Abstract
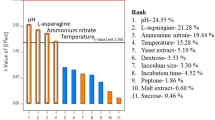
L-asparaginase has been used in the remission of malignant neoplasms such as acute lymphoblastic leukemia. The search for new sources of this enzyme has become attractive for therapeutics. Traditional methods for biomolecule purification involve several steps. A two-phase system may be a good strategy to anticipate one of these stages. This study aimed to produce and purify a fungal L-asparaginase through an aqueous two-phase micellar system (ATPMS) using Triton X-114. The fungus Penicillium sp.–encoded 2DSST1 was isolated from Cerrado soil. Plackett-Burman design followed by a 24 full factorial design was used to determine the best conditions to produce L-asparaginase. The evaluated variables were L-asparagine, L-proline, wheat bran, potato dextrose broth, ammonium sulfate, yeast extract, sucrose and glucose concentrations, incubation temperature, incubation period, and initial pH of the culture medium. L-asparaginase quantification was valued by the formation of β-aspartyl hydroxamate. The significant positive variables, L-asparagine, L-proline, potato dextrose broth, and sucrose concentrations, were evaluated at 2 levels (+ 1 and − 1) with triplicate of the central point. After 34 runs, maximum activity (2.33 IU/mL) was achieved at the factorial design central point. A central composite design was performed in ATPMS at two levels (+ 1 and − 1) varying Triton X-114 concentration (w/v), separation phase temperature, and crude extract concentration (w/v). The L-asparaginase partition coefficient (K) was considered the experimental design response. Out of the 16 systems that were examined, the most promising presented a purification factor of 1.4 and a yield of 100%.


Similar content being viewed by others
References
Lopes AM, de Oliveira-Nascimento L, Ribeiro A, Tairum CA Jr, Breyer CA, de Oliveira MA, Monteiro G, de Souza-Motta CM, de Oliveira Magalhães P, Avendaño JGF (2017) Therapeutic l-asparaginase: upstream, downstream and beyond. Crit Rev Biotechnol 37(1):82–99
Kebriaei P, Anastasi J, Larson RA (2002) Acute lymphoblastic leukaemia: diagnosis and classification. Best Pract Res Clin Haematol 15(4):597–621
Onciu M (2009) Acute lymphoblastic leukemia. Hematol Oncol Clin North Am 23(4):655–674
Moghrabi A, Levy DE, Asselin B, Barr R, Clavell L, Hurwitz C, Samson Y, Schorin M, Dalton VK, Lipshultz SE (2007) Results of the Dana-Farber Cancer Institute ALL Consortium Protocol 95-01 for children with acute lymphoblastic leukemia. Blood 109(3):896–904
Quintanilla-Flores DL, Flores-Caballero MÁ, Rodríguez-Gutiérrez R, Tamez-Pérez HE, González-González JG (2014) Acute pancreatitis and diabetic ketoacidosis following L-asparaginase/prednisone therapy in acute lymphoblastic leukemia. Case Rep Oncol Med 2014:139169. https://doi.org/10.1155/2014/139169
Egler RA, Ahuja SP, Matloub Y (2016) L-asparaginase in the treatment of patients with acute lymphoblastic leukemia. J Pharmacol Pharmacother 7(2):62–71
Pieters R, Hunger SP, Boos J, Rizzari C, Silverman L, Baruchel A, Goekbuget N, Schrappe M, Pui CH (2011) L-asparaginase treatment in acute lymphoblastic leukemia: a focus on Erwinia asparaginase. Cancer 117(2):238–249
Torres-Obreque K, Meneguetti GP, Custódio D, Monteiro G, Pessoa-Junior A, Rangel-Yagui CO (2018) Production of a novel N-terminal PEGylated crisantaspase. Biotechnol Appl Biochem 66(3):281–289. https://doi.org/10.1002/bab.1723
