Abstract
The conjunctiva is crucial in safeguarding the eye from harm or infection, thereby ensuring the preservation of the vision. The repair of infected conjunctival damage is necessary. The objective of this study is to develop copper-doped flexible silica nanofibers (SiO2@Cu NFs) with multifunctional antibacterial and anti-inflammatory characteristics. The continuous release of copper ions from electrospun membranes is shown to be effective to promote antibacterial and bioactive functions. Nanofiber membranes also exhibit biocompatibility and promote cell growth, angiogenesis, and inflammation modulation. In vivo evaluations further reveal the therapeutic efficacy of SiO2@Cu NFs to promote the structural and the functional recoveries of the conjunctiva. Taken together, SiO2@Cu NFs may hold significant promise for the fabrication of alternative ocular bandage to suppress bacterial infection and promote repair of ocular tissues and may potential be also used for related disciplines.
Graphical Abstract
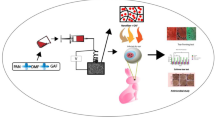
Schematic diagram showing the fabrication of SiO2@Cu NFs for the regeneration of infected conjunctiva.









Similar content being viewed by others
Data Availability
Data will be made available on request.
Abbreviations
- hAM:
-
Human amniotic membrane
- ECM:
-
Extracellular matrix
- PCL:
-
Poly(ε-caprolactone)
- PLCL:
-
Poly(l-lactate-co-ε-caprolactone)
- DDS:
-
Drug delivery systems
- NMs:
-
Nanomaterials
- NPs:
-
Nanoparticles
- TEOS:
-
Tetraethyl orthosilicate
- LPS:
-
Lipopolysaccharides
- DPPH:
-
2,2-Diohenyl-1-picrylhydrazyl
- RT:
-
Room temperature
- SEM:
-
Scanning electron microscopy
- EDS:
-
Energy-dispersive spectroscopy
- FTIR:
-
Fourier-transform infrared spectroscopy
- XRD:
-
X-ray diffraction
- WCA:
-
Water contact angle
- ICP:
-
Inductively coupled plasma atomic emission spectroscopy
- NIH3T3:
-
NIH-3T3 fibroblasts
- HUVEC:
-
Human umbilical vein endothelial cells
- hSCF:
-
Human stromal fibroblasts
- FBS:
-
Fetal bovine serum
- UV:
-
Ultraviolet
- CCK-8:
-
Cell counting kit-8
- VEGF:
-
Vascular endothelial growth factor
- ARVO:
-
Association of Vision and Ophthalmology Annual
- H&E:
-
Hematoxylin and eosin staining
- PAS:
-
Periodic acid-Schiff's
- GO:
-
Gene Ontology
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- OD:
-
Optical density
- DMEM:
-
Dulbecco’s modified Eagle medium
- BCA:
-
Bicinchoninic acid
References
Bandeira F, Yam GH, Fuest M, Ong HS, Liu YC, Seah XY, Shen SY, Mehta JS. Urea-de-epithelialized human amniotic membrane for ocular surface reconstruction. Stem Cells Transl Med. 2019;8:620–6.
Li H, Chen X, Lu WP, Wang J, Xu YS, Guo YC. Application of electrospinning in antibacterial field. Nanomaterials. 2021;11:1822.
Wei Z, Wang L, Zhang S, Chen T, Yang J, Long S, Wang X. Electrospun antibacterial nanofibers for wound dressings and tissue medicinal fields: a review. J Innovative Opt Health Sci. 2020;13:2030012.
Khunova V, Kovacova M, Olejnikova P, Ondreas F, Spitalsky Z, Ghosal K, Berkes D. Antibacterial electrospun polycaprolactone nanofibers reinforced by halloysite nanotubes for tissue engineering. Polymers. 2022;14:14746.
Zhou S, Yang D, Yang D, Guo Y, Hu R, Li Y, Zan X, Zhang X. Injectable, self-healing and multiple responsive histamine modified hyaluronic acid hydrogels with potentialities in drug delivery, antibacterial and tissue engineering. Macromol Rapid Commun. 2022;44:2200674.
Yi X, He J, Wang X, Zhang Y, Tan G, Zhou Z, Chen J, Chen D, Wang R, Tian W, Yu P, Zhou L, Ning C. Tunable mechanical, antibacterial, and cytocompatible hydrogels based on a functionalized dual network of metal coordination bonds and covalent crosslinking. ACS Appl Mater Interfaces. 2018;10:6190–8.
Lu H, Li X, Zhang M, Xu C, Li W, Wan L. Antibacterial cellulose nanocrystal-incorporated hydrogels with satisfactory vascularization for enhancing skin regeneration. Front Bioeng Biotechnol. 2022;10:876936.
