Abstract
Gasification of biomass produces a syngas containing trace amounts of viscous hydrocarbon tar, which causes serious problems in downstream pipelines, valves and processing equipment. This study focuses on the use of tire-derived pyrolysis char for tar conversion using biomass tar model compounds representative of tar. The catalytic decomposition of tar model compounds, including methylnaphthalene, furfural, phenol, and toluene, over tire char was investigated using a fixed bed reactor at a bed temperature of 700 °C and 60 min time on stream. The influence of temperature, reaction time, porous texture, and acidity of the tire char was investigated with the use of methylnaphthalene as the tar model compound. Oxygenated tar model compounds were found to have higher conversion than those containing a single or multi-aromatic ring. The reactivity of tar compounds followed the order of furfural > phenol > toluene > methylnaphthalene. The conversion of the model compounds in the presence of the tire char was much higher than tar thermal cracking. Gas production increased dramatically with the introduction of tire char. The H2 potential for the studied tar model compounds was found to be in the range of 40%–50%. The activity of tire char for naphthalene removal was compared with two commercial activated carbons possessing a very well-developed porous texture. The results suggest that the influence of Brunauer-Emmett-Teller surface area of the carbon on tar cracking is negligible compared with the mineral content in the carbon samples.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Biomass is a key renewable energy feedstock that responds to the vital environmental and societal need for a step change in the sustainability of energy production required to combat climate change. Gasification of biomass represents a major sustainable route to produce syngas from a source that is renewable and CO2-neutral. Gasification thermochemically converts the biomass waste to syngas composed of hydrogen and carbon monoxide. The product gas can potentially be used in furnaces, boilers, gas engines, gas turbines, and fuel cells. A further advantage of biomass gasification is that a range of biomass feedstocks can be used, including agricultural residues, forestry residues, byproducts from biorefineries, byproducts from the food industry such as brewery wastes and municipal solid waste. However, a major issue in the gasification process is the production of byproduct tar, for example, several recent review articles by Saleem et al. [1], Zhang et al. [2], and Rios et al. [3] have reported biomass gasification and tar removal technologies and highlighted the critical problematic issue of the presence of tar in syngas.
Tar is a viscous, complex mixture of high molecular weight organic material that causes serious operational issues with downstream syngas utilisation, such as plugging, fouling, and corrosion in fuel lines, filters, engine nozzles and turbines. Gasification tars tend to be refractory, i.e., very unreactive, and are difficult to remove by thermal, or physical processes. Condensed tars can also undergo polymerization to form more complex compounds and can also interact with particulates causing difficulty in particle removal systems [4]. These problems can result in high operational costs and plant shut-down. In addition, the tars contain a range of polycyclic aromatic hydrocarbons, some of which have shown to be carcinogenic [5]. Typical values for tar in syngas range from 2000 mg m−3 to 100,000 mg m−3. However, the quality requirement for syngas to be used in internal combustion engines is for a tar content in syngas to be <100 mg m−3; for gas turbines, the allowable tar content in syngas is <5 mg m−3; and for fuel cells, the tar content must be below 1 mg m−3 [6].
The pyrolysis of biomass and waste materials produces an oil, char and gas product [7]. Char is considered an ideal material for tar adsorption or catalytic cracking due to its unique properties, including its porous structure and specific surface area, as well as potentially the presence of transition metal species and oxygen functional groups on its surface. For example, pyrolysis chars and activated carbons have been investigated for the catalytic decomposition of tar and for tar reforming in several recent studies [8,9,10,11,12,13]. The catalytic activity of carbonaceous materials for tar cracking depends on many factors, such as the surface area and porosity and the surface chemistry of the chars, such as the surface functional groups on the carbon and on the nature of tar compounds [14, 15]. Feng et al. [16] used a two-stage fluidized bed/fixed bed reactor to examine the removal of in situ tar in the product gas using cornstalk biochar, rice husk biochar, and sawdust, and their catalytic activity was found to be 59%, 60%, and 66%, respectively. The high activity of sawdust biochar was attributed to its developed porous texture. Ravenni et al. [17] examined the performance of wood-derived chars for the reforming of toluene and naphthalene in the temperature range of 250–800 °C, and naphthalene was found to be cracked more extensively than toluene. Choi et al. [18] investigated the use of lignite-derived char for the decomposition of benzene at 900 °C and concluded that the char surface not only converted the benzene into carbon deposits but also to diaromatic and higher molecular weight aromatic compounds.
The composition of tar depends on the gasification temperature, with higher temperatures, above 750 °C, producing higher molecular weight compounds that are more condensable and cause the problematic blocking and fouling issues associated with tars in syngas. The composition of tar produced from the gasification of biomass at 750 °C has been reported to contain aromatic hydrocarbons such as phenol, cresol, indene, naphthalene, and methylnaphthalenes [19, 20]. Zhang et al. [21] also identified a wide range of 1–4 ring aromatic hydrocarbons in biomass derived gasification tar.
Because of the complexity of tar streams derived from biomass gasification, most studies use tar model compounds to study the reaction mechanism. For example, Coll et al. [22] investigated the steam reforming of five tar model compounds with commercial nickel catalysts and found the following order of reactivity: benzene > toluene > anthracene > pyrene > naphthalene. In another study conducted by Burhenne and Aicher [23], the decomposition of benzene as a tar model compound on different wood char samples and commercial activated carbon using a fixed bed reactor was investigated at high temperatures (850–1050 °C). The non-activated chars were found to exhibit a low activity, as the benzene conversion was almost similar to the experiments performed in the absence of the carbon sample. In contrast, char activated with CO2 exhibited a high benzene removal of about 100% at the start of the experiment, however, after 10 min of reaction time, it dropped to 25% due to the deactivation of the char caused by coking. In contrast, the commercial activated carbon was found to produce a higher benzene removal and the conversion of 50% was stable during a 2-h experimental time, which was attributed to the higher amount of mesopores in the activated carbon. Similar results were observed by Moliner et al. [24], who concluded that mesoporous carbon provided a more stable decomposition of methane compared to microporous carbon, which was found to deactivate rapidly. In contrast, Park et al. [25] reported a similar tar cracking behaviour for the investigated char samples despite the difference in the porous texture and mineral contents. The nature of the tar model compound has also been found to influence the char activity for tar decomposition [26]. Naphthalene is one of the most refractory compounds among the aromatic compounds [27] and represents the major fraction of two-ring aromatic compounds found in gasification tars [19], therefore, it is commonly used as a model tar compound [28]. In an investigation into the thermal decomposition of different tar model compounds, Jess [28] studied the thermal conversion of toluene, naphthalene and benzene in the presence of hydrogen and steam in a tubular flow reactor at temperatures of 700–1400 °C and residence times of 0.3–2 s. The reaction activity was found to follow the order of toluene > naphthalene > benzene. In another study conducted by Fuentes-Cano et al. [26], catalytic conversion of both toluene and naphthalene over chars produced from coal, coconut and sewage sludge was examined in a fixed bed reactor with the presence of 15 vol.% steam. Despite the difference in porous texture of the studied char materials, the tar conversion was found to be similar for the three types of chars. The initial conversion of naphthalene and toluene has been reported to be around 60% and 80%, respectively, however, as the reaction proceeds, a constant deactivation of char was observed, and after a 60-min reaction time, the conversion for both tar model compounds decreased to about 40%.
