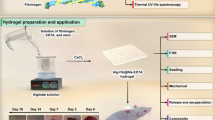
Abstract
Biomass-based supramolecular hydrogels are widely used in the biomedical field due to their favorable biocompatibility and outstanding mechanical properties. However, the preparation of injectable polysaccharide-based hydrogels has proven to be a significant challenge. Here we have employed a simple poor-solvent strategy to prepare alginate supramolecular hydrogels via a hierarchical self-assembly process, including micellization, micelles alignment to form nanofilament, and nanofibrils formation. The alginate supramolecular fibrillar hydrogels exhibit excellent mechanical properties and shear recoverability, meeting the requirements of injectable hydrogels. Furthermore, the presence of alginate and its fibrillar structures imparts superb hemostasis properties and the inherent biocompatibility to these hydrogels. Therefore, this simple and intriguing approach has the potential to develop polysaccharide-based hydrogels for hemostasis in wound within the biomedical fields.
Graphical Abstract








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding authors upon request.
References
Levin A, Mason TO, Adler-Abramovich L, Buell AK, Meisl G, Galvagnion C, Bram Y, Stratford SA, Dobson CM, Knowles TP, Gazit E. Ostwald’s rule of stages governs structural transitions and morphology of dipeptide supramolecular polymers. Nat Commun. 2014;5:5219.
Fichman G, Guterman T, Damron J, Adler-Abramovich L, Schmidt J, Kesselman E, Shimon LJW, Ramamoorthy A, Talmon Y, Gazit E. Spontaneous structural transition and crystal formation in minimal supramolecular polymer model. Sci Adv. 2016;2: e1500827.
Lehn J-M. Perspectives in supramolecular chemistry-from molecular recognition towards molecular information processing and self-organization. Angew Chem Int Ed. 1990;29:1304.
Burnworth M, Tang L, Kumpfer JR, Duncan AJ, Beyer FL, Fiore GL, Rowan SJ, Weder C. Optically healable supramolecular polymers. Nature. 2011;472:334.
Hou J, Liu M, Zhang H, Song Y, Jiang X, Yu A, Jiang L, Su B. Healable green hydrogen bonded networks for circuit repair, wearable sensor and flexible electronic devices. J Mater Chem A. 2017;5:13138.
Lokhande G, Carrow JK, Thakur T, Xavier JR, Parani M, Bayless KJ, Gaharwar AK. Nanoengineered injectable hydrogels for wound healing application. Acta Biomater. 2018;70:35.
Meng Y, Zou S, Jiang M, Xu X, Tang BZ, Zhang L. Dendritic nanotubes self-assembled from stiff polysaccharides as drug and probe carriers. J Mater Chem B. 2017;5:2616.
Sun Y, Zhang X, Wu T, Zhang Z, Yang R, Liu W. YAP-suppressive nanodrug crosslinked self-immunoregulatory polysaccharide injectable hydrogel for attenuating cardiac fibrosis to treat myocardial infarction. Adv Funct Mater. 2023;33:2214468.
Cha GD, Kim M, Park OK, Sunwoo SH, Kang T, Lee WH, Nam S, Hyeon T, Choi SH, Kim DH. Minimally-invasive and in-vivo hydrogel patterning method for in situ fabrication of implantable hydrogel devices. Small Methods. 2023;7:2300032.
Tan W, Long T, Wan Y, Li B, Xu Z, Zhao L, Mu C, Ge L, Li D. Dual-drug loaded polysaccharide-based self-healing hydrogels with multifunctionality for promoting diabetic wound healing. Carbohyd Polym. 2023;312: 120824.
Yuk H, Lu B, Zhao X. Hydrogel bioelectronics. Chem Soc Rev. 2019;48:1642.
Sunwoo SH, Han SI, Joo H, Cha GD, Kim D, Choi SH, Hyeon T, Kim DH. Advances in soft bioelectronics for brain research and clinical neuroengineering. Matter. 2020;3:1923.
Liu S, Rao Y, Jang H, Tan P, Lu N. Strategies for body-conformable electronics. Matter. 2022;5:1104.
Duan J, Liang X, Cao Y, Wang S, Zhang L. High strength chitosan hydrogels with biocompatibility via new avenue based on constructing nanofibrous architecture. Macromolecules. 2015;48:2706.
