofilm Viability Kit, and biofilm formation ability was tested using confocal laser scanning microscopy (CLSM). Green fluorescence indicates live cells; red fluorescence indicates dead cells. “***” represents adjusted p value <0.01; “*”represents adjusted p value <0.05. (a) Analysis of biofilm formation ability of PMML strain using CLSM. (b) Analysis of biofilm formation ability of PMGL strain using CLSM. (c) Analysis of biofilm formation ability of PMML and PMGL strain using CLSM (Fig. 5; Supplementary Figure 2).
Analysis of biofilm formation ability via crystal violet staining. The PMML and PMGL strains were cultured in 96-well polystyrene microtiter plates at 37 °C for 24 h. Biofilm forming ability was measured. The biofilms formed on the glass tubes were analyzed using crystal violet staining (a) and quantified by measuring the absorbance of crystal violet at 570 nm (b)
RNA-Seq mapping and comparative genomic analysis
According to the functional analysis of the KEGG pathway, all DEG clusters were analyzed. Both upregulated and downregulated genes were identified. In PMML, approximately 12 genes were upregulated, and 17 genes were downregulated (Supplementary Figure 3).
We found that the downregulated genes outnumbered the upregulated genes, suggesting that gene metabolism expression was inhibited in PMML. Additionally, most of the DEGs were associated with amino acid metabolism, sugar metabolism, transport, and signal transduction (Supplementary Figure 4; Table 3). Three DEGs related to biofilm metabolism were identified in PMML, including PMI_RS12550 (pstS), PMI_RS06750 (sodB), and PMI_RS06255 (fumC). The above results were verified using qPCR.
mRNA expression levels of key genes related to biofilm formation
The mRNA expression levels of key genes related to biofilm formation were analyzed to determine the mechanisms of biofilm formation by PMML and PMGL. PMML exhibited decreased pstS, sodB, and fumC levels compared to PMGL (p = 0.025, p = 0.0056, and p = 0.004, respectively) (Fig. 6).
Discussion
P. mirabilis was exposed to simulated microgravity for 14 days using HARV. In addition, its phenotypic characteristics, including morphology, growth rate, metabolism, and biofilm formation ability, were analyzed. P. mirabilis cultured under the condition of SMG showed a decrease in growth rate, metabolic ability, and biofilm formation ability. Further analysis showed that the decrease of biofilm forming ability may be related to the downregulated expression of genes related to biofilm formation (pstS, sodB, and fumC). It is the first study of P. mirabilis in a SMG environment, providing a certain basis for the prevention and treatment of P. mirabilis infectious diseases in the future space environment.
Compared with PMGL, the proliferation of PMML was significantly slower than that of PMGL in the logarithmic growth phase. In addition, the growth rate of PMML decreased, which may be related to the downregulation of the genes involved and changes in metabolism and ribosomal structure. Previous studies have also observed changes in the same rate of growth [11]. In addition, Nicholson et al. [12] analyzed the growth rate of Bacillus subtilis spores on the O/OREOS spacecraft. The flying group of B. subtilis cells also showed a lower growth rate compared with the ground control experiment.
The regulation of bacterial metabolism may involve many aspects, including changes in growth rate, utilization of nutrients, and changes in metabolites in a complex environment. Studies have shown that α-d-lactose and d-mannose provide necessary energy support in the process of biofilm formation [13,14,15,16]. In this study, it was found that in the simulated microgravity environment, the utilization rate of two carbon sources (α-d-lactose and d-mannose) decreased. However, the decrease in the utilization rate of α-d-lactose and d-mannose may slow down the biofilm formation or reduce the intensity of biofilm formation. As a result, it further affected the biofilm formation and reduced the growth rate of P. mirabilis.