The use of tire char for the reduction of tar model compounds has not been studied to any great extent. The product yield from the pyrolysis of waste tires can be very variable and depends on the process conditions, particularly temperature and heating rate, and the type of pyrolysis reactor used, but an average of yields from 25 different process systems gave yields of 45.3%, 36.7%, and 15.4% in weight for oil, char, and gas, respectively [7]. The carbonaceous char represents a major product of the tire pyrolysis process and may be considered as a low-cost catalyst material for applications such as a catalyst for tar cracking. The char derived from waste tires also has the added advantage of having a high content of metals and, as such, has the potential for catalytic cracking of tar components. The metal content of tire char is derived from the metals added to the tire to improve the properties of the product tire or to aid the manufacturing process. For example, tires contain high concentrations of Zn (zinc oxide), added to improve the rubber vulcanisation process, and Ca (clay) and Si (silica) as filler material [7]. These metals become concentrated in the residual char during the pyrolysis process due to loss of volatiles, resulting in ash contents in some cases of more than 10 wt.% and with a high metal content. There has been some limited work on the use of tire-derived chars as catalysts for cracking tar model compounds. For example, Husar et al. [31], investigated the use of tire char activated at three different activation temperatures as a tar cracking catalyst with the use of toluene as model tar compound. Tire char activated at a temperature of 900 °C and with the highest specific surface area (46.5 m2 g−1) exhibited the highest toluene conversion with 92% compared to char with a surface area of 43.4 m2 g−1, which achieved a conversion of 89%. Suhaj et al. [32] studied the use of tire pyrolysis char as a tar cracking catalyst during the gasification of refuse derived fuel, and the results showed that using the catalyst resulted in an increase in H2 concentration due to the reduction of tar. Char catalytic activity can be enhanced by impregnation with metals. Irfan et al. [33] reported a decrease in tar content from 9% (without catalyst) to 2.15% with the use of Ni supported on tire char, resulting in the conversion of heavy tar compounds into smaller tar compounds. The decrease in tar content was accompanied by an increase in gas yield.
We have previously shown that tire char is effective for the cracking of biomass pyrolysis volatiles using char at temperatures above 600 °C [29]. The tire char was able to reduce the condensable bio-oil/tar by approximately 70%. The tar reduction was attributed to the catalytic cracking of the tar volatiles and to physical adsorption onto the char. In a later report [30], we showed that tire-derived pyrolysis char could be used to both catalyse the cracking of biomass pyrolysis volatiles and catalyse the steam reforming of the volatiles to produce a syngas high in hydrogen content. Furthermore, the tire char also participated in producing hydrogen-rich syngas from the steam gasification of the char. Thereby, the tire char acted as a catalyst in enhancing the tar reforming, water gas shift and char-steam reactions.
Based on the observations obtained in our previous papers, we suggested that the catalytic activity of tire char for tar removal is influenced by the textural properties and surface chemistry of the tire char. To expand on this work and due to the complexity of tar composition, tar model compounds were used to simulate typical biomass tar compounds produced during biomass gasification. In this work, we extend our previous work by investigating the use of tire-derived pyrolysis char in relation to biomass bio-oil/tar reduction but specifically the use of model biomass tar compounds. Biomass bio-oil/tar model compounds comprising, phenol, furfural, toluene and methylnaphthalene were used in a two-stage vaporisation-cracking reactor system. Generally, benzene, phenol, toluene and naphthalene are commonly used as tar model compounds, which represent the main types of biomass gasification tar. These tar model compounds were chosen for the purpose to investigate how the structure of the aromatic molecule affects tar conversion and the char catalytic activity individually for each aromatic compound. The model compounds were vaporised at the 1st stage, and tire pyrolysis char was used in the 2nd stage reactor for catalytic cracking. Several process parameters were investigated, including char temperature, reaction time, char surface area and porosity and char surface chemistry in relation to the reduction in bio-oil/tar.
Materials and methods
Materials
Four model biomass tar compounds were used consisting of phenol, furfural, toluene and methylnaphthalene and were obtained from Sigma-Aldrich UK. Table 1 shows the characteristics of the model compounds, including their tar class designation. Tar compounds may be grouped based on a classification system in which the tar compounds are classified into five classes depending on the number of aromatic rings and their molecular weight [34, 35]. As naphthalene is one of the main tar components produced during the gasification of biomass, further experiments were carried out with methylnaphthalene as the tar model compound. The tar model compounds were dissolved in methanol at a 1:1 molar ratio to enable injection of the model compound solution into the vaporisation reactor. The effect of temperature, reaction time, the porous texture of the tire char, and the acidity of carbon on tar conversion was evaluated.
Tire pyrolysis char was used as a catalyst for cracking tar model compounds. Tire char was prepared using a fixed bed reactor at a temperature of 800 °C. Details of the production of tire char are reported in detail elsewhere [29] but briefly described here. The waste tire (10 g) was pyrolysed in a fixed bed nitrogen purged reactor heated at 10 °C min−1 to a final temperature of 800 °C and held at that temperature for one hour. The residual tire pyrolysis char was collected at the end of the experiment, weighed, ground and sieved to produce a particle size between 1.5 mm and 2.0 mm and dried overnight at a temperature of 105 ºC. The tire pyrolysis experiments were repeated several times to enable sufficient char to be produced for the experimental programme and to ensure the uniformity of the product tire char as much as possible. Table 2 shows the product yield and mass balance experiments carried out to determine the repeatability of the tire pyrolysis process for the production of tire char. The produced batch of tire chars was mixed thoroughly for the subsequent biomass tar model compound work. The characteristics of the product tire char in terms of proximate, ultimate and ash metal content are shown in Table 3. Proximate analysis of the char samples was determined by thermogravimetric analysis (TGA) Shimadzu TGA-50H (Shimadzu UK, Ltd., Milton Keynes, UK). Elemental analysis was performed using a CE Instruments CE Instruments Flash EA2000 (CE Instruments Ltd., Wigan, UK). The metals present in the ash were determined by ashing the tire char, followed by dissolution of the ash in concentrated nitric acid at 240 °C and analysis using a Varian Instruments UK, fast sequential atomic absorption spectrophotometer Varian AA240FS (Varian Medical Systems Ltd., Crawley, UK).
