Abstract
Pachycrepoideus vindemiae is a generalist wasp parasitoid released for biological control of the pupal stage for several species of Tephritoidea and Muscoidea flies that limit production of fruits and poultry commodities, respectively, worldwide. The parasitoid wasp must find buried host pupae to oviposit on them, and several factors may influence this outcome. The objectives of this study were to determine the capacity of first, host larvae to burrow to pupate according to substrate humidity and second, the parasitoid wasp to burrow and parasitize buried host pupae according to different conditions (substrate type, host species and parasitization time exposure). Moreover, comparison of chemical profiles between host’s pupae potentially involved for host location by the parasitoid was realized by GC-FID. Peat humidity significantly affected the burrowing depth of Dasiops inedulis and Anastrepha striata and both species buried significantly deeper in peat with 50% humidity than in dry conditions. The number of emerged parasitoids is not different between the two tested host species. P. vindemiae performance was better in peat than in crop soil. In peat wasps’ parasitism is similar between buried pupae located at 0 mm or 10 mm depth regardless of the time of exposure to the parasitoid. Cuticular chemical profiles showed that the four-host pupa species studied did not share compounds. However, A. striata shared some compounds with D. inedulis and, M. domestica shared some with C. capitata. Common compounds were found in the extracts of parasitized and non-parasitized pupae of C. capitata, D. inedulis and M. domestica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The wasp Pachycrepoideus vindemiae (Rondani) (Hymenoptera: Pteromalidae) parasitizes more than 60 species of Diptera (Noyes 2014); it attacks pupae of several species of cyclorrhaphous Diptera as a primary parasitoid (Wang and Messing 2004; Marchiori and Barbaresco 2007; Schetelig et al. 2018) and it is a facultative hyperparasitoid of other primary tephritid fruit fly parasitoids (Wang and Messing 2004). P. vindemiae is released mainly for augmentative biological control of Tephritoidea pests in orchards as fly infestation limits market access due to international biosecurity restrictions (Wyckhuys et al. 2010; Langford et al. 2014). After consuming the fruits or flower buds, the larvae of certain Tephritoidea species (Han and Ro 2005), move into the soil to pupate and remain concealed under the soil. Thus, it is in the soil that P. vindemiae must find and parasitize their host. Tephritoidea species include fruit flies (for example, Anastrepha, Ceratitis) and lance flies of the genus Dasiops, among others. The frugivorous larvae of Anastrepha species are known to cause significant damage to the fruits of numerous species of economic impact around the world, such as citrus Citrus spp. (Rutaceae), mango Mangifera indica L. (Anacardiaceae), guava Psidium guajava L. (Myrtaceae), apple Malus domestica Borkh (Rosaceae) and papaya Carica papaya L. (Caricaceae) (Aluja and Mangan 2008). The Mediterranean fruit fly (medfly), Ceratitis capitata (Wiedemann), infests more than 25 plant families that include some families of economic value such as Rutaceae, Rosaceae, Lauraceae and Caricaceae (CABI 2019). Larvae of the lance fly Dasiops inedulis Steyskal (Diptera: Lonchaeidae) consume the floral bud of Passifloraceae crops (Wyckhuys et al. 2012; Santamaría Galindo et al. 2014). In Latin America, P. vindemiae was introduced for classical biological control programs of fruit flies, which include Argentina (Greco et al. 2020), Belize (Sosa et al. 2020), Caribbean Islands (van Lenteren and Bueno 2020) Costa Rica (Blanco-Metzler and Morera-Montoya 2020), Dominica (van Lenteren 2020), Jamaica (Sherwood and van Lenteren 2020), Mexico (Arredondo-Bernal and Rodríguez-Vélez 2020) and Peru (Mujica and Whu 2020). Besides orchards, P. vindemiae is released to control Musca domestica L. in poultry and livestock production (Rueda and Axtell 1985; Bueno et al. 2020). In Colombia, P. vindemiae was introduced from the USA and released during 1984–1985 to control fruit flies in guava (Löhr et al. 2018). Presently, the parasitoid is mass produced and marketed in Colombia and recommended as part of an IPM strategy to control D. inedulis (Kondo et al. 2020). Recently, worldwide interest is devoted to the use of P. vindemiae to control the invasive Drosophila suzukii Matsumura (Miller et al. 2015; Zengin and Karaca 2019; Mariano-Macedo et al. 2020) and the invasive Bactrocera dorsalis Hendel (Zhao et al. 2013). P. vindemiae is an idiobiont generalist wasp that develops as a solitary ectoparasitoid enclosed by a protective puparium formed from the fly’s hardened exoskeleton (Tormos et al. 2009).