The biofilm formation in bacteria was first carried out under the action of microgravity in 2001 [17]. Three genes related to biofilm formation, including pstS, sodB, and fumC, were downregulated in SMG. In theory, pstS is a factor related to the ability of bacteria to form biofilm [18]. Some people think that the formation of bacterial biofilm may be induced by sodB [19]. Gabryszewski et al. confirmed the existence of fumC in biofilm and its ability to promote biofilm formation [20]. It has been found that pstS, sodB, and fumC may affect the growth rate of bacteria [21,22,23]. Studies have shown that the reduction of biofilm formation will affect the viability of bacteria and reduce the growth rate of bacteria [21]. A study reports that pstS is involved in the formation of biofilms [24]. The main genes involved in the formation of bacterial biofilm may be pstS [25, 26]. We believe that due to the stimulation of microgravity, the expression of pstS is downregulated, the biofilm forming ability is decreased, and the biofilm becomes thinner, which may affect the growth rate of P. mirabilis. Hassett et al. observed that sodB significantly affected the growth rate of Pseudomonas aeruginosa [22]. SodB is a factor related to biofilm formation, and the decrease of sodB expression will reduce the biofilm forming ability of bacteria [27]. In P. mirabilis, the environmental changes that inhibit the activity of sodB also lead to the weakening of biofilm, which may reduce the growth rate of P. mirabilis. Studies have shown that the growth rate of bacteria is affected by the expression of fumC gene [23]. Fumc is a kind of II enzyme, which is related to the formation of biofilm [28]. In our opinion, the decrease of fumC expression may weaken the formation of biofilm, resulting in a decrease in the growth rate of P. mirabilis. As a result, it is speculated that the decrease in the growth rate of P. mirabilis under SMG may be attributed to the downregulation of genes related to biofilm formation.
The growth of bacteria on the surface of microorganisms and the ability of biofilm formation under different growth conditions are affected by many factors, including the type of culture, cutting methods, and nutrient utilization. As a result, it showed that Pseudomonas aeruginosa showed increased biofilm formation and new structure formation during spaceflight [29]. In addition, studies have shown that spaceflight reduces the biofilm forming ability of the multidrug-resistant Acinetobacter baumannii [30]. Studies have shown that the uncertainty in the treatment of P. mirabilis is closely related to the intensity of biofilm formation [31]. Crystal violet staining and confocal scanning electron microscopy were used to detect the biofilm forming ability of P. mirabilis. Compared with PMGL, the biofilm forming ability of PMML decreased. For further analysis at the transcriptional level, a series of biofilm forming–related genes were detected in the SMG group. As a result, the decrease of biofilm formation ability of P. mirabilis in the SMG environment could be attributed to the downregulation of the expression of the above genes.
Conclusion
The study provided data showing a decline in growth rate, metabolism, and biofilm formation of P. mirabilis after 14 days of exposure to simulated microgravity. In addition, further analysis showed that the decrease of growth rate, metabolic ability, and biofilm forming ability may be due to the downregulation of biofilm formation and synthesis (pstS, sodB, and fumC) gene expression. The SMG condition enables us to explore the potential relationship between bacterial phenotype and molecular biology, thus opening up a suitable and constructive method for medical fields that have not been explored before. It provides a certain strategy for the treatment of P. mirabilis infectious diseases in space environment by exploring the microgravity of P. mirabilis.
References
Thirsk R, Kuipers A, Mukai C, Williams D (2009) The space-flight environment: the International Space Station and beyond. CMAJ 180(12):1216–1220. https://doi.org/10.1503/cmaj.081125