Arrizabalaga JH, Nollert MU. Human amniotic membrane: a versatile scaffold for tissue engineering. ACS Biomater Sci Eng. 2018;4:2226–36.
Fenelon M, Catros S, Meyer C, Fricain JC, Obert L, Auber F, Louvrier A, Gindraux F. Applications of human amniotic membrane for tissue engineering. Membranes. 2021;11:387.
Witt J, Dietrich J, Mertsch S, Schrader S, Spaniol K, Geerling G. Decellularized porcine conjunctiva as an alternative substrate for tissue-engineered epithelialized conjunctiva. Ocul Surf. 2020;18:901–11.
Chen Y, Dong X, Shafiq M, Myles G, Radacsi N, Mo X. Recent advancements on three-dimensional electrospun nanofiber scaffolds for tissue engineering. Adv Fiber Mater. 2022;4:959–86.
Bosworth LA, Doherty KG, Hsuan JD, Cray SP, D’Sa RA, Pineda Molina C, Badylak SF, Williams RL. Material characterisation and stratification of conjunctival epithelial cells on electrospun poly(epsilon-caprolactone) fibres loaded with decellularised tissue matrices. Pharmaceutics. 2021;13:318.
Yao Q, Zhang W, Hu Y, Chen J, Shao C, Fan X, Fu Y. Electrospun collagen/poly(l-lactic acid-co-epsilon-caprolactone) scaffolds for conjunctival tissue engineering. Exp Ther Med. 2017;14:4141–7.
Yan D, Zhang S, Yu F, Gong D, Lin J, Yao Q, Fu Y. Insight into levofloxacin loaded biocompatible electrospun scaffolds for their potential as conjunctival substitutes. Carbohydr Polym. 2021;269:118341.
Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis-part II: Cr, Si, Zn, Cu, and S. Crit Rev Oncol/Hematol. 2015;96:143–55.
Zhang X, Cheng X, Si Y, Yu J, Ding B. Elastic and highly fatigue resistant ZrO2–SiO2 nanofibrous aerogel with low energy dissipation for thermal insulation. Chem Eng J. 2022;433:133628.
Zulkiflee I, Masri S, Zawani M, Salleh A, Amirrah IN, Wee M, Yusop SM, Fauzi MB. Silicon-based scaffold for wound healing skin regeneration applications: a concise review. Polymers. 2022;14:4219.
Uriu-Adams JY, Keen CL. Copper, oxidative stress, and human health. Mol Aspects Med. 2005;26:268–98.
Das A, Sudhahar V, Chen GF, Kim HW, Youn SW, Finney L, Vogt S, Yang J, Kweon J, Surenkhuu B, Ushio-Fukai M, Fukai T. Endothelial antioxidant-1: a key mediator of copper-dependent wound healing in vivo. Sci Rep. 2016;6:33783.
Wang S, Wang Y, Peng Y, Yang X. Exploring the antibacteria performance of multicolor Ag, Au, and Cu nanoclusters. ACS Appl Mater Interfaces. 2019;11:8461–9.
Shen Q, Qi Y, Kong Y, Bao H, Wang Y, Dong A, Wu H, Xu Y. Advances in copper-based biomaterials with antibacterial and osteogenic properties for bone tissue engineering. Front Bioeng Biotechnol. 2021;9:795425.
Liu M, Shafiq M, Sun B, Wu J, Wang W, El-Newehy M, El-Hamshary H, Morsi Y, Ali O, Khan AUR, Mo X. Composite superelastic aerogel scaffolds containing flexible SiO2 nanofibers promote bone regeneration. Adv Healthcare Mater. 2022;11:2200499.
Wu N, Chen L, Yan D, Zhou M, Shao C, Lu Y, Yao Q, Sun H, Fu Y. Trehalose attenuates TGF-beta1-induced fibrosis of hscfs by activating autophagy. Mol Cell Biochem. 2020;470:175–88.
Wu N, Yan C, Chen J, Yao Q, Lu Y, Yu F, Sun H, Fu Y. Conjunctival reconstruction via enrichment of human conjunctival epithelial stem cells by p75 through the NGF-p75-SALL2 signaling axis. Stem Cells Transl Med. 2020;9:1448–61.
Wang L, Qiu Y, Guo Y, Si Y, Liu L, Cao J, Yu J, Li X, Zhang Q, Ding B. Smart, elastic, and nanofiber-based 3d scaffolds with self-deploying capability for osteoporotic bone regeneration. Nano Lett. 2019;19:9112–20.
Awadh SM, Yaseen ZM. Investigation of silica polymorphs stratified in siliceous geode using FTIR and XRD methods. Mater Chem Phys. 2019;228:45–50.
Wang Y, Zhang W, Yao Q. Copper-based biomaterials for bone and cartilage tissue engineering. J Orthop Transl. 2021;29:60–71.