Since the host pupae are buried in the soil, so our main interest was to study the effect of some variables associated with this habitat on both the host pupae and the parasitoid. Abiotic variables associated with soil may affect the process of host searching and parasitization, for example, the pupation depth of certain species of Tephritidae (Hennessey 1994; Dimou et al. 2003), which can hinder the process of finding and parasitization by their natural enemies (Guillén et al. 2002).
In the present study, we investigated if soil gravimetric humidity affects A. striata and D. inedulis pupal burrowing depth. We also studied whether the type of substrate, host species, pupal burrowing depth and host exposure time for parasitization influence the parasitization and emergence of P. vindemiae.
When hosts are hidden, parasitoids may rely on vibration sounds combined with visual cues (Wäckers et al.1998) or on olfactory stimuli (Vet and Dicke 1992) to find its hosts. For pupal parasitoids, host finding is also a challenge, as pupae stop feeding and defecating, reducing environmental odour emission and consequently detection (Vet and Dicke 1992). In the current study we explored the presence of potential chemical information in the host-parasitoid interaction. To achieve this, we used a first-step comparative approach of the chemical compound profiles between the pupae of some species of Tephritoidea hosts and besides between host pupae and parasitized host pupae to search for the presence of shared compounds that potentially could be used by the generalist P. vindemiae during its host searching process.
Materials and methods
Origin of Diptera pupae
Floral buds of yellow passion fruit (Passiflora edulis f. flavicarpa O. Deg.) damaged by the larvae of the lance fly Dasiops inedulis (symptoms are chlorosis and easy bud detachment) were collected from crops located in Valle del Cauca, Colombia: Palmira, Village La Torre, La Cabaña Farm (3.62N 76.43W; 977 m above sea level (m a.s.l.)); Bolívar, La Herradura Village (4.28 N,76.21 W; 941 m.a.s.l); Trujillo, Robledo Village (4.27N, 76.22 W; 953 m a.s.l); Palmira, El Bolo San Isidro Village, La Trocha Alley (3.47 N, 76.34 W; 1000 m.a.s.l). Mature guava (P. guajava) fruits infested with A. striata larvae were collected at Corporación Colombiana de Investigación Agropecuaria Palmira (Agrosavia), Palmira (3.5 N, 76.3 W; 999 m.a.s.l.), and Palmira, La Cascada, Tienda Nueva Village (3.56 N, 76.23W; 1000 m.a.s.l). All crop samples were collected in the department of Valle del Cauca, Colombia.
Passion fruit and floral buds were transported to the laboratory and disinfected using a 0.1% NaClO solution for two min and washed with tap water. To permit D. inedulis larvae to abandon fruits and transform into pupae, passion fruit floral buds were laid over a 1 cm layer (200 g) of sterile peat at 50% saturation inside a plastic cage (20 cm × 10 cm × 5 cm) with a mesh cover to permit air flow.
Guava fruits were laid over tissue paper inside plastic containers (40 × 28 × 13 cm) with a mesh cover. When the fruits became brown, they were opened, and the last larval stages were recovered and set on a sterile soil layer (4 cm) in plastic cages (22 × 14 × 6 cm) to pupate. Pupae of A. striata and D. inedulis of 24–48 h were used for burrowing depth parasitization experiments. Pupae of A. striata, C. capitata, and M. domestica were stored in glass vials (5 mL) and frozen at -20 °C until used for chemical profile experiments.
Some pupae were allowed to develop into adults for taxonomic identification. Diptera species were identified by the entomologist Mónica Hernandez (Universidad del Valle), and the identification was supported by the Diptera collection of the Museum of Entomology, Universidad del Valle Cali Colombia (MUSENUV, register 077 of the Alexander von Humboldt Institute, Colombia).
The company Productos Biológicos Perkins Ltda in Palmira, Colombia, provided the pupae of M. domestica and Francisco Beitia of the Instituto Valenciano de Investigaciones Agrarias (IVIA), Valencia, Spain provided the pupae of Ceratitis capitata (Wiedemann).