Two-stage vaporisation-tar cracking reactor system
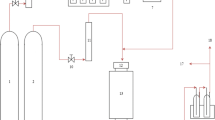
The effectiveness of tire char for the decomposition of the model biomass tar compounds was investigated using a two-stage fixed bed reactor, and a schematic diagram of the reactor is shown in Fig. 1. The reactor was constructed of stainless steel and comprised a first stage reactor of 25 mm diameter and a length of 160 mm used to vaporise the model compounds. The second stage was also 25 mm in diameter and 160 mm in length and contained tire char. The vaporisation reactor was held at a temperature of 250 °C, and the tire char (2 g) located in the tar cracking reactor was held at a temperature of 700 °C.
The tar model compounds dissolved in methanol (1:1 molar ratio) were fed continuously into the vaporisation reactor to vaporize the tar model compounds before entering the second reactor where the decomposition reactions with the tire char occurred. The tar compounds were fed into the reactor with a flow rate of 3.8 mL h−1 using a syringe pump and the total experimental time for injection was 60 min. Nitrogen was used as the carrier gas with a continuous flow rate of 90 mL min−1. The volatile gases exiting the reactor were passed to a series of dry ice condensers where the unconverted model compounds were collected. The amount of unconverted reactant was calculated by weighing both condensers and injection syringes before and after the experiments. The product gases were collected in a Tedler™ gas sample bag and later analysed using packed column gas chromatography.
Product analysis
The gases collected in the gas sample bag were analysed for permanent and hydrocarbons. Permanent gases (N2, H2, CO, CO2) were analysed using a Varian CP-3380 gas chromatograph with a thermal conductivity detector (GC/TCD) equipped with a 2 m long × 2 mm diameter column packed with 60–80 mesh molecular sieve. Argon was used as the carrier gas. Nitrogen, the continuous purge gas used in the experiments was determined, and the volumetric flow rates of the evolved product gases were calculated by comparison with the N2 flow rate. Carbon dioxide was analysed by a second Varian CP-3380 (GC/TCD) on a 2 m length by 2 mm diameter column and packed with a HayeSep 80–100 molecular mesh, with argon as the carrier gas. Hydrocarbon gases from C1 to C4 were analysed using a third Varian CP-3380 GC with a flame ionisation detector (FID). The column used was 2 m long with 2 mm diameter packed with 80–100 mesh HayeSep with nitrogen carrier gas. The mass of each gas produced and hence the total mass of gas was calculated based on the known flow rates and the molecular mass of each gas.
To quantify the products of the cracking process, carbon conversion and product yields were calculated. The carbon conversion was defined as the moles of carbon in the gaseous products divided by the moles of carbon fed. The total amount of moles of carbon in the gas was calculated from the moles of CO, CO2 and C1–C4 hydrocarbons formed during the reaction which were determined from the GC analyses. The moles of carbon in the feed were calculated form the total amount of model compound fed into the reactor.
The yield of gaseous species was calculated as follows:
The hydrogen yield potential is defined as the sum of the measured hydrogen and the hydrogen that could theoretically be formed by completely shifting carbon monoxide. Hydrogen yield potential was calculated as the ratio between the concentration of H2 in the effluent gas and the maximum allowed by stoichiometry:
Char characterization
Porous texture
The physical properties of the carbonaceous materials used, including BET surface area and porosity were determined by the adsorption of N2 at 77 K using a Micromeritics Tristar 3000 (Micromeritics Instruments Corp., London, UK). The apparatus measures the quantity of nitrogen adsorbed onto or desorbed from a solid sample at different equilibrium vapour pressures. Prior to the analysis, the samples (0.1 g) were degassed under Nitrogen for 3 h at 250 °C. The Micropore and Mesopore volumes were determined by the Dubinin Radushkevich (DR) and Barret-Joyner-Halenda (BJH) methods, respectively.
TGA
TGA of tire char was performed using a Stanton Redcroft thermogravimetric analyser (Stanton Redcroft Ltd., East Grinstead, UK) interfaced to a Nicolet Magna IR-560 Fourier-transform infra-red Nicolet Magna IR-560 Fourier-transform infra-red spectrometer (ThermoFisher Scientific Ltd., Loughborough, UK). About 28 mg of sample was heated from 25 °C to 900 °C with a heating rate of 20 °C min−1 using nitrogen as the carrier gas and hooding time of 30 min at the final temperature. The sample weight loss, together with time and temperature and CO, CO2 detected by FTIR were continuously monitored.
Acidic groups
The Boehm titration method was used to determine the acidic oxygen groups of the treated activated carbon. The acidic sites were determined by mixing 1 g of activated carbon with 50 mL of 0.05 mol/L sodium hydroxide (NaOH) in a closed flask and shaken for 24 h. The solution was then filtered. An aliquot of 5 mL was taken and titrated with 0.05 mol/L hydrochloric acid (HCl). The amounts of acidic groups were calculated as
Results and discussion
Catalytic activity of tire char for cracking tar model compounds
The effectiveness of tire char for cracking the model biomass tar compounds at a temperature of 700 °C in terms of aromatic model compound carbon conversion is displayed in Fig. 2. In addition, blank experiments were carried out with each tar model compound, where sand was used as an inert bed material. This was to assess the influence of thermal cracking. The conversion was calculated from the carbon contained in the tar model feed that was converted to gaseous products (CO2, CO, and C1–C4 hydrocarbons). Lower carbon conversion was obtained for phenol, toluene, and methylnaphthalene. The reactivity of the oxygenated model compounds (phenol and furfural) appeared to be higher than that of the non-oxygenated model compounds (toluene and methylnaphthalene). Oxygenated compounds are considered to be more thermally unstable, as a result, they undergo thermal decomposition, as well as cracking reactions on the char surface. In addition, larger cyclic hydrocarbons appeared to be less reactive. The results suggest that the cracking mechanisms of aromatic hydrocarbons and oxygenated compounds differ significantly. In relation to both catalytic (tire char) and thermal cracking (sand), the reactivity of tar compounds followed the order of furfural > phenol > toluene > methylnaphthalene. The results showed that the decomposition rate of the studied model tar compounds was much lower with the use of sand, highlighting the catalytic effectiveness of the tire char.
The highest carbon conversion of 75% was obtained with furfural, followed by phenol with 54% carbon conversion. Abu El-Rub et al. [36] showed that commercial biomass char is an effective catalyst for phenol conversion. They achieved 82% conversion with commercial biomass char at a reforming temperature of 800 °C and a gas residence time of 3 s in the presence of steam. The difference among the results obtained in this work is due to the presence of steam, which is known to promote the tar cracking process [36]. The toluene conversion obtained in this work (47%) was lower than that of other work, such as that of Lu et al. [37], who reported a 63% toluene conversion at a reaction time of 60 min in the presence of sewage sludge derived char at 750 °C under an inert atmosphere and with a retention time of 0.3 s. However, the toluene conversion reported is similar to that reported by Coll et al. [22] over a commercial nickel-based catalyst at 700 °C using a steam to carbon ratio of 6.5.