Han W, Cummings H, Duvuuru MK, Fleck S, Vahabzadeh S, Elsawa SF. In vitro osteogenic, angiogenic, and inflammatory effects of copper in β-tricalcium phosphate. MRS Adv. 2019;4:1253–9.
Majewski M, Ognik K, Juskiewicz J. Copper nanoparticles modify the blood plasma antioxidant status and modulate the vascular mechanisms with nitric oxide and prostanoids involved in wistar rats. Pharmacol Rep. 2019;71:509–16.
Abramovič H, Grobin B, Poklar Ulrih N, Cigić B. Relevance and standardization of in vitro antioxidant assays: ABTS, DPPH, and Folin-Ciocalteu. J Chem. 2018;2018:1–9.
Tan Z, Zhou B, Zheng J, Huang Y, Zeng H, Xue L, Wang D. Lithium and copper induce the osteogenesis-angiogenesis coupling of bone marrow mesenchymal stem cells via crosstalk between canonical wnt and hif-1alpha signaling pathways. Stem Cells Int. 2021;2021:6662164.
Li J, Zhai D, Lv F, Yu Q, Ma H, Yin J, Yi Z, Liu M, Chang J, Wu C. Preparation of copper-containing bioactive glass/eggshell membrane nanocomposites for improving angiogenesis, antibacterial activity and wound healing. Acta Biomater. 2016;36:254–66.
Giacomelli C, Trincavelli ML, Satriano C, Hansson O, La Mendola D, Rizzarelli E, Martini C. Copper(II) ions modulate angiogenin activity in human endothelial cells. Int J Biochem Cell Biol. 2015;60:185–96.
Wang X, Gao L, Han Y, Xing M, Zhao C, Peng J, Chang J. Silicon-enhanced adipogenesis and angiogenesis for vascularized adipose tissue engineering. Adv Sci. 2018;5:1800776.
Kong N, Lin K, Li H, Chang J. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J Mater Chem B. 2014;2:1100–10.
Wu Q, Xu S, Wang X, Jia B, Han Y, Zhuang Y, Sun Y, Sun Z, Guo Y, Kou H, Ning C, Dai K. Complementary and synergistic effects on osteogenic and angiogenic properties of copper-incorporated silicocarnotite bioceramic: in vitro and in vivo studies. Biomaterials. 2021;268:120553.
Liu M, Wang X, Cui J, Wang H, Sun B, Zhang J, Rolauffs B, Shafiq M, Mo X-M, Zhu Z, Wu J. Electrospun flexible magnesium-doped silica bioactive glass nanofiber membranes with anti-inflammatory and pro-angiogenic effects for infected wounds. J Mater Chem B. 2022;11:359.
Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–33.
Jaidev LR, Kumar S, Chatterjee K. Multi-biofunctional polymer graphene composite for bone tissue regeneration that elutes copper ions to impart angiogenic, osteogenic and bactericidal properties. Colloids Surf B. 2017;159:293–302.
Raffi M, Mehrwan S, Bhatti TM, Akhter JI, Hameed A, Yawar W, ul Hasan MM. Investigations into the antibacterial behavior of copper nanoparticles against Escherichia coli. Ann Microbiol. 2010;60:75–80.
Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–66.
Chen Y, Hu M, Wang L, Chen W. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090.
Lin R, Deng C, Li X, Liu Y, Zhang M, Qin C, Yao Q, Wang L, Wu C. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics. 2019;9:6300–13.
Chen Y, Yuan Z, Sun W, Shafiq M, Zhu J, Chen J, Tang H, Hu L, Lin W, Zeng Y, Wang L, Zhang L, She Y, Zheng H, Zhao G, Xie D, Mo X, Chen C. Vascular endothelial growth factor-recruiting nanofiber bandages promote multifunctional skin regeneration via improved angiogenesis and immunomodulation. Adv Fiber Mater. 2023;5:327–48.
Shafiq M, Rafique M, Cui Y, Pan L, Do C-W, Ho EA. An insight on ophthalmic drug delivery systems: focus on polymeric biomaterials-based carriers. J Control Release. 2023;362:446–67.
Suzuki T, Yamamoto T, Ohashi Y. The antibacterial activity of levofloxacin eye drops against staphylococci using an in vitro pharmacokinetic model in the bulbar conjunctiva. J Infect Chemother. 2016;22:360–5.
Ai F, Chen L, Yan J, Yang K, Li S, Duan H, Cao C, Li W, Zhou K. Hydroxyapatite scaffolds containing copper for bone tissue engineering. J Sol-Gel Sci Technol. 2020;95:168–79.