Origin of pupae and adults of Pachycrepoideus vindemiae
Pupae of P. vindemiae using M. domestica as a host were provided by Productos Biológicos Perkins Ltda. (Palmira, Colombia). The remaining pupae were kept in plastic cages (22 × 14 × 6 cm) until parasitoid emergence. As P. vindemiae carries Wolbachia and only females are produced (K. Wyckhuys, personal communication), 24 to 48-day-old unmated females were fed a 50% honey solution and used to parasitize A. striata and D. inedulis pupae. Pupae of D. inedulis and A. striata were laid in different plastic containers (20 cm × 14 cm × 5 cm) on a 3 cm humid sand layer and exposed for three days to parasitization by P. vindemiae (proportion of one wasp to four pupae). Then, wasps were removed, and pupae were allowed to develop until showing parasitization symptoms. Parasitization was confirmed by checking the presence of the developing parasitoid inside the pupae using a stereomicroscope (Nikon SMZ-745). Parasitoid pupae were kept in glass vials (5 mL) and frozen at -20 °C until chemical analysis.
Pupae of P. vindemiae using the Medfly C. capitata as a host were provided by F. Beitia.
Substrate characteristics and substrate gravimetric humidity
To assess the effect of the substrate on pupa burrowing depth, PGX peat (sphagnum peat 65–75% p/p, vermiculite, macro- and micronutrients, lime, and moisturizer agents from a commercial company) and crop soil (passion fruit crop from Rozo, Valle, Colombia, 3.56 N, 76.41W; 1000 m.a.s.l) were tested. Leaf litter-free crop soil samples of 100 g (10 samples/ha) were randomly taken, for a total of 1000 g of soil. The soil was analyzed in the soil laboratory of the CIAT International Center for Tropical Agriculture and by relating the different textures (Table 1) according to the graph presented by (Bachouche et al. 2018) it can be characterized as a silt loam soil.
To assess the gravimetric humidity both peat and crop soil were autoclaved (All American) for 20 min at 15 psi. Later 100 g of soil and peat were oven dried at 105 °C for 24 h, and the dry weight value obtained was used to calculate the substrate humidity content (H %) using the following formula:
where Psh is moist soil weight (g) and Pss is dry soil weight (g).
To maintain constant saturation of the substrates throughout the study, the experimental units were weighed daily. In each experiment the humidity level was maintained with the addition of distilled water, and the quantity was calculated by the above formula.
The saturation percentage allocated to substrates (0% and 50%) corresponded to field observations for passion fruit and guava crops. After daily drip irrigation, 100% soil humidity close to the root system (inundation) and 0% humidity three m away without a crop canopy and covering vegetation were assumed. As Diptera pupae were found buried in this humidity gradient, an intermediate value of 50% for the soil gravimetric humidity was assumed and tested for the P. vindemiae-Diptera pupae interaction. To compare with extreme dry soil conditions, the parasitoid performance was also tested at 0% soil gravimetric humidity.
Diptera pupation depth in peat
Experiments were carried out under room conditions (25 °C, 63.4% R.H.) to allow larvae of D. inedulis and A. striata to freely burrow in peat (0% and 50%) as substratum. Five floral buds of P. edulis damaged by D. inedulis larvae were set over a 3 cm peat layer inside a Petri dish (100/20 mm) covered with clear plastic wrap (Darnel Wrap®) to avoid water loss. Larvae were allowed to abandon the floral bud over three days, floral buds were removed, and two days later, the burrowing depth was determined by using a ruler (mm). For each treatment 12 experimental units were tested (one Petri dish with five floral buds) and 120 floral buds were used.
As guava fruits contain water that would impact substrate humidity, A. striata last instar larvae were manually removed from fruits. Guava fruits infested with A. striata larvae were laid on a plastic grill inside a plastic box (40 × 28 × 13 cm) to avoid fruit contact with free liquid coming from fruits. When fruits became brown, they were opened, and the last larval stages of A. striata were transferred to plastic cages (23 × 17 × 12 cm) containing a 9 cm peat layer to bury freely to pupate. Seven days later the burrowing depth of pupae was determined by using a ruler (cm). The results were used to determine the pupal burrowing depth for the parasitism experiments.
Parasitization experiments
Diptera pupae were set on peat or crop soil both at 50% saturation, and placed either on the surface (0 mm) or buried at 5.0 or 10 mm according to the results of pupation depth experiments that showed that A. striata and D. inedulis could pupate at least until 10 mm; 5 mm was chosen as an intermediate value between surface and 10 mm. The experimental unit was a glass Petri dish (100/20 mm) filled with a 30 mm substratum layer with five Diptera pupae (24–48 h old) distributed equidistant in a circular pattern and exposed to five P. vindemiae females. Parasitoid females were released on the surface and allowed to parasitize the host pupae between 24 and 48 h. Experiments with D. inedulis had 24 repetitions for each depth (surface, 5 mm, and 10 mm) in combination with the substrates (peat and soil) and the exposure time to parasitism (24 h and 48 h) which meant 72 experimental units. For A. striata the experiments had 12 repetitions (surface, 5 mm and 10 mm) which meant 36 experimental units. In all cases pupae remained until parasitoid/host emergence. The variable measured was number of emerged adults for both flies and wasps.