The difference in carbon conversion between toluene and 2-methylnaphthalene was negligible. This result agrees with the results obtained by Fuentes-Cano et al. [26], where toluene and naphthalene achieved almost similar conversion of about 40% using coconut char at a temperature of 750 °C and with an inlet gas composition of 15% H2O in volume and 8% H2 in volume. However, methylnaphthalene seems to be stable and hard to remove, where about 26.9% in weight of condensed product was obtained. Figure 2b displays the hydrogen potential for the studied tar model compounds. The results clearly show that tire char promotes the yield of hydrogen. The H2 potential for the studied model compounds was found to be in the range of 40%–50%.
The decomposition of high molecular hydrocarbons in an inert atmosphere results in the formation of gaseous products and carbon according to the following equations [26],
Cracking reaction: \({\text{C}}_{{{n}}} {\text{H}}_{{{m}}} + {\text{H}}_{2} \leftrightarrow {\text{C}}_{{\text{x}}} {\text{H}}_{y} + {\text{H}}_{2}\).
Carbon formation: \({\text{C}}_{n} {\text{H}}_{m} \leftrightarrow n{\text{C}}^{*} + \frac{1}{2}{\text{H}}_{2}\).
Methane formation: \({\text{C}} + {\text{H}}_{2} \leftrightarrow {\text{CH}}_{4}\).
Hydrocracking: \({\text{C}}_{n} {\text{H}}_{m} + {\text{H}}_{2} \leftrightarrow {\text{CH}}_{4}\).
The main reactions involved during the catalytic cracking of tar compounds are tar cracking and hydrocarbon reforming. Therefore, the change in the gas composition with/without the use of catalyst is due to these reactions. The results in relation to the gas composition with the presence of tire char and sand in the second stage are shown in Fig. 3. The yield (%) of gases was calculated using Eq. 2, based on the gaseous yield (%) from the cracking of the tar model compounds. As presented in Fig. 3, the gas production increased dramatically with the introduction of tire char in the second-stage reactor in comparison to the experiments with sand. For example, with the use of furfural as a tar model compound, the gas yield increased from 39% to 78% in weight, and the hydrogen production increased from 8 mmol H2 g−1 to 18.54 mmol H2 g−1 furfural. The same trend was observed with the other tar model compounds. The results suggest that the tire char significantly improved the hydrogen production during the catalytic cracking of tar model compounds. The analysis of gaseous products during the experiments confirms that cracking reactions were taking place with the use of tire char. The cumulative yields of gases in the presence of tire char showed a significant difference from that with the use of sand only. For example, at a cracking temperature of 700 °C and with the use of furfural, the yields of H2, CH4, CO and CO2 with the presence of tire char increased by 59%, 71%, 43% and 84%, respectively, compared to the non-catalytic experiment. An increase in gases was observed with all the tar model compounds used. Char might adsorb the tar components and then catalyze them on active metal sites in the char. In general, the strong catalytic activity of tire char in tar catalytic reforming is due to its surface area, internal surface texture, amorphous carbon structure, active surface functional groups, and alkali and alkaline earth metal oxides. The highest yields of CO were obtained for the oxygenated hydrocarbons, i.e., phenol (45% in weight) and furfural (42% in weight). The presence of oxygen atoms in these molecules would promote the generation of CO in the gaseous stream. In addition, CH4 was also generated with the use of tire char compared to thermal cracking which confirms the high cracking activity of tire char. CH4 could also be derived from CO through methanation or from the decomposition of the model compound.
The oxygen functional groups on the char could interact with tar molecules and enhance the cracking process. Oxygenated functional groups found on carbon surfaces include, carboxylic, lactone, phenol, carbonyl, anhydride, ether and quinoline groups [38]. It has been reported that strong acidic functionalities such as carboxylic, anhydrides and lactones decompose at lower temperatures and weakly acidic functionalities decompose at higher temperatures. Therefore, heating the char can produce basicity due to the decomposition of acid functional groups [38]. In the study by Wang et al. [39], char was found to undergo thermal cracking leading to the production of carbon containing gases, such as CO and CO2, which was also observed from the negative mass balance after the experiment. Surface oxygen groups present on the carbon surface are believed to decompose upon heating by releasing CO and CO2 [40]. In addition, coke could also be produced due to tar cracking. According to Wang et al. [39], the produced coke may react with the oxygen present on the char surface resulting in the formation of carbon–oxygen compounds that could also decompose to CO.
Many factors might influence the catalytic activity of carbonaceous materials for tar decomposition, including the thermal effect, mineral content, surface chemistry and porous texture. The increase in gas yield and the change in gas compositions with the use of tire char suggest that the tar decomposition is mainly due to the catalytic effect of tire char and not due to thermal cracking, as more gases were produced over tire char than with the use of sand. This trend was observed with all the studied tar model compounds. In addition, the amount of condensed liquid was found to be much higher with the use of sand only. For example, in the experiment investigating the cracking of phenol, the condensed liquid collected in the presence of sand was found to be 73% and decreased to 24% with the introduction of tire char. In the non-catalytic cracking experiment, tar cracking and poly-condensation reactions were prevalent, resulting in the formation of non-condensable gas components and soot. With the introduction of tire char, tar compounds may be decomposed by the hydrogenation and ring-opening reaction, producing a large amount of non-condensable gaseous product. The decrease in the condensed liquid with the use of tire char could also be due to the adsorption of tar compounds on the char surface or soot formation. Tars are expected to adsorb on the surface of char. The active sites present in char are believed to combine with the formed tar radicals and facilitate the cracking process. Tar radicals could also react with each other through a polymerization reaction and form soot [41]. This was also observed from the mass balance results.
To understand the main role that tire char plays during the cracking of tar compounds, the fresh unused tire char and the spent tire chars after reaction were examined by TGA-FTIR, and the results are shown in Fig. 4. The spent tire char had some weight loss at a temperature of 900 °C (about 4%), which may be related to the adsorption of heavy tar compounds formed due to the polymerization reaction. However, the difference in weight loss between the fresh and spent tire char is not significant, suggesting that the decrease in the liquid content with the use of tire char is not due to the adsorption mechanism and could be mainly due to cracking of tar compounds on active sites of the char surface into gases. The same conclusion was reported by Jin et al. [42]. The weight loss observed with the spent tire char at 50 min was accompanied by the release of more CO2 than that with fresh tire char.