Ren H, Pan C, Liu Y, Liu D, He X, Li X, Sun X. Fabrication, in vitro and in vivo properties of porous Zn–Cu alloy scaffolds for bone tissue engineering. Mater Chem Phys. 2022;289:126458.
Ning C, Wang X, Li L, Zhu Y, Li M, Yu P, Zhou L, Zhou Z, Chen J, Tan G, Zhang Y, Wang Y, Mao C. Concentration ranges of antibacterial cations for showing the highest antibacterial efficacy but the least cytotoxicity against mammalian cells: Implications for a new antibacterial mechanism. Chem Res Toxicol. 2015;28:1815–22.
Shi Q, Luo X, Huang Z, Midgley AC, Wang B, Liu R, Zhi D, Wei T, Zhou X, Qiao M, Zhang J, Kong D, Wang K. Cobalt-mediated multi-functional dressings promote bacteria-infected wound healing. Acta Biomater. 2019;86:465–79.
Huang Z, Zhang Y, Liu R, Li Y, Rafique M, Midgley AC, Wan Y, Yan H, Si J, Wang T, Chen C, Wang P, Shafiq M, Li J, Zhao L, Kong D, Wang K. Cobalt loaded electrospun poly(epsilon-caprolactone) grafts promote antibacterial activity and vascular regeneration in a diabetic rat model. Biomaterials. 2022;291:121901.
Zidan G, Rupenthal ID, Greene C, Seyfoddin A. Medicated ocular bandages and corneal health: potential excipients and active pharmaceutical ingredients. Pharm Dev Technol. 2018;23:255–60.
Kumari S, Singh BN, Srivastava P. Effect of copper nanoparticles on physico-chemical properties of chitosan and gelatin-based scaffold developed for skin tissue engineering application. 3 Biotech. 2019;9:102.
Gerard C, Bordeleau LJ, Barralet J, Doillon CJ. The stimulation of angiogenesis and collagen deposition by copper. Biomaterials. 2010;31:824–31.
Hemmati S, Ahmeda A, Salehabadi Y, Zangeneh A, Zangeneh MM. Synthesis, characterization, and evaluation of cytotoxicity, antioxidant, antifungal, antibacterial, and cutaneous wound healing effects of copper nanoparticles using the aqueous extract of Strawberry fruit and L-Ascorbic acid. Polyhedron. 2020;180:114425.
Ginting B, Maulana I, Karnila I. Biosynthesis copper nanoparticles using blumea balsamifera leaf extracts: characterization of its antioxidant and cytotoxicity activities. Surf Interfaces. 2020;21:100799.
Shafiq M, Chen Y, Hashim R, He C, Mo X, Zhou X. Reactive oxygen species-based biomaterials for regenerative medicine and tissue engineering applications. Front Bioeng Biotechnol. 2021;9:821288.
Hassanshahi A, Moradzad M, Ghalamkari S, Fadaei M, Cowin AJ, Hassanshahi M. Macrophage-mediated inflammation in skin wound healing. Cells. 2022;11:2953.
Gu G, Erisen DE, Yang K, Zhang B, Shen M, Zou J, Qi X, Chen S, Xu X. Antibacterial and anti-inflammatory activities of chitosan/copper complex coating on medical catheters: in vitro and in vivo. J Biomed Mater Res Part B. 2022;110:1899–910.
Maher P. Modulation of the neuroprotective and anti-inflammatory activities of the flavonol fisetin by the transition metals iron and copper. Antioxidants. 2020;9:1113.
Funding
This research was supported by Science and Technology Commission of Shanghai Municipality, China (Nos. 20S31900900, 20DZ2254900), Sino German Science Foundation Research Exchange Center, China (M-0263), and China Education Association for International Exchange (2022181). This project was also supported by Researchers Supporting Project Number (RSP2024R65), King Saud University, Riyadh, Saudi Arabia. Moreover, this project was supported by the State Key Laboratory for Modification of Chemical Fibers and Polymer Materials, Donghua University (KF2109), National Natural Science Foundation of China (No.82070919 and 82271041), the Program of Shanghai Academic/Technology Research Leader (22XD1401800); the Biomaterials and Regenerative Medicine Institute Cooperative Research Project, Shanghai Jiao Tong University School of Medicine (2022LHA06), Shanghai Key Clinical Specialty, and Shanghai Eye Disease Research Center (2022ZZ01003). This project was also supported by the Fundamental Research Funds for the Central Universities (CUSF-DH- T-2023064).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 56392 KB)
Supplementary file3 (MP4 15543 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cui, J., Cai, Y., Yu, X. et al. Flexible Copper-Doped Silica Fibers Promote Infected Conjunctival Tissue Repair Through Antibacterial and Anti-inflammatory Effects. Adv. Fiber Mater. 6, 278–296 (2024). https://doi.org/10.1007/s42765-023-00358-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-023-00358-5