Abstract
Fruit fly surveillance remains essential for international and domestic trade. The dry cuelure baited Lynfield trap has been the Australian standard since the early 1990s. Here, we tested the two versions of Biotraps against the Lynfield traps in the Riverina area of New South Wales. The Biotraps using a protein gel performed significantly better in trapping Island fly and female Queensland fruit fly. Also, Biotraps were assessed as at least equal to or superior to Lynfield traps for trapping male Queensland fruit fly. However, the number of Newman fly trapped exhibited no significant difference between the two trap types in both time periods A and B. We discuss differences in trap architecture, toxicants and lures between the two traps, along with benefits for storage and transport.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many tephritids are pests of fruit crops and an impediment to trade throughout the world (Vargas et al. 2015). Fortunately, many pest Dacus and Bactrocera species are well suited to eradication or management because the males are attracted to lures such as methyl eugenol and cuelure (Suckling et al. 2016). In Australia, Queensland fruit fly (Qfly), Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), is the main pest of most fruits and fruiting vegetables along the east coast, including the Riverina region in south east New South Wales (NSW) (Dominiak and Mapson 2017). Qfly has 297 known hosts in native and commercial fruit and vegetables (Dominiak 2023). Female Qfly can lay hundred’s of eggs in their lifetime and usually, eggs are laid in ripening fruit, often leaving little surface indication that fruit is infested. Infested fruit is transported long distances by human assisted methods, frequently in vehicles by road and air (Dominiak et al. 2000). The estimated natural dispersal of Qfly is about 400 m in their lifetime, mostly recently reviewed by Dominiak and Fanson (2020). The detection of larvae in commercial fruit consignments requires the fruit to be disinfested before trade in the domestic or international markets can be completed (Jessup et al. 1998; IPPC 1999, 2006, 2007). All these events cause considerable loss of income for producers or the destruction of food (Dominiak and Mapson 2017).
Other tephritids trapped in the Riverina include Newman fly (Dacus newmani (Perkins), an non economic tephritid, which was trapped in large numbers in cuelure traps (Dominiak 2019b). Additionally, Island fly (Dirioxa pornia (Walker) is a pest of damaged fruit and is attracted to protein lure (Dominiak et al. 2003).
Lures are essential for surveillance. Additionally, trap design is important and continues to evolve in Australia. Cuelure is an attractive male lure of Qfly (Monro and Richardson 1969). Cuelure, {4-(p-acetoxyphenol)-2-butanone} is a stabilised precursor of raspberry ketone and cuelure breaks down to raspberry ketone, particularly in the presence of moisture (Alexander et al. 1962; Dominiak et al. 2003). Jackson traps (delta design) baited with cuelure using sticky mats were originally used in the late 1980s in NSW. Cowley et al. (1990) demonstrated the Lynfield traps, baited with cuelure and malathion, were superior to the Jackson traps and subsequently, Lynfield traps (cuelure and malathion) became the standard trap design in southern Australia (Dominiak et al. 2003). However, cuelure-baited Lynfield traps only trapped male flies.
Both male and female Qfly require protein in their diet to reach sexual maturity (Fletcher 1987) and therefore traps that contain protein are able to monitor both sexes. The liquid protein-baited McPhail traps did attract both males and females but were only about one-seventh as effective as Lynfield traps (Dominiak and Nicol 2010). Protein-baited McPhail traps are not used in standard trapping arrays but were used more frequently in incursion surveillance to find the epicentre of an incursion or outbreak (Dominiak et al. 2003). Both traps are recognised in international trade agreements with the cuelure baited Lynfield trap being the mainstay for Qfly surveillance.