Chemical profiles
Pupae of parasitized and unparasitized species were stored in glass containers at -20 °C. Chemical compounds were extracted by submerging sixty pupae into 1.5 mL of dichloromethane at room temperature overnight and agitating several times for 30 s. After pupae removal, the solvent was reduced to 50 μL with a stream of N2 (Caravantes-Villatoro et al. 2021) and 2 μL of solution was injected into a gas chromatography apparatus (Agilent, 7890AGC) equipped with a flame ionization detector and interfaced with an HP ChemStation. Splitless injection was performed into a 30 m × 0.32 mm × 1 μm HP-5 capillary column operated at 45 °C for two minutes, increased at 15 °C/min to 250 °C, held for two minutes, then at 10 °C/min to 310 °C and held at this temperature for ten minutes. The injector and detector temperatures were modified from Bosorang et al. (2016) and were held at 80 °C and 300 °C, respectively.
For comparison of the chemical profiles, unparasitized pupae of A. striata (pool of ten pupae, N = 4), C. capitata (pool of 50 pupae, N = 5), D. inedulis (pool of ten pupae, N = 5), M. domestica (pool of ten pupae, N = 5); and chemical profiles of pupae of P. vindemiae when D. inedulis was the host: D. inedulis (pool of ten pupae, N = 5) and when Ceratitis capitata was the host (pool of ten pupae, N = 5) were analyzed. Chemical profiles were compared qualitatively.
Statistical analysis
The normality and homoscedasticity of the data were assessed (SAS system 9.4) prior to data analysis. Differences in burrowing depth between A. striata and D. inedulis pupae was analyzed by the Mann Whitney test because data did not follow the normality distribution. For parasitization experiments data were analyzed using a Generalized Linear Model (SAS system 9.4) that followed a Poisson distribution because response values were small (between zero and five emerged wasps) as five pupae were exposed to each tested wasp. The Tukey-Kramer test was used to determine which pairwise comparisons and variable interactions (LS means) were significantly different (Alpha = 0.05).
Results
Diptera pupation depth in peat
Peat humidity significantly affected the burrowing depth of D. inedulis and A. striata larvae to pupate (Fig. 1). Indeed, larvae of both species buried significantly deeper in peat with 50% humidity than in dry conditions (Mann Whitney U = 1 090.5, P < 0.0001, for D. inedulis and U = 1 183.5, P < 0.0001, for A. striata).
Parasitization experiments
The number of emerged wasps was not influenced by the Diptera host species, and more wasps emerged from peat than from soil independently of exposure time for parasitization by P. vindemiae (Table 2).
P. vindemiae performance was better in peat than in soil regardless of the time of exposure to parasitism (Table 3). P. vindemiae similarly parasitized pupae located on the surface (0 mm) and at 10 mm depth in peat indicating that the parasitoid was able to burrow up to 10 mm with less difficulty in peat (Table 3). To burrow in soil was more difficult and less wasps emerged from this substrate (Table 3). In soil, more wasps emerged from pupae placed in the soil surface than beneath, but a similarly low number of wasps emerged from pupae located at 5 and 10 mm (Table 3). To burrow 0.5 mm beneath the surface is apparently a short distance and the parasitoid can do it either in peat or in soil.
Chemical profiles
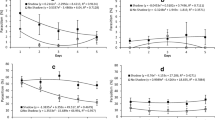
The unparasitized pupae of the four fly species did not share similar compounds. However, extractions of unparasitized D. inedulis and A. striata shared four compounds (a, b, c, and f), both with high signal intensity (Fig. 2).
Extractions of M. domestica and C. capitata pupae analysis showed that they shared some compounds (group a) (Figs. 3A and 4A). On the other hand, shared compounds were found in the extracts of parasitized and non-parasitized pupae. When unparasitized D. inedulis and parasitized D. inedulis pupal profiles were compared, extracts shared seven compounds (a to g) (Fig. 2). Extracts of unparasitized and parasitized pupae of M. domestica shared few compounds, a group of ten peaks (a: Retention Times from 5.5 to 6.4 min), and two other peaks (d, e). Compound d had the highest signal intensity in the parasitized M. domestica extract (Fig. 3).
Extracts of unparasitized C. capitata and parasitized C. capitata analysis revealed that they also shared two groups of ten peaks with low retention times (RT from 5.5 to 6.4 min and RT from 22.2 to 25 min) and six other compounds that are present only in parasitized C. capitata pupal extracts (Fig. 4).
