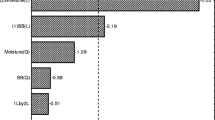
Abstract
Starch is one of the most abundant renewable carbohydrate reserves of higher plants. It can be used to produce many valuable food products in the food processing industry. Furthermore, starch is also used as an important feedstock in the fermentation industry to produce value-added products. Lignocellulosic materials such as agriculture and forestry wastes are considered as a renewable feedstock for bioenergy production through a biochemical conversion process. Converting lignocellulosic biomass into fuels and chemicals entail a physicochemical pretreatment of the biomass, followed by enzymatic hydrolysis of the polysaccharide components such as cellulose and hemicellulose into monomeric sugars. These sugars can then be further fermented into other desired compounds of interest. During the deconstruction processes, various inhibitory compounds are released due to the partial over-degradation of lignocellulose biomass, which inhibits the cell growth and metabolic capacity of fermenting strains. Cellulosic materials such as waste paper in large quantities can also be used as potentially cheap feedstock for sustainable production of value-added products. The present investigation is mainly focused on the utility of starchy hydrolysates (wheat, potato, and rice) and lignocellulosics hydrolysates (bagasse and wheat straw) in cellulase production under liquid state fermentation. It also depicts the potential of cellulosic hydrolysate (waste newspaper) in product formation.






Similar content being viewed by others
Change history
27 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s42768-021-00078-9
References
Ali N, Athar MA, Khan YH, et al. Regulation and improvement of cellulase production: recent advances. Nat Res. 2014;5:857–63.
Oszust K, Pawlik A, Siczek A, et al. Efficient cellulases production by Trichoderma atroviride G79/11 in submerged culture based on soy flour-cellulose-lactose. BioRes. 2017;12(4):8468–89.
Adelabu BA, Kareem SO, Oluwafemi F, et al. Bioconversion of corn straw to ethanol by cellulolytic yeasts immobilized in Mucuna urens matrix. J King Saud Univ Sci. 2017;31:136.
Verma N, Bansal MC, Kumar V. Utility of Luffa cylindrica and Litchi chinensis peel, an agricultural waste biomass in cellulase production by Trichoderma reesei under solid state cultivation. Biocatal Agric Biotechnol. 2018;16:483–92.
Carvalho JFA. Starch: major sources, properties and applications as thermoplastic materials, handbook of biopolymers and biodegradable plastics. Boston: William Andrew Publishing; 2013.
Razdan N, Kocher GS. Utilization of damaged and spoiled wheat grains for bioethanol production. Biosci Biotech Res Comm. 2018;11(4):658–73.
Perez S, Bertoft E. The molecular structures of starch components and their contribution to the architecture of starch granules: a comprehensive review. Starch-Starke. 2010;62(8):389–420.
Miao M, Jiang B, Jin Z, et al. Microbial starch-converting enzymes: recent insights and perspectives. Compr Rev Food Sci Food Saf. 2018;17(15):1238–60.
Singh SV, Ali SZ. Properties of starches modified by different acid. Int J Food Prop. 2008;11:495–507.
Luo W, Zhao Z, Pan H, et al. Feasibility of butanol production from wheat starch wastewater by Clostridium acetobutylicum. Energy. 2018;154:240–8.
Chen S, Wayman M. Novel inducers derived from starch for cellulase production by Trichoderma reesei. Process Biochem. 1992;27:327–34.
Izmirlioglu G, Demirci A. Enhanced bio-ethanol production from industrial potato waste by statistical medium optimization. Int J Mol Sci. 2015;16(10):24490–505.
Tasić MB, Konstantinović BV, Lazic ML, et al. The acid hydrolysis of potato tuber mash in bioethanol production. Biochem Eng J. 2009;43(2):208–11.
Yadav P, Majumder CB. Production of glucose syrup by the hydrolysis of starch made from rotten potato. J Integr Sci Technol. 2017;5(1):19–22.
Soest JJGV, Benes K, Wit DD. The influence of acid hydrolysis of potato starch on the stress-strain properties of thermoplastic starch. Starch. 1995;47(11):429–34.
Miao M, Jiang B, Zhang T, et al. Impact of mild acid hydrolysis on structure and digestion properties of waxy maize starch. Food Chem. 2011;126(2):506–13.
Moniruzzaman M, Ingram LO. Ethanol production from dilute acid hydrolysate of rice hulls using genetically engineered Escherichia coli. Biotechnol Lett. 1998;20(10):943–7.
Wang CH, Hseu TH, Huang CM. Induction of cellulases by cellooligosaccharides in Trichoderma koninghii G-39. J Biotechnol. 1998;9:47–60.
Wayman M, Chen S. Cellulase production by Trichodermma reesei using whole wheat flour as a carbon source. Enzyme Microbial Technol. 1992;14:825–31.