Qfly is native to Australia and currently present in Australia and in several Pacific islands (Vargas et al. 2015). In Australia, Qfly is established in the eastern states/territories of Northern Territory, Australian capital Territory, Queensland, New South Wales and Victoria (Dominiak and Mapson 2017). The presence of Qfly in production areas means that fruit for some domestic or all international market access must be disinfested (Jessup 1988; Jessup et al. 1998; De Lima et al. 2007; Haynes and Dominiak 2018) and this treatment comes at a significant cost in financial and time delay terms. Some trade protocols do not require disinfestation if producers can demonstrate pest freedom at regional or local areas (Dominiak et al. 2015; Dominiak and Worsley 2016; Dominiak and Mapson 2017; IPPC 1999, 2007). In the early 1990’s, Australia developed a protocol to demonstrate the presence or absence of Qfly and this was accepted internationally in the mid 1990’s (see Dominiak and Mapson (2017) for details). Essentially, a trapping array of Lynfield traps was established on a 1000 m array in orchards and 400 m array in urban areas. The threshold for an outbreak is based primarily on the detection of five male Qfly (Dominiak et al. 2011a) and the five male Qfly detection was the most frequent event resulting in the declaration of an outbreak (Dominiak and Mapson 2017). Detections of female Qfly or larval detections in fruit usually happened after an outbreak was declared and there was a breeding population. Wet protein-baited McPhail traps were used to help find the epicentre of an outbreak but were less effective and more time consuming to service (Dominiak and Nicol 2010).
However, the Lynfield trap shells have some drawbacks. The holes for insect ingress and drainage have to be drilled before use. The 1 L shells do not stack efficiently into each other and take up considerable space in storage or during transport to surveillance centres, particularly when thousands of traps are stored in preparation for scheduled trap replacement. Additionally, regulators must build each trap and source the components separately. By comparison, cone traps can be flat packed and use considerably less storage space but must be assembled prior to use (Dominiak et al. 2019). The Biotrap components stack efficiently into each other and require less space for storage or transport compared to Lynfield traps. The production of the 1 L plastic Lynfield container is declining and the availability of these containers is becoming more challenging. There is a need to develop alternative traps to service fruit fly surveillance.
Growers and fruit fly managers/regulators continue to seek to optimise Qfly surveillance. Alternative traps continue to be developed but need to be equivalent to current international standards. These alternative traps will provide growers with a choice in traps to suit individual farming enterprises. In an initial assessment in Victoria, Bain and Dominiak (2022) found that the BioTrap, baited with a protein gel and dichlorvos toxicant, was a suitable alternative to the Lynfield Trap. Here, we conducted a more extensive scale assessment to compare the performance of Biotraps (protein gel-baited) with the Lynfield traps (cuelure-baited) in southern NSW for trapping Qfly (male and female), Newman fly and Island fly.
Materials and methods
The Riverina is a major fruit producing region in southern NSW, Australia and was a part of the Fruit Fly Exclusion Zone (FFEZ) for trade optimisation by demonstrating pest freedom (IPPC 1999; Dominiak and Mapson 2017). Prior to 2013, the FFEZ trapping array in fruiting hosts existed throughout the Riverina on either 1 km (orchard) or 400 m (urban) arrays. However, after the FFEZ was discontinued in 2013 (Dominiak and Mapson 2017), and a 5 km array was in place and operational during our trial (Quilici and Donner 2012). The trial was conducted at 11 sites (replicates) in the Riverina in the districts of Hanwood, Tharbogang, Somerton Park, Corbie Hill, Paynter’s Siding, Nericon, Darlington Point, and Leeton. Sites were located in orchards where the existing cuelure-baited Lynfield traps were already operational. At each site, we selected a fruiting tree next to the Lynfield trap tree to hang the Biotrap. The assessment was conducted between 30 and 2014 and 22 June 2016. This period was after area wide management of Qfly had ceased in July 2013. Pest management at each site was conducted by individual growers.
Lynfield traps (Fig. 1a) are a 1 L cylindrical clear plastic container (120 mm in depth and diameter), a yellow lid, and a cotton wick lure dispenser (Cowley et al. 1990; Dominiak and Nicol 2010). In the trap’s body, there were four 25 mm holes drilled at equally spaced locations into the sides to allow the lure vapour to exit the trap and for insects to enter. Four additional 2 mm holes were drilled into the bottom to prevent any water accumulation. Lynfield lure dispensers comprised of cotton wicks [four dental cotton rolls (each 10 mm x 40 mm long) held together by a wire clamp] suspended from the middle of the trap lid. The wick was hung at about the same level as the ingress holes in the side wall of the trap body. The cuelure treatment was a 5 mL solution containing eight parts cuelure and one part malathion (1150 gL− 1 active ingredient). Wicks were changed every six months.