Parametric study
Naphthalene is one of the main tar components produced during the gasification of biomass [43] and is considered as the most representative model compound of biomass gasification tar [44, 45]. According to Han and Kim [34], the naphthalene content in tar is generally above 9%. In another study reported by Michel et al. [44], steam reforming of methylnaphthalene over olivine and olivine supported nickel was investigated at various temperatures. At 900 °C, the 2-methylnaphthalene conversion was about 4% in the presence of olivine and 31% in relation to Ni/olivine. The low activity of olivine was attributed to its low BET surface area. Therefore, many factors could influence the tar conversion, including the BET, porosity and the reaction atmosphere of the catalytic material. The main aim of the parametric study here was to determine the main factors that could influence the tar conversion of the representative biomass tar model compounds with the use of tire char as a catalyst. The conversion of methylnaphthalene in the presence of tire char was carried out as a function of reaction time and reaction temperature and in terms of the char characteristics of porosity and acidity.
The effect of varying the reaction temperature using 2-methylnaphthalene is shown in Fig. 5. It shows that with increasing temperature from 700 °C to 900 °C, the carbon conversion increased slightly from 46% to 50%. The results also show that with increasing reaction temperature, the generation rates of CO and H2 were promoted with temperature, while the hydrocarbons decreased. The reforming and cracking reactions of tar are enhanced with increasing the temperature. The minerals within the tire char were responsible for catalyzing the ring-opening reaction. However, CO2 showed the opposite trend, which could be attributed to char gasification at high temperatures.
The yield of condensed liquid gradually decreased, in which at a tire char temperature of 900 °C about 5% of the condensed liquid was obtained compared to 26.9% at a tire char temperature of 700 °C. As a consequence, the gaseous products increased, for example, a high hydrogen yield (35 mmol g−1) was found at a temperature of 900 °C compared to 28 mmol g−1 at a temperature of 700 °C. Therefore, increasing the tire char temperature from 700 °C to 900 °C had no major influence on the carbon conversion and the total gas yield. However, the collected liquid product decreased by about 80%. The decrease in condensed liquid at high temperature was associated with the formation of soot, which was the highest at this temperature and accounted for 18% of the total obtained product. The results suggest that tar compounds could be trapped on the char surface and accumulate to form coke or soot, without being converted. The analysis of BET surface area agreed with this suggestion, as the BET surface area of the used tire char at a temperature of 900 °C decreased to 58.8 m2 g−1 compared to about 71.7 m2 g−1 for the fresh char at 700 °C. During tar conversion, macromolecular compounds and the generated soot can block the pores, resulting in a decrease in surface area. However, in a real-world actual gasifier, the evaporation of inherent water in fuel particles and the creation of pyrolytic water will produce a certain amount of steam, therefore, char may be auto-activated.
Anis et al. [46] found that the amount of condensed products collected during the catalytic treatment of naphthalene at a temperature of 700 °C and with the use of dolomite and Y-zeolite were about 67% and 25%, respectively, which agreed with the results found in this study. They also showed that the thermal treatment of naphthalene is highly stable at a temperature below 1000 °C, and about 70% of the condensed product was obtained at 1050 °C. However, increasing the temperature to 1200 °C led to an increase in the naphthalene removal efficiency and decrease in the condensed product to about 9%.
Methylnaphthalene has been found to decompose mostly to naphthalene and accounted for 50% of the total products at a reaction temperature of 900 °C. The first step in the decomposition of 2-methylnaphthalene is the separation of the methyl group from the aromatic ring [47]. The same trend has been reported by Parsland et al. [48]. However, both Parsland et al. [48] and Leinger et al. [49] reported the formation of low concentrations of other products, such as benzene and styrene, which was not observed in this study. This is due to the difference in the reaction atmosphere and the catalysts used. In the study carried out by Parsland et al. [48], the activity of BaNi(1) hexa-aluminate catalyst for methylnaphthalene conversion was investigated with the use of steam as a gasifying agent, while in this study the reaction was carried out in an inert atmosphere. The influence of the presence of H2O, CO2, and H2 on the naphthalene decomposition using dolomite as a catalyst has been studied by Alden et al. [50]. They concluded that both CO2 and H2O promote the cracking process. The de-alkylation of methylnaphthalene resulted in the formation of naphthalene, which can then be catalytically dissociated into radicals of naphthyl and hydrogen due to the cleavage of C–C or C–H bonds. In the presence of CO2 and H2O, naphthyl reacts with the oxidative radicals (which form from CO2 and H2O), resulting in the formation fewer aliphatic and single aromatic compounds. However, in an inert atmosphere, the catalytic decomposition of 2-methylnaphthalene could result only in the formation of naphthalene which can then undergo polymerization reactions to produce soot [27].
One of the main issues to consider while using a catalyst for tar conversion is maintaining the activity over a long period of time. Therefore, the influence of the tire char time on-stream was investigated for the feeding time of the 2-methylnaphthalene. The results are shown in Fig. 6 in terms of the volume percent of each gas in the product gas yield. Increasing the time on-stream of the 2-methylnaphthalene interaction with the tire char from 40 min to 80 min had no influence on the catalytic activity of tire char for 2-methylnaphthalene decomposition. As illustrated in Fig. 6, the 2-methylnaphthalene conversion was maintained at around 46% over the studied time on-stream. In the study carried out by Hosokai et al. [27], it was reported that charcoal exhibited a high naphthalene conversion (96%) at the beginning of the reaction time and decreased to about 50% after 45 min. The authors ascribed this to coke deposition on the surface of the charcoal, which resulted in a decrease in the BET surface area and pore volume. The stability of biomass char for naphthalene conversion has also been reported by Zhang et al. [51] in which the char maintained its activity for about 5 h. Hosokai et al. [27] concluded that gaseous species formed with the use of charcoal as a catalyst for naphthalene and benzene cracking are due to the slow thermal cracking of charcoal and that coking is the main mechanism for the decomposition of the studied tar compounds.
The influence of porosity on 2-methhylnaphthalene conversion
To assess the influence of char porosity on methyl conversion, two commercial activated carbons (obtained from Norit Ltd., Amersfoort, The Netherlands) with various porous textures were investigated for 2-methylnaphthalene cracking at a reaction temperature of 700 °C. Tire char is a mesoporous carbon, but to eliminate the influence of mineral content of tire char and to be able to understand the influence of porosity on 2-methylnaphthalene conversion, a mesoporous activated carbon (AC2) with an ash content of 2% in weight was used for the comparison along with a microporous activated carbon (AC1). The textural properties in terms of surface area and porosity of the tire char and commercial activated carbon (AC1 and AC2) samples are displayed in Table 4. Table 4 shows the influence of the textural properties of the tire char and activated carbons on the 2-methylnaphthalene conversion, gas yield and hydrogen production.