Gerald S (2005) The immune system in space, including Earth-based benefits of space based research. Curr Pharm Biotechnol 6(4):343–349. https://doi.org/10.2174/1389201054553699
Song B, Leff LG (2005) Identification and characterization of bacterial isolates from the Mir space station. Microbiol Res 60(2):111–117. https://doi.org/10.1016/j.micres
Branka B, Nataša F, Vesna EG, Marija K, Krešimir M, Iva Š, Josefa L, Jasmina V, Tomislav M, Gernot Z, Andrea G (2016) Emergence of multidrug-resistant Proteus mirabilis in a long-term care facility in Croatia. Wien Klin Wochenschr 128(11-12):404–413. https://doi.org/10.1007/s00508-016-1005-x
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331:1559–1564. https://doi.org/10.1126/science.1203543
Castro VA, Thrasher AN, Healy M, Ott CM, Pierson DL (2004) Microbial characterization during the early habitation of the International Space Station. Microb Ecol 47:119–126. https://doi.org/10.1007/s00248-003-1030-y
Nickerson CA, Ott CM, Mister SJ, Morrow BJ, Burns-Keliher L, Pierson DL (2000) Microgravity as a novel environmental signal affecting Salmonella enterica serovar Typhimurium virulence. Infect Immun 68(6):3147–3152. https://doi.org/10.1128/iai.68.6.3147-3152.2000
Langmead B, Salzberg SL (2012) Fast gapped-read alignment with Bowtie 2. Nat Methods 9(4):357–359. https://doi.org/10.1038/nmeth.1923
Li B, Dewey CN (2011) RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 4(12):323. https://doi.org/10.1186/1471-2105-12-323
Saldanha AJ (2004) Java Treeview--extensible visualization of microarray data. Bioinformatics 20(17):3246–3248. https://doi.org/10.1093/bioinformatics/bth349
Su L, Zhou L, Liu J, Cen Z, Wu C, Wang T, Zhou T, Guo T, Fang X, Wang J, Li T, Yin S, Dai S, Zhou P, Zhao J, Fang C, Yang R, Liu C (2014) Phenotypic, genomic, transcriptomic and proteomic changes in Bacillus cereus after a short-term space flight. Adv Space Res 53:18–29. https://doi.org/10.1016/j.asr.2013.08.001
Nicholson WL, Ricco AJ, Agasid E, Beasley C, Diazaguado M, Ehrenfreund P, Friedericks C, Ghassemieh S, Henschke M, Hines JW, Kitts C, Luzzi E, Ly D, Mai N, Mancinelli R, McIntyre M, Minelli G, Neumann M, Parra M, Piccini M, Rasay RM, Ricks R, Santos O, Schooley A, Squires D, Timucin L, Yost B, Young A (2011) The O/OREOS mission: first science data from the Space Environment Survivability of Living Organisms (SESLO) payload. Astrobiology 11:951–958. https://doi.org/10.1089/ast.2011.0714
Varela JA, Montini N, Scully D, Van der Ploeg R, Oreb M, Boles E, Hirota J, Akada R, Hoshida H, Morrissey JP (2017) Polymorphisms in the LAC12 gene explain lactose utilisation variability in Kluyveromyces marxianus strains. FEMS Yeast Res 17(3):1–13. https://doi.org/10.1093/femsyr/fox021
Zhang L, Xu Z, Patel BKC (2007) Bacillus decisifrondis sp. nov., isolated from soil underlying decaying leaf foliage. Int J Syst Evol Microbiol 57(Pt 5):974–978. https://doi.org/10.1099/ijs.0.64440-0
Collier WA, De Miranda JC (1955) Bacterien-Haemagglutination. Antonie Van Leeuwenhoek 21:133–140. https://doi.org/10.1007/bf02543806
De Graft-Hanson JA, Heath JL (1990) Effect of D-mannose on motility, presence of flagella, and attachment of Pseudomonas aeruginosa to chicken skin cells. Poult Sci 69(8):1404–1409. https://doi.org/10.3382/ps.0691404
McLean RJ, Cassanto JM, Barnes MB, Koo JH (2001) Bacterial biofilm formation under microgravity conditions. FEMS Microbiol Lett 195(2):115–119. https://doi.org/10.1111/j.1574-6968.200
Cabral MP, Soares NC, Aranda J, Parreira JR, Rumbo C, Poza M, Valle J, Calamia V, Lasa I, Bou G (2011) Proteomic and functional analyses reveal a unique lifestyle for Acinetobacter baumannii biofilms and a key role for histidine metabolism. J Proteome Res 10(8):3399–3417. https://doi.org/10.1021/pr101299j
DePas WH, Hufnagel DA, Lee JS, Blanco LP, Bernstein HC, Fisher ST, James GA, Stewart PS, Chapman MR (2013) Iron induces bimodal population development by Escherichia coli. Proc Natl Acad 110(7):2629–2634. https://doi.org/10.1073/pnas.1218703110
Gabryszewski SJ, Wong Fok Lung T, Annavajhala MK, Tomlinson KL, Riquelme SA, Khan IN, Noguera LP, Wickersham M, Zhao A, Mulenos AM, Peaper D, Koff JL, Uhlemann AC, Prince A (2019) Staphylococcus aureus metabolic adaptation in methicillin-resistant pneumonia. Am J Respir Cell Mol Biol 61(2):85–197. https://doi.org/10.1165/rcmb.2018-0389OC