Biotraps were designed and manufactured by Biotrap Pty Ltd (BioTrap Australia Pty Ltd, Ocean Grove, Victoria, 3226, Australia) (Fig. 1b and c). Both versions (minor variations in base design) of the Biotrap consisted of two individual plastic bodies, the top and the base. The top was produced from clear PVC with a rounded skirt allowing it to be pushed (clipped together) onto the base. This rounded skirt had additional internal protrusions to ensure a firm grip on the base. A small centrally positioned hole in the top allowed a clip to be pushed through to enable the trap to be hung from a suitable tree branch or similar.
The Biotrap base was made by injection moulding using PMS 803 yellow HIPS (High Impact Poly Styrene), which resulted in a structurally ridged body. The base had a lip to fit within the skirt of the top. The base contained five vertically inclined entry holes of 10 mm diameter, four around the circumference and one in the centre. The four cardinal positioned holes within the trap ended abruptly to reduce the potential for insects exiting, and their entry was via a scalloped section. The centre hole in the base was a cone type with an entry size of 25 mm, rising 50 mm within the base to end in a 10 mm hole (Fig. 1b and c). The width of the trap was 150 mm, the base was 75 mm and the top was 75 mm making for a compact design that allowed for easy stacking of each component. The base had a capacity for 250 mL of liquid.
The female-biased protein lure was demonstrated to primarily attract the female Qfly, although males were also attracted (Dominiak and Nicol 2010). The Biotrap attractant was a gel produced from a stabilised protein concentrate, ammonium compounds and a thickening agent (Xanthan gum). The lure was changed every three months. The toxicant was DDVP (dichlorvos) carried on a cube and DDVP cubes were replaced every three months. For the DDVP cubes, Biotrap purchased stripes from AMVAC Chemical Corporation, Newport Beach, USA. Each strip was 60 × 165 × 3.5 mm, weighed 65 g and contained 186 g/kg DDVP. Strips were cut into 15 × 15 × 3.5 cubes and sealed immediately into an approved foil bag. This product is registered under the Australian Pesticides and Veterinary Medicines Authority as “Biotrap DDVP Cubes”, Product 68,989. DDVP cubes were removed from their sealed bags immediately before placement in traps.
Two Biotrap versions were tested; version 1 (see Fig. 1b) between 30 and 2014 to 28 April 2015 (7 months: time period A) and version 2 (Fig. 1c) between 11 and 2015 to 22 June 2016 (13 months: time period B).
In each comparison, a Biotrap was hung in adjacent trees and were about 10 m apart from existing Lynfield traps. Trap sites were selected to be representative of the Riverina district and not selected for high or low Qfly populations. Traps were inspected weekly in the summer cycle (September to May) and fortnightly in winter (June to August). Flies in traps were placed in individual containers and labelled with the trap number and date and were sent to Orange Agricultural Institute at Orange for further analysis. Entomologists identified fly species using standard texts and numbers of each species was entered data on the state database “PestMon” (see Dominiak et al. 2007 for details). Data was extracted for analysis after the trial was terminated.
All statistical analysis was carried out using R version 4.0.3 (R Core Team 2013). There were 11 sites where the Biotraps were tested against the standard Lynfield traps in the field in two test periods (A and B). We applied a generalised linear model with Poisson distribution (Cameron and Trivedi 1998) and log as the link function to estimate if (a) the total number of flies, (b) number of Qfly male, (c) number of Qfly female, (d) number of Newman fly and (e) number of Island fly, trapped were affected by the trap type in two time periods A and B. The total number of flies trapped at each site between the two time periods A and B is presented in Fig. 2. All statistical comparisons resulting in p-values smaller than 0.001 are expressed as < 0.001. Overdispersion in poisson regression models was checked using check_overdispersion() function from performance package in R (Lüdecke et al. 2021). Also, all five comparisons (as mentioned above) were made using a zero-inflated negative binomial (ZINB) model (Perumean-Chaney et al. 2013) using zeroinfl() function from pscl package (http://github.com/atahk/pscl) (Fávero et al. 2021).