There is an obvious difference between tire char and the commercial activated carbons used in terms of the BET surface area and the well-developed porous texture. The BET surface area of the commercial activated carbons was much larger than that of tire char. The nitrogen adsorption–desorption isotherms shown in Fig. 7 suggest that AC1 contained both micropores and mesopores, and the isotherms obtained for tire char and AC2 are similar to type IV adsorption isotherms, which indicate more mesoporous carbon. Despite the large difference in the BET surface area between the tire char and the commercial activated carbons, the 2-methylnaphthalene removal efficiency with both commercial activated carbons was slightly higher than that with the use of tire char. There was no obvious correlation between the hydrogen yield (mmol g−1) and the surface area, mesoporosity or microporosity of the different carbons. The influence of the porosity of the different carbons and with sand as a blank material on the composition of the product gases is shown in Fig. 8. The yield (%) of gases was calculated using Eq. 2. The gas composition produced indicates an increase in CO, CO2, and H2 gas formation with the use of AC1, as shown in Fig. 8. Fuentes-Cano et al. [26] investigated the catalytic activity of various char materials with different porous textures for toluene and naphthalene reforming in an atmosphere of steam and H2 and concluded that the textural properties of char had no major influence on tar removal. In contrast, Jin et al. [36] reported that the difference in the performance of the studied carbon samples during the upgrading of coal oil is due to the difference in BET surface area. Additionally, the authors concluded that activated carbon had more structural defects than char, in which these defects could serve as active sites for cracking tar compounds. Hosokai et al. [27] investigated the decomposition of tar model compounds using charcoal and observed that coke deposited mainly occurred in the micropores causing the loss of the catalytic activity of char. The mesopore volume of the charcoal remained unaffected. However, in this study, a decrease was found with both micropores and mesopores volumes.
Both commercial activated carbons used in this work have a well-developed pore structure with a high BET surface area, however, the difference in the catalytic activity of the activated carbons in comparison to the tire char was not significant. This could be related to the effect of the mineral content of tire char (18% ash content in weight) which could provide active sites for cracking of 2-methylnaphthalene. For example, the catalytic activity of inorganic metals for heavy hydrocarbon conversion to light components has been reported [52, 53]. The results suggest that the influence of the BET surface area of carbon on tar cracking is negligible compared with the mineral content in the carbon samples. The results are consistent with the conclusion reported by Li et al. [53], in which semi-cokes with a higher mineral content (35% in weight) had a higher activity in tar cracking than the semi-coke with a well-developed porous structure but had a lower mineral content (8.3% in weight). The authors suggested that both mineral content and porous texture affect the catalytic activity of the studied semi-cokes for cracking tar oil. In contrast, Zeng et al. [52] concluded that char with a higher BET surface area exhibited higher catalytic activity for tar removal. Demirbas [54] investigated the catalytic pyrolysis of various biomass samples impregnated with different catalysts and concluded that the highest hydrogen yield was obtained with the samples impregnated with ZnCl2. As shown in Fig. 8, the difference in the H2 yield produced, as a result of cracking of 2-methylnaphthalene, between AC1 and tire char was only about 1%. Additionally, the H2 yield found with tire char was higher than that with AC2 with a much higher BET surface area which suggests that the influence of mineral matter is more important than the porous texture.
The influence of char surface acidity on 2-methylnaphthalene conversion
To assess the influence of the presence of oxygen functional groups present in the tire char in relation to tar conversion, a commercial activated carbon, designated AC1, provided by Norit Ltd. (Amersfoort, The Netherlands), was treated with HNO3 with molar concentrations between 1 and 4 Molar. The treatment was carried out in a 25 mL Teflon bottle containing 7 g AC and 50 mL 1–4 mol/L HNO3. The treatment was carried out at 70 °C for 8 h and 150 rpm. The treated carbons were washed several times with distilled water and dried at 105 °C for 48 h.
According to Radovic et al. [55], the adsorption efficiency of carbonaceous material depends to a high extent on the surface chemistry and ash mineral composition. Both Rodriguez-Reinoso et al. [56] and Moreno-Castilla [57] agreed that the effectiveness of activated carbon for the adsorption of organic compounds is mainly determined by the carbon surface chemistry. The nature of surface functional groups can be modified via chemical and physical treatment. The influence of carbon acidity on tar conversion during pyrolysis-gasification has not been studied before. We have reported previously [29] that the higher efficiency of tire char for tar conversion could be due to the carbon acidity. Buchireddy et al. [58] concluded that zeolite with a higher acidity had a better performance toward naphthalene conversion. One of the ways to form acidic sites on a carbon is to heat the sample in an oxidising environment. Therefore, AC1 was treated with HNO3 at different molar concentrations. The textural properties of the original and treated activated carbon are displayed in Table 5. Comparing the treated samples with the starting material (AC1), the physical properties change little after the treatment. The decrease in BET surface area of the oxygenated samples has been reported previously by others [59].
The results presented in Table 5, suggest that the acidic treatment was not effective in enhancing the 2-methylnaphthalene removal efficiency. The carbon conversion of the acid treated activated carbons was not significantly different from that of the original activated carbon (AC1). Additionally, the gas concentration did not change, and it was almost the same as all the tested samples, which further suggests that the acid treatment had no influence on enhancing the effectiveness of carbon for the cracking of tar compounds. This is probably due to the desorption of the oxygen functional groups while heating the char in nitrogen at 700 °C in the second furnace where the reaction occurs. According to Li et al. [53], the oxidised samples release more CO2 at a temperature of 400 °C. The heat treatment of biomass char at a temperature of 750 °C under N2 for 10 h has been found to decompose most of the surface oxygenated functional groups [60]. Therefore, the oxygen surface groups produced due to the acid treatment could be desorbed in the early stages of the experiment before the char reacts with the 2-methylnaphthalene. The results are in agreement with those reported by Klinghoffer et al. [59], in which the influence of the acidic groups of char have been investigated for methane decomposition at 850 °C, and the authors concluded that the acidic functional groups have no influence on the catalytic activity of char. According to the study carried out by Bhandari et al. [61], the acidic activated carbon exhibited lower toluene removal (79%) than the original activated carbon (82%) at a temperature of 700 °C. The authors suggested that this could be due to polar properties of the catalysts, formed because of the acidic groups, which can reduce the reactivity of active sites of carbon with toluene. The oxygenated groups are polar while the aromatic ring is non polar [49]. In contrast, Sechandri and Shamsi [62] concluded that a char/dolomite mixture, due to its low BET surface area and surface acidity, had a lower activity for coal tar decomposition than zeolites. The influence of oxygen functional groups on catalytic activity of char for methane decomposition has been investigated by Klinghoffer et al. [59], and it was reported that the acidic groups desorb at a low temperature.
Conclusions
This study investigated the catalytic cracking of phenol, furfural, toluene, and methylnaphthalene using a tire-derived pyrolysis char in a fixed bed reactor. The selected model biomass gasification tar compounds cover the main detected compounds identified from biomass gasification. High carbon conversion (46%–75%) and hydrogen yield (15–28 mmol g−1) were observed in comparison to the non-catalytic experiments. Furfural was found to be the most reactive compound, followed by phenol and toluene, with methylnaphthalene being the most refractory model tar compound. Further experiments were carried out to evaluate the influence of tire char temperature, reaction time on-stream, porous texture and the acidity of carbon on tar conversion using methylnaphthalene as the tar model compound. An increase in the reactor char bed temperature from 700 °C to 900 °C had a marginal increase in carbon conversion from 46% to 50%. Neither the surface area nor the surface acidity of the carbons investigated had a significant effect on tar model compound cracking. The mineral content in the tire char played a more important role in the cracking of the tar model compounds.