Yang Y, Hu M, Tang Y, et al. Progress and perspective on lignocellulosic hydrolysate inhibitor tolerance improvement in Zymomonas mobilis. Biores Bioprocess. 2018;5:6.
Jönsson LJ, Alriksson B, Nilvebrant NO. Bioconversion of lignocellulose: inhibitors and detoxification. Biotechnol Biofuels. 2013;6:16.
Canilha L, Rodrigues RDCLB, Antunes FAF, et al. Bioconversion of sugarcane biomass into ethanol: an overview about composition, pretreatment methods, detoxification of hydrolysates, enzymatic saccharification and ethanol fermentation. J Biomed Biotechnol. 2012;2012:15.
Pessoa A Jr, Mancilha IM, Sato S. Acid hydrolysis of hemicellulose from sugarcane bagasse. Brazil J Chem Eng. 1997;14:3.
Lavarack BP, Griffin GJ, Rodman D. The acid hydrolysis of sugarcane bagasse hemicellulose to produce xylose, arabinose, glucose and other products. Biomass Bioenergy. 2002;23(5):367–80.
Larsson S, Palmqvist E, Hahn-Hagerdal B, et al. The generation of fermentation inhibitors during dilute acid hydrolysis of softwood. Enzym Microb Technol. 1999;24:151–9.
Adeboye PT, Bettiga M, Olsson L. The chemical nature of phenolic compounds determines their toxicity and induces distinct physiological responses in Saccharomyces cerevisiae in lignocellulose hydrolysates. AMB Express. 2014;4:46.
Paniagua AI, Diez-Antolinez R, Hijosa-Valsero M, et al. Response surface optimization of dilute sulfuric acid pretreatment of switchgrass (Panicum virgatum l.) for fermentable sugars production. Chem Eng Trans. 2016;49:223–8.
Sritrakul N, Nitisinprasert S, Keawsompong S. Evaluation of dilute acid pretreatment for bioethanol fermentation from sugarcane bagasse pith. Agric Nat Res. 2017;51(6):512–9.
Luo Y, Li Z, Li X, et al. The production of furfural directly from hemicellulose in lignocellulosic biomass: a review. Catal Today. 2019;319:14–24.
Stagge S, Adnan Cavka A, Jönsson LJ. Identification of benzoquinones in pretreated lignocellulosic feedstocks and inhibitory effects on yeast. AMB Express. 2015;5:62.
Luís C, DuarteL C, Silva-Fernandes T, et al. Dilute acid hydrolysis of wheat straw oligosaccharides. Appl Biochem Biotechnol. 2009;153:116.
Dussan KJ, Silva DDV, Moraes EJC, et al. Dilute-acid hydrolysis of cellulose to glucose from sugarcane bagasse. Chem Eng Trans. 2014;38:433–8.
Zhuang J, Liu Y, Wu Z, et al. Hydrolysis of wheat straw hemicellulose and detoxification of the hydrolysate for xylitol production. BioResources. 2009;4(2):674–86.
Guerra-Rodríguez E, Portilla-RiveraO M, Jarquín-Enríquez L, et al. Acid hydrolysis of wheat straw: a kinetic study. Biomass Bioenergy. 2012;36:346–55.
Marđetko N, Novak M, Trontel A, et al. Bioethanol production from dilute-acid pre-treated wheat straw liquor hydrolysate by genetically engineered Saccharomyces cerevisiae. Chem Biochem Eng Q. 2018;32(4):483–99.
Baig MZ, Dharmadhikari SM. Optimization of detoxification with over liming and charcoal treatment for increasing the fermentability of cotton stalk hydrolyzate. Microbiology. 2014;4(7):8–10.
Carvalheiro F, Duarte LC, Lopes S, et al. Evaluation of the detoxification of brewery’s spent grain hydrolysate for xylitol production by Debaryomyces hansenii CCMI 941. Process Biochem. 2005;40:1215–23.
Pattra S, Sangyoka S, Boonmee M, et al. Bio-hydrogen production from the fermentation of sugarcane bagasse hydrolysate by Clostridium butyricum. Int J Hydrogen Energy. 2008;33:5256–65.
Llano T, Quijorna N, Coz A. Detoxification of a lignocellulosic waste from a pulp mill to enhance its fermentation prospects. Energies. 2017;10(3):348.
Bahiru TB, Balomajumder C, Roy P. Microbial delignification and hydrolysis of lignocellulosic biomass to enhance biofuel production: an overview and future prospect. Bull Natl Res Centre. 2019;43:51.
Chandel AK, Silva SSRD, Singh O (2011) Detoxification of lignocellulosic hydrolysatesfor improved bioethanol production. In: MarcoAurelio Dos Santos Bernardes (Ed.) Biofuel production-recent developments and prospects, InTech, (ISBN: 978-953-307- 478-8).