Rogers AH, Pilowsky K, Zilm PS (1984) The effect of growth rate on the adhesion of the oral bacteria Streptococcus mutans and Streptococcus milleri. Arch Oral Biol 29(2):147–150. https://doi.org/10.1016/0003-9969(84)90119-5
Hassett DJ, Schweizer HP, Ohman DE (1995) Pseudomonas aeruginosa sodA and sodB mutans defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol 177(22):6330–6337. https://doi.org/10.1128/jb.177.22.6330-6337
Park SJ, Gunsalus RP (1995) Oxygen, iron, carbon, and superoxide control of the fumarase fumA and fumC genes of Escherichia coli: role of the arcA, fnr, and soxR gene products. Microb Pathog 177(21):6255–6262. https://doi.org/10.1128/jb.177.21
O’May GA, Jacobsen SM, Longwell M, Stoodley P, Mobley HLT, Shirtliff ME (2009) The high-affinity phosphate transporter Pst in Proteus mirabilis HI4320 and its importance in biofilm formation. Microbiology 155(Pt 5):1523–1535. https://doi.org/10.1099/mic.0.026500-0
Mudrak B, Tamayo R (2012) The Vibrio cholerae Pst2 phosphate transport system is upregulated in biofilms and contributes to biofilm-induced hyperinfectivity. Infect Immun 80(5):1794–1802. https://doi.org/10.1128/IAI.06277-11
Blus-Kadosh I, Zilka A, Yerushalmi G, Banin E (2013) The effect of pstS and phoB on quorum sensing and swarming motility in Pseudomonas aeruginosa. PLoS One 8(9):e74444. https://doi.org/10.1371/journal.pone.0074444
Chen Y, Wu F, Pang H, Tang J, Cai S, Jian J (2019) Superoxide dismutase B (sodB), an important virulence factor of Vibrio alginolyticus, contributes to antioxidative stress and its potential application for live attenuated vaccine. Fish Shellfish Immunol 89:354–360. https://doi.org/10.1016/j.fsi.2019.03.061
Woods SA, Schwartzbach SD, Guest JR (1988) Two biochemically distinct classes of fumarase in Escherichia coli. Biochim Biophys Acta 954(1):14–26. https://doi.org/10.1016/0167-4838(88)90050-7
Kim W, Tengra FK, Young Z, Shong J, Marchand N, Chan HK, Pangule RC, Parra M, Dordick JS, Plawsky JL, Collins CH (2013) Spaceflight promotes biofilm formation by Pseudomonas aeruginosa. PLoS One 8(4):e62437. https://doi.org/10.1371/journal.pone.0062437
Zhao X, Yu Y, Zhang X, Huang B, Bai P, Xu C, Li D, Zhang B, Liu C (2019) Decreased biofilm formation ability of Acinetobacter baumannii after spaceflight on China’s Shenzhou 11 spacecraft. MicrobiologyOpen 8(6):e00763. https://doi.org/10.1002/mbo3.763
Deva AK, Adams WP, Vickery K (2013) The role of bacterial biofilms in device-associate infection. Plast Reconstr Surg 132(5):1319–1328. https://doi.org/10.1097/PRS.0b013e3182a3c105
Funding
This work was supported by the Key Program of Logistics Research (BWS17J030).
Author information
Authors and Affiliations
Contributions
Dapeng Wang and Po Bai performed the experiments, analyzed the data, prepared figures and/or tables, and authored or reviewed drafts of the manuscript. Bin Zhang and Xiaolei Su performed the experiments and contributed reagents/materials/analysis tools. Xuege Jiang, Tingzheng Fang, and Li Xu performed the experiments and prepared figures and/or tables. Junfeng Wang conceived and designed the experiments, authored or reviewed drafts of the manuscript, and approved the final manuscript draft. Changting Liu conceived and designed the experiments, authored or reviewed drafts of the manuscript, and approved the final manuscript draft.
Corresponding authors
Ethics declarations
Ethics approval
The study was approved by the institutional review board (CWO) of the Chinese PLA General Hospital, Beijing, China (ethical number: S2019-327-01).
Conflict of interest
The authors declare no competing interests.
Additional information
Responsible Editor: Jorge Luiz Mello Sampaio.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Bai, P., Zhang, B. et al. Decreased biofilm formation in Proteus mirabilis after short-term exposure to a simulated microgravity environment. Braz J Microbiol 52, 2021–2030 (2021). https://doi.org/10.1007/s42770-021-00588-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-021-00588-y