Discussion
Peat humidity influences the burrowing depth of both A. striata and D. inedulis larvae and consequently the pupa depth location. At lower substrate humidity, the compaction of the substrate increases, which negatively affects the excavation capacity of Tephritidae larvae (Hennessey 1994; Sharma et al. 2021). For example, in a dry sand soil, most pupae of Bactrocera dorsalis (Hendel) occurred at 0–5.5 mm depth (Jackson et al. 1998). To increase substrate humidity, a crop scale, during the dry seasons, it is necessary to implement a localized irrigation system (Cleves et al. 2009). Precise drip irrigation, which is one of the most used systems determines greater soil moisture in the rooting zone (Mmolawa and Or 2000). Soil humidity can be highly variable during cropping season as it’s dependent on forecast and crop irrigation regimens. Variation in soil moisture and burrowing conditions can negatively affect the presence and development of pupae of Tephritidae species (Yee 2013). Based on the information that larvae of Anastrepha ludens (Loew) and A. obliqua (Macquart) burrow into loamy-sand soil by 1.0 to 2.0 cm depth (Montoya et al. 2008), thus the experimental depth (10 mm) used in the present study was appropriate. However, in our experiment A. striata burrowed up to 70 mm in loamy sand with 50% saturation to form the pupae. No information is available on the depth of pupation of Dasiops species.
Concerning P. vindemiae, our results showed that the parasitoid successfully parasitized A. striata and D. inedulis pupae as expected for a generalist parasitoid (Wang and Messing 2004) resulting on similar emergence values and these results were not linked to the duration of host exposure to parasitization but to the substrate.
In relation to pupal burrowing depth, in our experiments the parasitoid searched for pupae of A. striata on both peat and crop soil and to a maximum depth of 10 mm. The parasitoid P. vindemiae performed better in peat as more wasps emerged from peat that from soil. Among the best physical properties, peat is characterized by low bulk density and high porosity (Markoska et al. 2018), which decrease substrate compaction and probably could allowed the wasp to burrow more easily. In the opposite way, the crop soil had a medium value of organic matter (4.38%) below the optimum value (> 5%), indicating a soil condition not compatible with low compaction (Minasny and McBratney 2018). High soil compaction would hinder the excavation capacity of P. vindemiae. Thus, our results are in line with a previous study showing that P. vindemiae can easily burrow into soft substrates such as poultry manure and burrow up to 3 cm beneath it (Rueda and Axtell 1985). However, another study found that P. vindemiae only parasitizes pupae found on the surface (Guillén et al. 2002), while in our experiment it burrowed up to 10 mm although with more difficulty in crop soil than in peat. For practical reasons, we suggest considering augmentative releases of P. vindemiae to control Tephritoidea pests on crops without compact soil or receiving good irrigation to enhance parasitoid burrowing.
For host searching by a generalist parasitoid such as P. vindemiae, physiological pressure on chemical information processing is expected due to the great diversity of chemical information present, resulting in extremely limited shared chemical components (Vet and Dicke 1992). In fact, the cuticular profiles of the four unparasitized host pupae species studied did not share common compounds; however, the neotropical species A. striata and D. inedulis shared four compounds, while the Mediterranean C. capitata and the globally distributed M. domestica shared few compounds. Lonchaeidae and Tephritidae are sister groups of the superfamily Tephritoidea (Sivinski 1999) and this could explain the presence of shared compounds between D. inedulis and A. striata. Furthermore, in our parasitism experiments, the number of emerged wasps was not influenced by the species of pupae (D. inedulis or A. striata) within the same type of substrate. Parasitoids may attack closely related hosts that share similar physiological properties and defense mechanisms, and even more if they have similar feeding niches (Godfray 1994).
Parasitoid pupae and host pupae shared several chemical compounds but presented differences in the host’s cuticular profile. This outcome is interesting because the pupa of P. vindemiae is not protected by any cocoon but instead is protected inside the puparium of its host (Tormos et al. 2009). We did not find similar chemical compounds between host pupae of the four studied species to suggest parasitoid attraction to specific host chemical compounds even if they are all parasitized by the same species P. vindemiae (Wang and Messing 2004). Considering A. striata and D. inedulis, both pupae species share some chemical compounds and we found that they have similar parasitism level (similar number of emerged wasps). Thus, the presence of specific chemical compounds is not mandatory for host finding but could facilitate it for other host species and could be associated with some preference and further investigation will be necessary to understand the role of chemical in host finding for the generalist parasitoid P. vindemiae.