Mingming LM, Tu M. Effect of overliming and activated carbon detoxification on inhibitors removal and butanol fermentation of poplar prehydrolysates. Biotechnol Biofuels. 2018;11:178.
Millati R, Niklasson C, Taherzadeh MJ. Effect of pH, time and temperature of overliming on detoxification of dilute-acid hydrolyzates for fermentation by Saccharomyces cerevisiae. Process Biochem. 2002;38(4):515–22.
Ranatunga TD, Jervis J, Helm RF, et al. The effect of overliming on the toxicity of dilute acid pretreated lignocellulosics: the role of inorganics, uronic acids and ether-soluble organics. Enzyme Microb Technol. 2000;27(3–5):240–7.
Persson P, Andersson J, Gorton L, et al. Effect of different forms of alkali treatment on specific fermentation inhibitors and on the fermentability of lignocellulose hydrolysates for production of fuel ethanol. J Agric Food Chem. 2012;50:5318.
Chi Z, Rover M, Jun E, et al. Overliming detoxification of pyrolytic sugar syrup fordirect fermentation of levoglucosan to ethanol. Biores Technol. 2013;150:220–7.
Kashi G. On the analysis of phenol removal from drinking water by batch reactor using powdered eggshell. Biosci Biotech Res Comm. 2017;10(2):287–96.
Cavka A, Guo X, Tang S-J, et al. Production of bacterial cellulose and enzyme from waste fiber sludge. Biotechnol Biofuels. 2013;6:25.
Pecar D, Gorsek A. Enzymatic treatment of paper sludge. Chem Eng Trans. 2015;43:631–6.
Varotkar P, Tumane PM, Wasnik DD. Bioconversion of waste paper into bio-ethanol by co-culture of fungi isolated from lignocellulosic waste. J Pure App Biosci. 2016;4(4):264–74.
Ju LK, Afolabi OA. Waste paper hydrolysate as soluble inducing substrate for cellulase production in continuous culture of Trichoderma reesei. Biotechnol Progg. 1999;5:91–7.
Balagurumurthy B, Singh R, Bhaskar T. Catalysts for thermochemical conversion of biomass. Recent Adv Thermo-Chem Conv Biomass. 2015;2015:109–32.
Joksimovic G, Markovic Z. Investigation of the mechanism of acidic hydrolysis of cellulose. Acta Agric Serbica. 2007;12(24):51.
Franceschin G, Favaron C, Bertucco A. Waste paper as carbohydrate source for biofuel production: an experimental investigation. Chem Engg Trans. 2010;20:279–84.
Domingues FC, Queinoz JA, Cabral JMS, et al. The influence of culture conditions on mycelial structure and cellulase production by Trichoderma reesei RUT C 30. Enzyme Microb Technol. 2004;26:394.
Ghose TK. Measurement of cellulase activities. Pure Appl Chem. 1987;59(2):257–68.
Chen S, Wayman M. Use of sorbose to enhance cellobiose activity in a Trichoderma reesei cellulase system produced on wheat hydrolysates. Biotechnol Technique. 1993;7:345–50.
Cornejo-Ramírez YI, Martínez-Cruz O, Toro-Sánchez CLD, et al. The structural characteristics of starches and their functional properties. CyTA J Food. 2018;16(1):1003–17.
Chandel AK, Kapoor RK, Singh A, et al. Detoxification of sugarcane bagasse hydrolysate improves ethanol production by Candida shehatae NCIM3501. Biores Technol. 2007;98:1947–50.
Nitsos CK, Lazaridis PA, Mach-Aigner A, et al. Enhancing lignocellulosic biomass hydrolysis by hydrothermal pretreatment, extraction of surface lignin, wet milling and production of cellulolytic enzymes. ChemSusChem. 2019;12(6):1179–95.
Talebnia F, Karakashev D, Angelidaki I. Production of bioethanol from wheat straw: an overview on pretreatment, hydrolysis and fermentation. Biores Technol. 2010;101:4744–53.
Cardona CA, Quintero JA, Paz IC. Production of bioethanol from sugarcane bagasse: status and perspectives. Biores Technol. 2010;101:4754–66.
Chansoliya PR, Anand R, Sharma S. Analysis of Bioethanol production from Newspaper by Saccharomyces cerevisiae and Zymomonas mobilis. Int J Eng Develop Res. 2016;4(3):517.
Wyman M, Chen S. Cellulase production induced by carbon source derived from waste news paper. Process Biochem. 1991;26:93–100.
Acknowledgement
Authors gratefully acknowledged the ministry of human resource and development, India for providing fellowship to carry out present research work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, N., Kumar, V. & Bansal, M.C. Utility of starchy, lignocellulosics and cellulosics hydrolysates on cellulase production under liquid state fermentation. Waste Dispos. Sustain. Energy 1, 289–299 (2019). https://doi.org/10.1007/s42768-019-00019-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42768-019-00019-7