Results
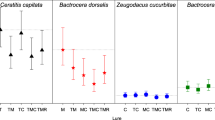
Figure 3 presents the total number of flies trapped at 11 sites and Table 1 gives the information about the total number of flies trapped in Biotrap and Lynfifield traps in two time periods. Table 1 shows that a higher number of flies (male and female Qfly, and Island fly) were trapped with Biotraps in comparison to Lynfield traps. Poisson and negative binomial regression results in Table 2 demonstrated that the total number of flies trapped by Lynfield and Biotraps was significantly different in both time periods A and B (p-value < 0.05; Table 2). There was significant overdispersion detected for poisson regression models and we relied on the results of ZINB for our interpretations. In addition, Lynfield traps exhibited a negative estimate in all comparisons (Table 2), and therefore, we suggest that Biotraps performed better than Lynfield traps in both time periods A and B. Also, Table 1 records the number of Qfly male, Qfly female, Newman fly and Island fly trapped by Lynfield and Biotraps in time periods A and B. Statistical comparisons for the Qfly males, Qfly females and Island fly revealed that the number of flies trapped differed significantly for the two trap types in both time periods (Table 2).
However, the number of Newman fly trapped exhibited no significant difference between the two trap types in both time periods A and B (Table 2, p-value > 0.05). Moreover, the overall number of trapped Newman fly remained low compared to other fly types. In dry years, Newman fly were trapped in larger numbers than Qfly (Dominiak 2019a) however this was not the case during our research period. Also, the Lynfield traps did not capture any female Qfly during both periods (Table 1). Additionally, we tested our dataset for the presence of excessive zeros in the counts of flies captured by the two trap types using ZINB. The zero inflated model detected the presence of excessive zeros for Newman fly and female Qfly. For Island fly, the number of flies trapped in Lynfield traps was lower than the number of flies trapped by Biotraps (Table 1). On further analysis, it was detected that most Island fly and Qfly were trapped in the months of April and May (Table 3). October was the peak month for Newman fly trappings.
Bactrocera neohumeralis (Hardy) was not detected during our trial and this is consistent with the known range (Dominiak and Worsely 2016). Dacus newmani was trapped and is consistent with the known distribution (Dominiak 2019a).
Discussion
Traditional tephritid surveillance relies on trapping males using a male attractant. It is likely that protein lures may be effective as in-canopy lures but may fail to attract flies from adjacent trees and rarely further than 10 m (Balagawi et al. 2012; Shelly & Manoukis 2018). Conversely, male parapheromones are found in nature and are known to attract male flies over much longer distance. Fletcher (1974) used a trap spacing of 0.4 km and this spacing seems to have been adopted every since. This distance may be based on the natural Qfly dispersal distance of 0.3–0.4 km, advocated by Dominiak and Fanson (2020).
Here, we demonstrated that the Biotrap, baited with a protein gel, trapped more male Qfly than the traditional Australian Lynfield trap. In addition, Biotraps had the benefit of trapping female Qfly. Dominiak and Nicol (2010) reported that the male:female ratio was about 7:1 with the McPhail male:female Qfly captures. We found that the ratios for the Biotrap version 1 and version 2 ratios were 11.5:1 and 2.5:1, respectively. So for Qfly surveillance, we propose that the protein gel baited Biotrap combines the utility of both the Lynfield and the McPhail traps in one design. For orchardists, Biotraps will provide a better insight into the female Qfly, that may be potentially egg-laying.
During both versions/time periods, we found that Biotraps had significantly better performance than the cuelure Lynfield trap for male Qfly and for female Qfly. Therefore, Biotraps are an effective alternative surveillance option for Qfly monitoring.
The cuelure-baited dry Lynfield traps became the standard Australian trap in about 1993 and are likely to remain the standard for regional pest freedom monitoring (IPPC 1999). However, new traps are emerging, particularly as some growers move towards the domestic trade agreements (IPPC 2007). Dominiak et al. (2019) demonstrated that cone traps were one alternative and had the advantage of being flat packed for transport or storage. Ladd traps had advantages in some circumstances (Schutze et al. 2016). Similarly, Bain and Dominiak (2022) demonstrated that Biotraps were equivalent to Lynfield traps in Victoria. Fay et al. (2022) used Biotraps to monitor Zeugodacus cucumis (French) in northern NSW and defined the southern range of Z. cucumis. Here, we demonstrated that Biotraps were better than Lynfield traps in the Riverina region for the monitoring of male Qfly. The added benefit was that Biotraps captured female Qfly while Lynfield traps do not. This added benefit may better inform fruit fly management regarding female populations in orchards and help fruit growers make a better choices to manage Qfly. Recently, climate change made large regional pest free areas difficult to maintain (Dominiak and Mapson 2017; Simpson et al. 2020). These trap improvements will assist fruit growers transition from regional pest free standards to the newer trade standards of systems approach, areas of low pest prevalence and pest free places of production (Dominiak et al. 2015; Dominiak 2019b; IPPC 2007).