Change history
11 April 2023
A Correction to this paper has been published: https://doi.org/10.1007/s42768-023-00150-6
References
Saleem, F., Harris, J., Zhang, K., et al. 2020. Non-thermal plasma as a promising route for the removal of tar from the product gas of biomass gasification—a critical review. Chemical Engineering Journal 382: 122761.
Zhang, Z., Liu, L., Shen, B., et al. 2018. Preparation, modification and development of Ni-based catalysts for catalytic reforming of tar produced from biomass gasification. Renewable & Sustainable Energy Reviews 94: 1086–1109.
Rios, M.L.V., Gonzalez, A.M., Lora, E.E.S., et al. 2018. Reduction of tar generated during biomass gasification: a review. Biomass and Bioenergy 108: 345–370.
Moud, P.H., Lanza, R., and Pettersson, J.B.C. 2015. Effect of gas phase alkali species on tar reforming catalyst performance: initial characterisation and method development. Fuel 154: 95–106.
Lee, M.L., Novotny, M., and Bartle, K.D. 1981. Analytical chemistry of PAC. Cambridge, MA, USA: Academic Press.
Liu, Z. 2019. Gasification of municipal solid wastes: a review on the tar yields. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects 41 (11): 1296–1304.
Williams, P.T. 2013. Pyrolysis of waste tyres: a review. Waste Management 33: 1714–1728.
Jiang, Y., Zong, P.J., Bao, Y., et al. 2022. Catalytic conversion of gaseous tar using coal char catalyst in the two-stage downer reactor. Energy 242: 123013.
Hu, D.D., Zeng, X., Wang, F., et al. 2021. Comparison of tar thermal cracking and catalytic reforming by char in a micro fluidised bed reaction analyser. Fuel 290: 120038.
Ravenni, G., Sarossy, Z., Sanna, S., et al. 2020. Residual gasification char applied to tar reforming in a pilot-scale gasifier: performance and evolution of char properties for perspective cascade uses. Fuel Processing Technology 210: 106546.
Wang, S.X., Shan, R., Lu, T., et al. 2020. Pyrolysis char derived from waste peat for catalytic reforming of tar model compound. Applied Energy 263: 114565.
Gilbert, P., Ryu, C., Sharifi, V., et al. 2009. Tar reduction in pyrolysis vapours from biomass over a hot char bed. Bioresource Technology 100 (23): 6045–6051.
Wang, F.-J., Zhang, S., Chen, Z.-D., et al. 2014. Tar reforming using char as catalyst during pyrolysis and gasification of Shengli brown coal. Journal of Analytical and Applied Pyrolysis 105: 269–275.
Meng, L.-Y., and Park, S.-J. 2012. MgO-templated porous carbons-based CO2 adsorbents produced by KOH activation. Materials Chemistry and Physics 137 (1): 91–96.
Guan, G., Kaewpanha, M., Hao, X., et al. 2016. Catalytic steam reforming of biomass tar: prospects and challenges. Renewable and Sustainable Energy Reviews 58: 450–461.
Feng, D.D., Zhao, Y.J., Zhang, Y., et al. 2017. Experimental comparison of biochar species on in-situ biomass tar H2O reforming over biochar. International Journal of Hydrogen Energy 42: 24035–32404.
Ravenni, G., Elhami, O.H., Ahrenfeldt, J., et al. 2019. Adsorption and decomposition of tar model compounds over the surface of gasification char and active carbon within the temperature range 250–800 °C. Applied Energy 241: 139–151.
Choi, C., Shima, K., Kudo, S., et al. 2019. Continuous monitoring of char surface activity toward benzene. Carbon Resource Conversion 2 (1): 43–50.
Elliott, D.C., and Baker, E.G. 1986. The effect of catalysts on wood-gasification tar composition. Biomass 9: 195–203.
Dufour, A., Masson, E., Girods, P., et al. 2011. Evolution of Aromatic Tar Composition in Relation to Methane and Ethylene from Biomass Pyrolysis-Gasification. Energy & Fuels 25 (9): 4182–4189.
Zhang, L., Yao, Z., Zhao, L., et al. 2021. Synthesis and characterisation of different activated biochar catalysts for removal of biomass pyrolysis tar. Energy 232: 120927.
Coll, R., Salvadó, J., Farriol, X., et al. 2001. Steam reforming model compounds of biomass gasification tars: Conversion at different operating conditions and tendency towards coke formation. Fuel Processing Technology 74 (1): 19–31.
Burhenne, L., and Aicher, T. 2014. Benzene removal over a fixed bed of wood char: The effect of pyrolysis temperature and activation with CO2 on the char reactivity. Fuel Processing Technology 127: 140–148.
Moliner, R., Suelves, I., Lázaro, M.J., et al. 2005. Thermocatalytic decomposition of methane over activated carbons: influence of textural properties and surface chemistry. International Journal of Hydrogen Energy 30 (3): 293–300.
Park, J., Lee, Y., and Ryu, C. 2016. Reduction of primary tar vapor from biomass by hot char particles in fixed bed gasification. Biomass and Bioenergy 90: 114–121.
Fuentes-Cano, D., Gómez-Barea, A., Nilsson, S., et al. 2013. Decomposition kinetics of model tar compounds over chars with different internal structure to model hot tar removal in biomass gasification. Chemical Engineering Journal 228: 1223–1233.
Hosokai, S., Kumabe, K., Ohshita, M., et al. 2008. Mechanism of decomposition of aromatics over charcoal and necessary condition for maintaining its activity. Fuel 87 (13–14): 2914–2922.
Jess, A. 1996. Mechanisms and kinetics of thermal reactions of aromatic hydrocarbons from pyrolysis of solid fuels. Fuel 75 (12): 1441–1448.
Al-Rahbi, A.S., Onwudili, J.A., and Williams, P.T. 2016. Thermal decomposition and gasification of biomass pyrolysis gases using a hot bed of waste derived pyrolysis char. Bioresource Technology 204: 71–79.
Al-Rahbi, A.S., and Williams, P.T. 2017. Hydrogen-rich syngas production and tar removal from biomass gasification using sacrificial tire pyrolysis char. Applied Energy 190: 501–509.
Husár, J., Haydary, J., and Šuhaj, P. 2019. Potential of tire pyrolysis char as tar-cracking catalyst in solid waste and biomass gasification. Chemical Papers 73: 2091–2101.