Chan WK, Lorenzi PL, Anishkin A, Purwaha P, Rogers DM, Sukharev S, Rempe SB, Weinstein JN (2014) The glutaminase activity of L-asparaginase is not required for anticancer activity against ASNS-negative cells. Blood 123(23):3596–3606
Warrell RP, Chou T-C, Gordon C, Tan C, Roberts J, Sternberg SS, Philips FS, Young CW (1980) Phase I evaluation of succinylated Acinetobacter glutaminase-asparaginase in adults. Cancer Res 40(12):4546–4551
Mazzola PG, Lopes AM, Hasmann FA, Jozala AF, Penna TC, Magalhaes PO, Rangel-Yagui CO, Pessoa A Jr (2008) Liquid–liquid extraction of biomolecules: an overview and update of the main techniques. J Chem Technol Biotechnol 83(2):143–157
Nadar SS, Pawar RG, Rathod VK (2017) Recent advances in enzyme extraction strategies: a comprehensive review. Int J Biol Macromol 101:931–957
Malpiedi LP, Nerli BB, Abdala DS, de Alcântara Pessôa-Filho P, Pessoa A Jr (2014) Aqueous micellar systems containing Triton X-114 and Pichia pastoris fermentation supernatant: a novel alternative for single chain-antibody fragment purification. Sep Purif Technol 132:295–301
Chuo SC, Mohd-Setapar SH, Mohamad-Aziz SN, Starov VM (2014) A new method of extraction of amoxicillin using mixed reverse micelles. Colloids Surf A Physicochem Eng Asp 460:137–144
Liu Y, Sun M-H, Shao S-K, Deng G (2017) An affinity-based aqueous two-phase mixed micellar system and its purification of Yeast 3′, 5′-bisphosphate nucleotidase. J Chromatogr B 1060:215–220
Liu CL, Nikas Y, Blankschtein D (1996) Novel bioseparations using two-phase aqueous micellar systems. Biotechnol Bioeng 52(2):185–192
Shiomori K, Ebuchi N, Kawano Y, Kuboi R, Komasawa I (1998) Extraction characteristic of bovine serum albumin using sodium bis (2-ethylhexyl) sulfosuccinate reverse micelles. J Ferment Bioeng 86(6):581–587
Rundlett KL, Armstrong DW (1995) Effect of micelles and mixed micelles on efficiency and selectivity of antibiotic-based capillary electrophoretic enantioseparations. Anal Chem 67(13):2088–2095
Soto A, Arce A, Khoshkbarchi MK (2005) Partitioning of antibiotics in a two-liquid phase system formed by water and a room temperature ionic liquid. Sep Purif Technol 44(3):242–246
Rangel-Yagui C, Pessoa-Jr A, Blankschtein D (2004) Two-phase aqueous micellar systems: an alternative method for protein purification. Braz J Chem Eng 21(4):531–544
Rangel-Yagui CO, Lam H, Kamei DT, Wang DI, Pessoa A Jr, Blankschtein D (2003) Glucose-6-phosphate dehydrogenase partitioning in two-phase aqueous mixed (nonionic/cationic) micellar systems. Biotechnol Bioeng 82(4):445–456
Saitoh T, Hinze WL (1991) Concentration of hydrophobic organic compounds and extraction of protein using alkylammoniosulfate zwitterionic surfactant mediated phase separations (cloud point extractions). Anal Chem 63(21):2520–2525
Drainas C, Kinghorn J, Pateman J (1977) Aspartic hydroxamate resistance and asparaginase regulation in the fungus Aspergillus nidulans. Microbiology 98(2):493–501
Van Der Meeren P, Cocquyt J, Flores S, Demeyere H, Declercq M (2002) Quantifying wetting and wicking phenomena in cotton terry as affected by fabric conditioner treatment. Text Res J 72(5):423–428
Imada A, Igarasi S, Nakahama K, Isono M (1973) Asparaginase and glutaminase activities of micro-organisms. Microbiology 76(1):85–99
Badoei-Dalfard A (2015) Purification and characterization of l-asparaginase from Pseudomonas aeruginosa strain SN004: production optimization by statistical methods. Biocatalysis Agric Biotechnol 4(3):388–397