On the other hand, some authors suggest that pupal parasitoids may use larval trails of several Tephritidae species to find hidden pupa (Guillén et al. 2002; Granchietti et al. 2012) and this could be investigated for P. vindemiae.
Conclusion
Substrate moisture affects the burrowing depth of A. striata and D. inedulis larvae and it is expected that during dry periods the pupae are found more superficially, although the frequency and strategy of irrigation in crops could cause variations in pupation depth. P. vindemiae burrows more easily into porous substrates such as peat where it can find and parasitize pupae buried to at least 10 mm. On the contrary, the soil of the crop was more compact, which would hinder its excavation capacity and its performance as a parasitoid. P. vindemiae parasitized similar numbers of A. striata and D. inedulis pupae, and further investigation will be necessary to know if chemical compounds shared by both pupal species influenced its behavior. No similar chemical compounds were found between the pupae of these species and the pupae of M. domestica or C. capitata.
References
Aluja M, Mangan RL (2008) Fruit fly (Diptera: Tephritidae) host status determination: critical conceptual, methodological, and regulatory considerations. Annu Rev Entomol 53:473–502. https://doi.org/10.1146/annurev.ento.53.103106.093350
Arredondo-Bernal HC, Rodríguez-Vélez, (2020) Biological control in Mexico. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 308–335
Bachouche N, Kellouche A, Lamine S (2018) Effects of soil texture and burial depth on the biological parameters of overwintering pupae of Bactrocera oleae (Diptera: Tephritidae). Biosci Res 15(2):663–671
Blanco-Metzler H, Morera-Montoya R (2020) Biological control in Costa Rica. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 162–175
Bosorang R, Assim Z, Hanapi S, Abang F (2016) Potential use of cuticular hydrocarbons in estimating the age of Blowfly Pupae Chrysomya megacephala (Diptera: Calliphoridae). Borneo J Resour Sci Technol 6(2):11–20. https://doi.org/10.33736/bjrst.339.2016
Bueno VHP, Parra JRP, Bettiol W, van Lenteren JC (2020) Biological control in Brazil. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 78–107
CABI (2019) Ceratitis capitata (Mediterranean fruit fly). Invasive Species Compendium. CAB International, Wallingford. https://www.cabi.org/isc/datasheet/12367. Accessed 18 Aug 2022
Caravantes-Villatoro LA, Cruz-Esteban S, Rojas JC (2021) Cuticular hydrocarbons of Anastrepha obliqua (Diptera: Tephritidae) as influenced by extraction method, natal host, and age. Fla Entomol 104(4):289–296. https://doi.org/10.1653/024.104.0406
Cleves A, Jarma A, Fonseca J (2009) Manejo integrado del cultivo de maracuyá (Passiflora edulis f. flavicarpa). In: Miranda D, Fischer G, Carranza C, Magnitskiy S, Casierra-Posada FC, Piedrahíta W and Flórez LE (eds) Cultivo, poscosecha y comercialización de las pasifloráceas en Colombia: maracuyá, granadilla, gulupa y curuba. Ruben’s Impresores Editores, Bogotá, pp 98–119
Dimou I, Koutsikopoulos C, Economopoulos AP, Lykakis J (2003) Depth of pupation of the wild olive fruit fly, Bactrocera (Dacus) oleae (Gmel.) (Dipt., Tephritidae), as affected by soil abiotic factors. J Appl Entomol 127(1):12–17. https://doi.org/10.1046/j.1439-0418.2003.00686.x
Godfray HCJ (1994) Parasitoids: behavioral and evolutionary ecology. Princeton Univiersity Press, New Jersey
Granchietti A, Sacchetti P, Rosi MC, Belcari A (2012) Fruit fly larval trail acts as a cue in the host location process of the pupal parasitoid Coptera occidentalis. Biol Control 61:7–14. https://doi.org/10.1016/j.biocontrol.2011.10.015