Additionally, we demonstrated that protein-baited Biotraps captured large numbers of D. pornia flies in both time periods. This observation was consistent with Dominiak and Nicol (2010). Cuelure-baited Lynfield traps captured low numbers of D. pornia and this is consistent with earlier reports (Osborne et al. 1997; Dominiak et al. 2003; Dominiak and Nicol 2010; Lloyd et al. 2010). Dirioxa pornia does not infest undamaged fruit (Morrow et al. 2015) and is not a problem in well-managed orchards.
Regarding D. newmani, Gillespie (2003) claimed this species was likely to be univoltine with a major flight in spring. Dominiak et al. (2011b) found Newman fly was active mainly in August and also November and December but not in March. Dominiak (2019a) reported a population peak in September. We found the peak in October which may be related to seasonal variations: D. newmani populations increased during drier periods (Dominiak 2019a). The host of D. newmani remains unknown but is not a pest of commercial fruit. However, D. newmani was trapped in large numbers in some seasons (Dominiak 2019a).
Our research revealed some contradictions to the current perceptions in Qfly surveillance. The McPhail traps frequently use a liquid protein lure (protein hydrolysate or yeast autolysate) and have to be recharged twice per week (Dominiak and Nicol 2010). The liquid lure is smelly and unpleasant to handle. There is no toxicant and fruit flies drown in the solution. The retrieval of tephritids requires the solution to be drained through a sieve and the often partially decomposed fruit flies are placed in containers to be sent to identification services. The overall task of retrieving flies and recharging liquid lures is unpleasant and time consuming. By contrast, the Biotraps used a gel protein lure which had to be recharged every three months. Tephritids were killed by dichlorvos and therefore were dry samples, similar to samples in Lynfield traps. Clearing flies from Biotraps was as fast and easy as clearing flies from Lynfield traps. Therefore, the time taken to service Biotraps was similar to Lynfield traps and had none of the foul odours associated with liquid protein lures. Additionally, identification services did not have to deal with smelly samples in confined laboratory environments. Also, samples were not degraded, making identification much less challenging.
Another contradiction was with Newman fly. Previously, Newman fly were trapped in cuelure-baited Lynfield traps but not attracted to liquid protein lures in McPhail traps (Dominiak and Nicol 2010). Dominiak et al. (2011b) found that Newman fly were attracted to cuelure baited (or cuelure and methyl eugenol baited) Lynfield traps but not to McPhail traps baited with liquid protein autolysate or orange juice concentrate. Conversely in our comparison, the protein gel baited Biotrap trapped more Newman fly than the cuelure-baited Lynfield traps. We suspect than difference in protein bait formulation had an influence, or that the difference in yellow presentation between the trap architectures had an influence.
The trap architecture between Lynfield and Biotraps was different. Yellow is attractive to Qfly because it is indicative of ripening fruit (Meats 1983) and sugars are the main nutrient used by larvae (Dominiak and Fanson 2017). The Lynfield traps have a yellow screw on lid with a relatively small yellow presentation (see Fig. 1). Often, tephritids land on the clear shell and walk on the outer surface until they find one of the ingress holes. Once inside the Lynfield trap, they may startle and attempt to fly away. They are likely to fly towards the clear sides walls, where the vertical exist holes are. If flies become unwell after contacting the malathion, they may walk on the inside of the trap and find the exit holes, and die outside the trap. Conversely, the main ingress hole in Biotraps was in the floor of the yellow trap and on a raised cone, similar to the design of McPhail traps. If flies startle, they may fly to the clear side wall but will not find the exit holes. If flies fall to the floor of the trap, they are unlikely to fall out of the trap because of the raised peak in the floor where the ingress/outgress hole is (see Fig. 1c – Biotrap V2). Therefore, we think that the retention rate of flies is likely to be better in Biotraps compared to Lynfield traps. Future testing may evaluate if the Biotrap, baited with cuelure and toxicant, is a direct replacement for the cuelure baited Lynfield trap for regional pest monitoring.