Suhaj, P., Husar, J., and Haydary, J. 2020. Gasification of RDF and its components with tire pyrolysis char as tar-cracking catalyst. Sustainability 12: 6647.
Irfan, M., Aimin, L., Zhang, L., et al. 2021. Waste tire derived char supported Ni-Fe catalyst for catalytic thermochemical conversion of wet municipal solid waste. International of Energy Research 46(3): 3634–3646.
Han, J., and Kim, H. 2008. The reduction and control technology of tar during biomass gasification/pyrolysis: An overview. Renewable and Sustainable Energy Reviews 12 (2): 397–416.
Morf, P., Hasler, P., and Nussbaumer, T. 2002. Mechanisms and kinetics of homogeneous secondary reactions of tar from continuous pyrolysis of wood chips. Fuel 81 (7): 843–853.
Abu-El-Rub, Z., Bramer, E.A., and Brem, G. 2008. Experimental comparison of biomass chars with other catalysts for tar reduction. Fuel 87 (10–11): 2243–2252.
Lu, P., Qian, X., Huang, Q., et al. 2016. Catalytic cracking of toluene as a tar model compound using sewage-sludge-derived char. Energy & Fuels 30: 8327–8334.
Shafeeyan, M.S., Daud, W.M.A.W., Houshmand, A., et al. 2010. A review on surface modification of activated carbon for carbon dioxide adsorption. Journal of Analytical and Applied Pyrolysis 89 (2): 143–151.
Wang, D., Yuan, W., and Ji, W. 2011. Char and char-supported nickel catalysts for secondary syngas cleanup and conditioning. Applied Energy 88 (5): 1656–1663.
Figueiredo, J.L., Pereira, M.F.R., Freitas, M.M.A., et al. 1999. Modification of the surface chemistry of activated carbons. Carbon 37 (9): 1379–1389.
Huang, Q., Lu, P., Hu, B., et al. 2016. Cracking of model tar species from the gasification of municipal solid waste using commercial and waste-derived catalysts. Energy & Fuels 30 (7): 5740–5748.
Jin, L., Bai, X., Li, Y., et al. 2016. In-situ catalytic upgrading of coal pyrolysis tar on carbon-based catalyst in a fixed-bed reactor. Fuel Processing Technology 17 (17): 41–46.
Dou, B., Gao, J., Sha, X., et al. 2003. Catalytic cracking of tar component from high-temperature fuel gas. Applied Thermal Engineering 23 (17): 2229–2239.
Michel, R., Łamacz, A., Krzton, A., et al. 2013. Steam reforming of α-methylnaphthalene as a model tar compound over olivine and olivine supported nickel. Fuel 109: 653–660.
Wang, T.J., Chang, J., Wu, C.Z., et al. 2005. The steam reforming of naphthalene over a nickel–dolomite cracking catalyst. Biomass and Bioenergy 28 (5): 508–514.
Anis, S., Zainal, Z.A., and Bakar, M.Z.A. 2013. Thermocatalytic treatment of biomass tar model compounds via radio frequency. Bioresource Technology 136: 117–125.
Tunå, P., Bauer, F., Hulteberg, C., et al. 2013. Regenerative reverse-flow reactor system for cracking of producer gas tars. Biomass Conversion and Biorefinery 4 (1): 43–51.
Parsland, C., Larsson, A.-C., Benito, P., et al. 2015. Nickel-substituted bariumhexaaluminates as novel catalysts in steam reforming of tars. Fuel Processing Technology 140: 1–11.
Leininger, J.P., Lorant, F., Minot, C., et al. 2006. Mechanisms of 1-methylnaphthalene pyrolysis in a batch reactor. Energy and Fuels 20 (6): 2518–2530.
Aldén, H., Björkman, E., Carlsson, M., et al. 1993. Catalytic cracking of naphthalene on dolomite. In Advances in Thermochemical Biomass Conversion, Bridgwater, A.V., ed. 216–232. Dordrecht, Netherlands: Springer.
Zhang, Y.-L., Luo, Y.-H., Wu, W.-G., et al. 2014. Heterogeneous cracking reaction of tar over biomass char, using naphthalene as model biomass tar. Energy & Fuels 28 (5): 3129–3137.
Zeng, X., Wang, Y., Yu, J., et al. 2011. Gas upgrading in a downdraft fixed-bed reactor downstream of a fluidized-bed coal pyrolyzer. Energy and Fuels 25 (11): 5242–5249.
Li, B., Lei, Z., and Huang, Z. 2009. Surface-treated activated carbon for removal of aromatic compounds from water. Chemical Engineering & Technology 32 (5): 763–770.
Demirbaş, A. 2002. Gaseous products from biomass by pyrolysis and gasification: Effects of catalyst on hydrogen yield. Energy Conversion and Management 43 (7): 897–909.
Radovic, L.R., Silva, I.F., Ume, J.I., et al. 1997. An experimental and theoretical study of the adsorption of aromatics possessing electron-withdrawing and electron-donating functional groups by chemically modified activated carbons. Carbon 35 (9): 1339–1348.
Rodríguez-Reinoso, F. 1998. The role of carbon materials in heterogeneous catalysis. Carbon 36 (3): 159–175.
Moreno-Castilla, C. 2004. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 42 (1): 83–94.
Buchireddy, P.R., Bricka, R.M., Rodriguez, J., et al. 2010. Biomass gasification: Catalytic removal of tars over zeolites and nickel supported zeolites. Energy & Fuels 24 (4): 2707–2715.
Klinghoffer, N.B., Castaldi, M.J., and Nzihou, A. 2015. Influence of char composition and inorganics on catalytic activity of char from biomass gasification. Fuel 157: 37–47.
Yip, K., Xu, M., Li, C.-Z., et al. 2010. Biochar as a fuel: 3. Mechanistic understanding on biochar thermal annealing at mild temperatures and its effect on biochar reactivity. Energy & Fuels 25 (1): 406–414.
Bhandari, P.N., Kumar, A., Bellmer, D.D., et al. 2014. Synthesis and evaluation of biochar-derived catalysts for removal of toluene (model tar) from biomass-generated producer gas. Renewable Energy 66: 346–353.
Seshadri, K.S., and Shamsi, A. 1998. Effects of temperature, pressure, and carrier gas on the cracking of coal tar over a char-dolomite mixture and calcined dolomite in a fixed-bed reactor. Industrial and Engineering Chemistry Research 37 (10): 3830–3837.
Acknowledgements
The support of the Government of Oman through a scholarship for Amal S. Al-Rahbi is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Paul T. Williams is the Editorial Board member of Waste Disposal & Sustainable Energy. On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised to correct the Conflict of Interest section.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Rahbi, A.S., Williams, P.T. Decomposition of biomass gasification tar model compounds over waste tire pyrolysis char. Waste Dispos. Sustain. Energy 4, 75–89 (2022). https://doi.org/10.1007/s42768-022-00103-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-022-00103-5