Greco NM, Cabrera Walsh G, Luna MG (2020) Biological control in Argentina. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 21–42
Guillén L, Aluja M, Equihua M, Sivinski J (2002) Performance of two fruit fly (Diptera: Tephritidae) pupal parasitoids (Coptera haywardi [Hymenoptera: Diapriidae] and Pachycrepoideus vindemiae [Hymenoptera: Pteromalidae]) under different environmental soil conditions. Biol Control 23:219–227. https://doi.org/10.1006/bcon.2001.1011
Han HY, Ro KE (2005) Molecular phylogeny of the superfamily Tephritoidea (Insecta: Diptera): new evidence from the mitochondrial 12S, 16S, and COII genes. Mol Phylogenet Evol 34:416–430. https://doi.org/10.1016/j.ympev.2004.10.017
Hennessey MK (1994) Depth of pupation of Caribbean fruit fly (Diptera: Tephritidae) in soils in the laboratory. Environ Entomol 23(5):1119–1123. https://doi.org/10.1093/ee/23.5.1119
Jackson CG, Long JP, Klungness LM (1998) Depth of pupation in four species of fruit flies (Diptera: Tephritidae) in sand with and without moisture. J Econ Entomol 91(1):138–142. https://doi.org/10.1093/jee/91.1.138
Kondo T, Manzano MR, Cotes AM (2020) Biological control in Colombia. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 124–161
Langford EA, Nielsen UN, Johnson SN, Riegler M (2014) Susceptibility of Queensland fruit fly, Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), to entomopathogenic nematodes. Biol Control 69:34–39. https://doi.org/10.1016/j.biocontrol.2013.10.009
Löhr B, Díaz-Niño MF, Manzano MR, Narváez-Vásquez CA, Gómez-Jiménez MI, Carabalí A, Vargas G, Kondo T, Bustillo-Pardey AE (2018) Uso de parasitoides en el control biológico de insectos plaga en Colombia. In: Cotes AM (ed) Control biológico de fitopatógenos, insectos y ácaros, vol 1. Agentes de control biológico. AGROSAVIA editorial, Bogotá, pp 486–543
Marchiori CH, Barbaresco LF (2007) Occurrence of Pachycrepoideus vindemiae (Rondani, 1875) (Hymenoptera: Pteromalidae) as a parasitoid of Megaselia scalaris (Loew, 1866) (Diptera: Phoridae) in Brazil. Braz J Biol 67:577–578. https://doi.org/10.1590/S1519-69842007000300025
Mariano-Macedo A, Váquez González YM, Martínez AM, Rebollar-Alviter Á, Figueroa JI, Morales SI, Viñuela E, Pineda S (2020) Biological traits of a Pachycrepoideus vindemiae Mexican population on the host Drosophila suzukii. Bull Insectology 73(2):241–248
Markoska V, Spalevic V, Lisichkov K, Atkovsca K, Gulaboski R (2018) Determination of water retention characteristics of perlite and peat. Agric For 64:113–126. https://doi.org/10.17707/agricultforest.64.3.10
Miller B et al (2015) Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull Insectology 68:255–263
Minasny B, McBratney AB (2018) Limited effect of organic matter on soil available water capacity. Eur J Soil Sci 69:39–47. https://doi.org/10.1111/ejss.12475
Mmolawa K, Or D (2000) Root zone solute dynamics under drip irrigation: A review. Plant Soil 222:163–190. https://doi.org/10.1023/A:1004756832038
Montoya P, Salvador F, Jorge T (2008) Effect of rainfall and soil moisture on survival of adults and immature stage of Anastepha ludens and A. oliqua (Diptera: Tephritidae) under semi-field conditions. Fla Entomol 91(4):643–650. https://doi.org/10.1653/0015-4040-91.4.643
Mujica N, Whu M (2020) Biological control in Peru. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 369–389
Noyes JS (2014) Universal Chalcidoidea Database. Natural History Museum. http://www.nhm.ac.uk./chalcidoids. Accessed 19 Aug 2022.