There were differences in toxicant. In Lynfield traps, tephritids must fly to and walk on the wick to contact malathion, a contact poison. Subsequently, flies may leave the wick and trap before they die. In Biotraps, the toxicant is dichlorvos and flies are likely to be subjected to the fumigant action irrespective of where they are in the trap. Therefore, the toxicant in Biotraps is more utilitarian than the contact poison in Lynfield traps.
The final contradiction is the perception that cuelure-baited Lynfield traps capture more Qfly than protein baited traps (Dominiak et al. 2003; Dominiak and Nicol 2010). In our comparison, the protein baited Biotraps were at least equal the cuelure Lynfield traps for male Qfly. We can only speculate that the combination of protein (with thickening agent), the Biotrap trap architecture, and toxicant was equal to the cuelure-baited Lynfield trap architecture and malathion combination. More research is required to identify if this pattern is consistent across Australia, and to identify the key element of the Biotrap success.
Additionally, the Biotrap yellow base and clear top do stack into each other and have storage advantages over the Lynfield traps. The Biotrap halves were quickly clipped into each other for field deployment. For better storage and transport, the cone traps were flat packed for efficient storage and transport. However, they needed to be assembled before field deployment and take more time than the Biotrap to assemble. In summary, the Biotrap had merits compared to the Lynfield traps and is an example of the continuing evolution of tephritid surveillance in Australia. Currently, Lynfield traps cost about twice as much as Biotraps. The cost and surveillance advantages of Biotraps should facilitate the transition away from Lynfield traps in many situations.
References
Alexander BH, Beroza M, Oda TA, Steiner LF, Miyashita DH, Mitchell WC (1962) The development of male melon fly attractants. Agric Food Chem 10:270–276
Bain C, Dominiak BC (2022) Evaluating a new trap design for the surveillance of Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) in southern Australia. Gen Appl Entomol 50:21–24
Balagawi S, Jackson K, Hamacek EL, Clarke AR (2012) Spatial and temporal foraging patterns of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), for protein and implications for management. Aust J Entomol 51:279–288
Cameron AC, Trivedi PK (1998) Regression analysis of count data. Cambridge University Press. ISBN 978-0-521-63201-0
Cowley JM, Page FD, Nimmo PR, Cowley DR (1990) Comparison of the effectiveness of two traps for Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) and the implications for quarantine surveillance systems. Aust J Entomol 29:171–176
De Lima CPF, Jessup AJ, Cruickshank L, Walsh CJ, Mansfield ER (2007) Cold disinfestation of citrus (Citrus ssp.) for Mediterranean fruit fly (Ceratitis capitata) and Queensland fruit fly (Bactrocera tryoni) (Diptera: Tephritidae). New Zeal J Crop Hort Sci 35:39–50
Dominiak BC (2019a) Review of the biology and distribution of Newman fruit fly, Dacus newmani (Perkins) (Diptera: Tephritidae), a cryptic Dacinae species from the dry inland of Australia. Gen Appl Entomol 47:17–24
Dominiak BC (2019b) Components of a systems approach for the management of Queensland fruit fly Bactrocera tryoni (Froggatt) in a post dimethoate fenthion era. Crop Protect 116:56–67
Dominiak BC (2023) Priority host plants of the Queensland fruit fly, Bactrocera tryoni (Froggatt), based on the host reproduction number, for tephritid management, surveillance and trade. Int J Trop Insect Sci (in press).