Jones CD, Kennedy SR, Walker M, Yufit DS, Steed JW. Scrolling of supramolecular lamellae in the hierarchical self-assembly of fibrous gels. Chem. 2017;3:603.
Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171.
Aida T, Meijer EW, Stupp SI. Functional supramolecular polymers. Science. 2012;335:813.
Adler-Abramovich L, Gazit E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications. Chem Soc Rev. 2014;43:6881.
Ikezoe Y, Washino G, Uemura T, Kitagawa S, Matsui H. Autonomous motors of a metal–organic framework powered by reorganization of self-assembled peptides at interfaces. Nat Mater. 2012;11:1081.
Hauser CAE, Maurer-Stroh S, Martins IC. Amyloid-based nanosensors and nanodevices. Chem Soc Rev. 2014;43:5326.
Liu Y, Ling S, Wang S, Chen X, Shao Z. Thixotropic silk nanofibril-based hydrogel with extracellular matrix-like structure. Biomater Sci. 2014;2:1338.
Chen Z, Liu X, Ding J, Tian Y, Zhang Y, Wei D, Sun J, Luo F, Zhou L, Fan H. Tissue-like electrophysiological electrode interface construction by multiple crosslinked polysaccharide-based hydrogel. Carbohyd Polym. 2022;296: 119923.
Wei P, Yu X, Fang Y, Wang L, Zhang H, Zhu C, Cai J. Strong and tough cellulose hydrogels via solution annealing and dual cross-linking. Small. 2023;19:2301204.
Tong C, Jiang S, Ye D, Li K, Liu J, Zeng X, Wu C, Pang J. Enhanced mechanical property and freeze-thaw stability of alkali-induced heat-set konjac glucomannan hydrogel through anchoring interface effects of carboxylated cellulose nanocrystals. Food Hydrocolloid. 2023;142: 108812.
Yanaki T, Norisuye T, Fujita H. Triple helix of Schizophyllum commune polysaccharide in dilute solution. 3. Hydrodynamic properties in water. Macromolecules. 1980;13:1462.
Xu S, Lin Y, Huang J, Li Z, Xu X, Zhang L. Construction of high strength hollow fibers by self-assembly of a stiff polysaccharide with short branches in water. J Mater Chem A. 2013;1:4198.
Fang Y, Duan B, Lu A, Liu M, Liu H, Xu X, Zhang L. Intermolecular interaction and the extended wormlike chain conformation of chitin in NaOH/urea aqueous solution. Biomacromol. 2015;16:1410.
Zhang X, Sheng N, Wang L, Tan Y, Liu C, Xia Y, Nie Z, Sui K. Supramolecular nanofibrillar hydrogels as highly stretchable, elastic and sensitive ionic sensors. Mater Horiz. 2019;6:326.
Zhao C, Zhang C, Kang H, Xia Y, Sui K, Liu R. Gelation of Na-alginate aqueous solution: a study of sodium ion dynamics via NMR relaxometry. Carbohyd Polym. 2017;169:206.
Dou X, Zhou Q, Chen X, Tan Y, He X, Lu P, Sui K, Tang BZ, Zhang Y, Yuan WZ. Clustering-triggered emission and persistent room temperature phosphorescence of sodium alginate. Biomacromolecules. 2018;19:2014.
Cleland RL. Viscometry and sedimentation equilibrium of partially hydrolyzed hyaluronate: comparison with theoretical models of wormlike chains. Biopolymers. 1984;23:647.
Mourey T, Le K, Bryan T, Zheng S, Bennett G. Determining persistence length by size-exclusion chromatography. Polymer. 2005;46:9033.
Vold IMN, Kristiansen KA, Christensen BE. A study of the chain stiffness and extension of alginates, in vitro epimerized alginates, and periodate-oxidized alginates using size-exclusion chromatography combined with light scattering and viscosity detectors. Biomacromol. 2006;7:2136.
McIntire TM, Brant DA. Imaging of carrageenan macrocycles and amylose using noncontact atomic force microscopy. Int J Biol Macromol. 1999;26:303.
Schefer L, Adamcik J, Mezzenga R. Unravelling secondary structure changes on individual anionic polysaccharide chains by atomic force microscopy. Angew Chem Int Ed. 2014;53:5376.
Asadi-Korayem M, Akbari-Taemeh M, Mohammadian-Sabet F, Shayesteh A, Daemi H. How does counter-cation substitution influence inter- and intramolecular hydrogen bonding and electrospinnability of alginates. Int J Biol Macromol. 2021;171:234.