Sindhu R, Manonmani H (2018) Expression and characterization of recombinant l-asparaginase from Pseudomonas fluorescens. Protein Expr Purif 143:83–91
Souza PM, de Freitas MM, Cardoso SL, Pessoa A, Guerra ENS, Magalhaes PO (2017) Optimization and purification of L-asparaginase from fungi: a systematic review. Crit Rev Oncol Hematol 120:194–202
Baskar G, Renganathan S (2012) Optimization of L-asparaginase production by Aspergillus terreus MTCC 1782 using response surface methodology and artificial neural network-linked genetic algorithm. Asia Pac J Chem Eng 7(2):212–220
Dias FF, Sato HH (2016) Sequential optimization strategy for maximum l-asparaginase production from Aspergillus oryzae CCT 3940. Biocatalysis Agric Biotechnol 6:33–39
Farag AM, Hassan SW, Beltagy EA, El-Shenawy MA (2015) Optimization of production of anti-tumor l-asparaginase by free and immobilized marine Aspergillus terreus. Egypt J Aquat Res 41(4):295–302
Gurunathan B, Sahadevan R (2012) Optimization of culture conditions and bench-scale production of L-asparaginase by submerged fermentation of Aspergillus terreus MTCC 1782. J Microbiol Biotechnol 22(7):923–929
Huang L, Liu Y, Sun Y, Yan Q, Jiang Z (2014) Biochemical characterization of a novel L-Asparaginase with low glutaminase activity from Rhizomucor miehei and its application in food safety and leukemia treatment. Appl Environ Microbiol 80(5):1561–1569
Duarte AWF, Lopes AM, Molino JVD, Pessoa A, Sette LD (2015) Liquid–liquid extraction of lipase produced by psychrotrophic yeast Leucosporidium scottii L117 using aqueous two-phase systems. Sep Purif Technol 156:215–225
Spir LG, Ataide JA, De Lencastre Novaes LC, De Borba GD, Moriel P, Silveira E, Pessoa A Jr, Tambourgi EB, Mazzola PG (2015) Application of an aqueous two-phase micellar system to extract bromelain from pineapple (Ananas comosus) peel waste and analysis of bromelain stability in cosmetic formulations. Biotechnol Prog 31(4):937–945
Jaramillo PMD, Gomes HAR, de Siqueira FG, Homem-de-Mello M, Ferreira Filho EX, Magalhães PO (2013) Liquid–liquid extraction of pectinase produced by Aspergillus oryzae using aqueous two-phase micellar system. Sep Purif Technol 120:452–457
de Freitas MM, Souza PM, Cruvinel K, Barros T, Santos SN, Long PF, Pessoa A, Magalhães PO (2019) Interferences that impact measuring optimal l-asparaginase activity and consequent errors interpreting these data. Appl Microbiol Biotechnol 103(13):5161–5166
Drainas D, Drainas C (1985) A conductimetric method for assaying asparaginase activity in Aspergillus nidulans. Eur J Biochem 151(3):591–593
Acknowledgments
This work was carried out with the financial support of the State Funding Agency of Distrito Federal (FAPDF), National Council for Scientific and Technological Development (CNPq), Coordination of Superior Level Staff Improvement (CAPES), and University of Brasilia.
Funding
This work was supported by the State Funding Agency of Distrito Federal (FAPDF) – process number 193.001.661/2017 and the São Paulo Research Fundation (FAPESP) – process number: 13/08617-7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Solange I. Mussatto
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cardoso, S.L., de Freitas, M.M., de Souza, P.M. et al. Optimization of aqueous two-phase micellar system for partial purification of L-asparaginase from Penicillium sp. grown in wheat bran as agro-industrial residue. Braz J Microbiol 51, 979–988 (2020). https://doi.org/10.1007/s42770-020-00269-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42770-020-00269-2