Dominiak BC, Fanson BG (2017) Assessing the proportion of nutrients removed from the larval diet of Queensland fruit fly (Bactrocera tryoni) at a mass-rearing facility and possible uses of spent media. Gen Appl Entomol 45:71–75
Dominiak BC, Fanson BG (2020) Current quarantine and suspension distances are excessive for incipient populations of Queensland fruit fly (Bactrocera tryoni (Froggatt)) (Diptera: Tephritidae) in southern New South Wales, Australia. Crop Prot 138:105341
Dominiak BC, Mapson R (2017) Revised distribution of Bactrocera tryoni in Eastern Australia and effect on possible incursions of Mediterranean fruit fly: development of Australia’s Eastern Trading Block. J Econ Entomol 110:2459–2465
Dominiak BC, Nicol HI (2010) Field performance of Lynfield and McPhail traps for monitoring male and female sterile (Bactrocera tryoni Froggatt) and wild Dacus newmani (Perkins). Pest Manag Sci 66:741–744
Dominiak BC, Worsley P (2016) Lesser Queensland fruit fly Bactrocera neohumeralis (hardy) (Diptera: Tephritidae: Dacinae) not detected in inland New South Wales or south of Sydney. Gen Appl Entomol 44:9–15
Dominiak BC, Campbell M, Cameron G, Nicol H (2000) Review of vehicle inspection historical data as a tool to monitor the entry of hosts of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) into a fruit fly free area. Aust J Exper Agric 40:763–771
Dominiak BC, Gilmour AR, Kerruish B, Whitehead D (2003) Detecting low populations of Queensland fruit fly Bactrocera tryoni (Froggatt) with McPhail and Lynfield traps. Gen Appl Entomol 32:49–53
Dominiak BC, Gillespie PS, Tom G (2007) Changes in data management and the impediments to change – a case study for fruit fly surveillance. Plant Protect Q 22:95–98
Dominiak BC, Daniels D, Mapson R (2011a) Review of the outbreak threshold of Queensland fruit fly (Bactrocera tryoni Froggatt). Plant Protect Q 26:141–147
Dominiak BC, Kerrush B, Barchia I, Pradhan U, Gilchrist S, Nicol HI (2011b) The influence of mixtures of parapheromone lures on trapping of fruit fly in New South Wales, Australia. Plant Protect Q 26:136–140
Dominiak BC, Wiseman B, Anderson C, Walsh B, McMahon M, Duthie R (2015) Definition of and management for areas of low pest prevalence for Queensland fruit fly Bactrocera tryoni Froggatt. Crop Protect 72:41–46
Dominiak BC, Galvin T, Deane D, Fanson B (2019) Evaluation of Probodelt cone traps for surveillance of Dacinae in New South Wales, Australia. Crop Protect 126:104940
Fay HAC, DeFaveri SG, Dominiak BC (2022) A new southern detection of Zeugodacus Cucumis (French 1907) in northern New South Wales. Gen Appl Entomol 50:31–37
Fletcher BS (1974) The ecology of a natural population of the Queensland fruit fly, Dacus tryoni VI. Seasonal changes in the fruit fly numbers in the areas surrounding the orchard. Aust J Zool 22:353–363
Fletcher BS (1987) The biology of Dacine fruit flies. Ann Rev Entomol 32:155–144
Fávero LP, Souza RF, Belfiore P, Corrêa HL, Haddad MFC (2021) Count Data Regression Analysis: concepts, Overdispersion Detection, zero-inflation identification, and applications with R. Pract Assess Res Evaluation 26:13. https://doi.org/10.7275/44nn-cj68
Gillespie P (2003) Observations on fruit flies (Diptera: Tephritidae) in New South Wales. Gen Appl Entomol 32:41–47
Haynes FEM, Dominiak BC (2018) Irradiation for phytosanitary treatment of the Queensland fruit fly Bactrocera tryoni Froggatt benefits international trade. Crop Prot 112:125–132
IPPC (1999) International Plant Protection Convention. International Standards for Phytosanitary Measures ISPM No.10. Requirements for the establishment of pest free places of production and pest free production sites. 2006 Edition. 24 pp
IPPC (2006) International Plant Protection Convention. International Standards for Phytosanitary Measures ISPM No.8. Determination of pest status in an area (1998). 2005 Edition. 9 pp
IPPC (2007) International Plant Protection convention. International Standards for Phytosanitary Measures ISPM No.29. Recognition of pest free areas and areas of low pest prevalence. 12 pp
Jessup AJ (1988) Response of Lambert and Ron’s seedling sweet cherries to fumigation with methyl bromide plus cold storage. Aust J Exp Agric 28:431–434
Jessup AJ, Carswell LF, Dalton SP (1998) Disinfestation of fresh fruit from Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) with combination mild heat and modified atmosphere packaging. Aust J Entomol 37:186–188