Xu S, Xu X, Zhang L. Effect of heating on chain conformation of branched β-glucan in water. J Phys Chem B. 2013;117:8370.
Schefer L, Adamcik J, Diener M, Mezzenga R. Supramolecular chiral self-assembly and supercoiling behavior of carrageenans at varying salt conditions. Nanoscale. 2015;7:16182.
Kirwan LJ, Papastavrou G, Borkovec M, Behrens SH. Imaging the coil-to-globule conformational transition of a weak polyelectrolyte by tuning the polyelectrolyte charge density. Nano Lett. 2004;4:149.
Wang J, Liu K, Xing R, Yan X. Peptide self-assembly: thermodynamics and kinetics. Chem Soc Rev. 2016;45:5589.
Liu Y, Zhang Y, Wang Z, Wang J, Wei K, Chen G, Jiang M. Building nanowires from micelles: hierarchical self-assembly of alternating amphiphilic glycopolypeptide brushes with pendants of high-mannose glycodendron and oligophenylalanine. J Am Chem Soc. 2016;138:12387.
Lampel A, McPhee SA, Park HA, Scott GG, Humagain S, Hekstra DR, Yoo B, Frederix PWJM, Li TD, Abzalimov RR, Greenbaum SG, Tuttle T, Hu C, Bettinger CJ, Ulijn RV. Polymeric peptide pigments with sequence-encoded properties. Science. 2017;356:1064.
Wang J, Chao J, Liu H, Su S, Wang L, Huang W, Willner I, Fan C. Clamped hybridization chain reactions for the self-assembly of patterned DNA hydrogels. Angew Chem Int Ed. 2017;56:2171.
Levin A, Mason TO, Adler-Abramovich L, Buell AK, Meisl G, Galvagnion C, Bram Y, Stratford SA, Dobson CM, Knowles TPJ, Gazit E. Ostwald’s rule of stages governs structural transitions and morphology of dipeptide supramolecular polymers. Nat Commun. 2014;5:5219.
Meiboom S, Gill D. Modified spin-echo method for measuring nuclear relaxation times. Rev Sci Instrum. 2004;29:688.
Seale R, Morris ER, Rees DA. Interactions of alginates with univalent cations. Carbohyd Res. 1982;110:101.
Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable multidomain peptide nanofiber hydrogel as a delivery agent for stem cell secretome. Biomacromol. 2011;12:1651.
Dowling MB, Kumar R, Keibler MA, Hess JR, Bochicchio GV, Raghavan SR. A self-assembling hydrophobically modified chitosan capable of reversible hemostatic action. Biomaterials. 2011;32:3351.
Li J, Celiz AD, Yang J, Yang Q, Wamala I, Whyte W, Seo BR, Vasilyev NV, Vlassak JJ, Suo Z, Mooney DJ. Tough adhesives for diverse wet surfaces. Science. 2017;357:378.
Qiao H, Qi P, Zhang X, Wang L, Tan Y, Luan Z, Xia Y, Li Y, Sui K. Multiple weak h-bonds lead to highly sensitive, stretchable, self-adhesive, and self-healing ionic sensors. ACS Appl Mater Interfaces. 2019;11:7755.
Lih E, Lee JS, Park KM, Park KD. Rapidly curable chitosan–PEG hydrogels as tissue adhesives for hemostasis and wound healing. Acta Biomater. 2012;8:3261.
Ryu JH, Lee Y, Kong WH, Kim TG, Park TG, Lee H. Catechol-functionalized chitosan/pluronic hydrogels for tissue adhesives and hemostatic materials. Biomacromol. 2011;12:2653.
Shin M, Park SG, Oh BC, Kim K, Jo S, Lee MS, Oh SS, Hong SH, Shin EC, Kim KS, Kang SW, Lee H. Complete prevention of blood loss with self-sealing haemostatic needles. Nat Mater. 2017;16:147.
Acknowledgements
This research was supported by State Key Laboratory of Bio-Fibers and Eco-Textiles, Qingdao University (ZDKT202006)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Hou, W., Zhang, Q. et al. Hierarchical Self-Assembly of Injectable Alginate Supramolecular Nanofibril Hydrogels for Hemostasis In Vivo. Adv. Fiber Mater. 6, 489–500 (2024). https://doi.org/10.1007/s42765-023-00355-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42765-023-00355-8