Lüdecke D, Ben-Shachar MS, Patil I, Waggoner P, Makowski D (2021) Performance: an R Package for Assessment, comparison and testing of statistical models. J Open Source Softw 6:3139
Lloyd AC, Hamacek EL, Kopittke RA, Peek T, Wyatt PM, Neale CJ, Eelkema M, Gu H (2010) Area-wide management of fruit flies (Diptera: Tephritidae) in the Central Burnett district of Queensland, Australia. Crop Prot 29:462–469
Meats A (1983) The response of Queensland fruit fly, Dacus tryoni, to tree models. In: Cavalloro, R. (Ed.). Proceedings of the CEC/IOBC International Symposium, Athens/Greece/ 16–19 November 1982. Fruit Flies of Economic Importance. A.A. Balkema/Rotterdam. pp 285–289
Monro J, Richardson NL (1969) Traps, male lures, and a warning system for Queensland fruit fly, Dacus tryoni (Frogg.) (Diptera: Trypetidae). Aust J Agric Res 20:325–338
Morrow JL, Frommer M, Shearman DCA, Riegler M (2015) The micriobiome of field-caught and laboratory-adapted Australian tephritid fruit fly species with different host plant use and specialisation. Invertebr Microbiol 70:498–508
Osborne R, Meats A, Frommer M, Sved JA, Drew RAI, Robson MK (1997) Australian distribution of 17 species of fruit flies (Diptera: Tephritidae) caught in cue lure traps in February 1994. Aust J Entomol 36:45–50
Perumean-Chaney SE, Morgan C, McDowall D, Aban I (2013) Zero-inflated and overdispersed: what’s one to do? J Stat Comput Simul 83:1671–1683
Quilici S, Donner P (2012) Analysis of exotic fruit fly trapping networks. EPPO Bull 42:102–108
R Core Team (2013) R Language and Environment for Statistical Computing. R foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Schutze MK, Cribb BW, Cunningham JP, Newman J, Peek T, Clarke AR (2016) Ladd traps’ as a visual trap for male and female Queensland fruit fly, Bactrocera tryoni (Diptera: Tephritidae). Austral Entomol 55:324–329
Shelly TE, Manoukis NC (2018) Capture of melon flies, Zeugodacus cucurbitae (Diptera: Tephritidae), in a food-baited multilure trap: influence of distance, diet, and sex. J Asia-Pacific Entomol 21:288–292
Simpson M, Dominiak BC, McGowan IJ, Crean JJ, Sides TJF (2020) Bioclimatic niche modelling projects a potential shift in distribution and abundance of Queensland fruit fly Bactrocera tryoni in Australia. Gen Appl Entomol 48:61–74
Suckling DM, Kean JM, Stringer LD, Caceres-Barrios C, Hendrichs J, Reyes-Flores J, Dominiak BC (2016) Eradication of Tephritid Fruit fly populations: outcomes and prospects. Pest Manag Sci 72:456–465
Vargas RI, Pinero JD, Leblanc L (2015) An overview of pest species of Bactrocera fruit flies (Diptera: Tephritidae) and the intergration of biopesticides with other biological approaches for their management with a focus on the Pacific region. Insects 6:297–318
Acknowledgements
We thank the regulatory staff who inspected and cleared the traps. Identification staff at Orange Agricultural Institute are acknowledged for identification and data entry roles. Louise Rossiter and David Wheller reviewed the manuscript prior to submission. Two anonymous journal reviewers further improved the manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. NSW Department of Primary Industries funded this study. There were no specific grants or funded projects. Open access publishing was facilitated by New South Wales Department of Planning and Environment, as part of the Springer-New South Wales Department of Planning and Environment agreement via the Council of Australian University librarians.
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
BD conceived the original concept, managed the data collection, storage and retrieval, and created the first draft on the manuscript. CB and DC conducted the field research. NS performed the data analysis and contributed to the first draft of the manuscript. All authors read and approved the submitted manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dominiak, B.C., Bain, C., Sharma, N. et al. Evaluating trap and lure combinations using Biotraps and Lynfield traps for the surveillance of Queensland fruit fly Bactrocera tryoni (Froggatt) (Diptera: Tephritidae) and other tephritids in southern New South Wales, Australia. Int J Trop Insect Sci 43, 2083–2093 (2023). https://doi.org/10.1007/s42690-023-01121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01121-4