Rueda LM, Axtell RC (1985) Effect of depth of house fly pupae in poultry manure on parasitism by six species of Pteromalidae (Hymenoptera). J Entomol Sci 20:444–449. https://doi.org/10.18474/0749-8004-20.4.444
Santamaría Galindo MY, Castro Ávila ÁP, Ebratt Ravelo EE, Margarita Brochero HL (2014) Caracterización de daños de moscas del género Dasiops (Diptera: Lonchaeidae) en Passiflora spp. (Passifloraceae) cultivadas en Colombia. Rev Fac Nac Agron Medellin 67(1):7151–7162. https://doi.org/10.15446/rfnam.v67n1.42605
Schetelig MF, Lee KZ, Otto S, Talmann L, Stökl J, Degenkolb T, Halitschke R (2018) Environmentally sustainable pest control options for Drosophila suzukii. J Appl Entomol 142:3–17. https://doi.org/10.1111/jen.12469
Sharma A, Sayed S, Bala M, Kmet J, Horvath M (2021) Study on ascending and descending vertical dispersal behavior of third instar larvae of Chrysomya megacephala (Fabricius) (Diptera: Calliphoridae): An evidence that blowflies survive burrowing. Saudi J Biol Sci 28(6):3176–3182. https://doi.org/10.1016/j.sjbs.2021.03.062
Sherwood MA, van Lenteren JC (2020) Biological control in Jamaica. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 194–198
Sivinski J (1999) Breeding habits and sex in families closely related to the Tephritidae: Opportunities for comparative studies of the evolution of fruit fly behavior. In: Aluja M, Norrbom AL (eds) Fruit flies (Tephritidae): Phylogeny and evolution of behavior. CRC Press, Boca Raton, pp 23–37
Sosa EE, Blanco F, van Lenteren JC (2020) Biological control in Belize. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 58–63
Tormos J, Beitia F, Böckmann EA, Asís JD, Fernández S (2009) The preimaginal phases and development of Pachycrepoideus vindemiae (Hymenoptera, Pteromalidae) on Mediterranean fruit fly, Ceratitis capitata (Diptera, Tephritidae). Microsc Microanal 15(5):422–434. https://doi.org/10.1017/S1431927609090801
van Lenteren JC (2020) Biological control in Dominica. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 194–198
van Lenteren JC, Bueno VHP (2020) Biological control in the Remaining Caribbean Islands. In: van Lenteren JC, Bueno HP, Colmenarez YC, Luna MG (eds) Biological control in Latin America and the Caribbean: its rich history and bright future. CAB International, Wallingford, pp 403–425
Vet LE, Dicke M (1992) Ecology of infochemical use by natural enemies in a tritrophic context. Annu Rev Entomol 37:141–172
Wäckers FL, Mitter E, Dorn S (1998) Vibrational sounding by the pupal parasitoid Pimpla (Coccygomimus) turionellae: An additional solution to the reliability–detectability problem. Biol Control 11:141–146. https://doi.org/10.1006/bcon.1997.0592
Wang XG, Messing RH (2004) The ectoparasitic pupal parasitoid, Pachycrepoideus vindemiae (Hymenoptera: Pteromalidae), attacks other primary tephritid fruit fly parasitoids: host expansion and potential non-target impact. Biol Control 31:227–236. https://doi.org/10.1016/j.biocontrol.2004.04.019
Wyckhuys KA, Acosta FL, Rojas M, Ocampo J (2010) The relationship of farm surroundings and local infestation pressure to pest management in cultivated Passiflora species in Colombia. Int J Pest Manag 57:1–10. https://doi.org/10.1080/09670874.2010.506223
Wyckhuys KA, Korytkowski C, Martinez J, Herrera B, Rojas M, Ocampo J (2012) Species composition and seasonal occurrence of Diptera associated with passionfruit crops in Colombia. J Crop Prot 32:90–98. https://doi.org/10.1016/j.cropro.2011.10.003
Yee WL (2013) Soil moisture and relative humidity effects during post diapause on the emergence of western cherry fruit fly (Diptera: Tephritidae). Can Entomol 145:1–10. https://doi.org/10.4039/tce.2013.7
Zengin E, Karaca İ (2019) Dynamics of trapped adult populations of Drosophila suzukii Matsumura (Diptera: Drosophilidae) and its parasitoids in Uşak Province, Turkey. Egypt J Biol Pest Control 29(1):1–6. https://doi.org/10.1186/s41938-019-0147-3
Zhao HY, Liu K, Ali S, Lu YY, Zeng L, Liang GW (2013) Host suitability of different pupal ages of Oriental Fruit Fly, Bactrocera dorsalis, for the parasitoid, Pachycrepoideus vindemmiae. Pak J Zool 45:673–678
Acknowledgements
This work was supported by the Universidad Nacional de Colombia (HERMES 12623, HERMES 29863) and by the European Commission through the scholar mobility program, Erasmus Mundus Masters Course-International Master in Applied Ecology (EMMC-IMAE) (FPA 2023 – 0224/532524-1-FR- 2012-1-ERA MUNDUS-EMMC) – Coordination F-J Richard, Université de Poitiers. Francisco Freitas (Instituto Valenciano de Investigaciones Agrarias, IVIA, Spain) provided the Ceratitis capitata pupae unparasitized and parasitized by P. vindemiae. Biological Products Perkins Ltda. (Colombia) provided the P. vindemiae pupae and the Musca domestica pupae unparasitized and parasitized by P. vindemiae. Mónica Hernández and the Museum of Entomology of Universidad del Valle, Colombia supported the Diptera species identification. Takumasa Kondo (AGROSAVIA, Palmira) reviewed an early version of the manuscript. Colombian farmers are thanked for allowing us to sample their fruit crops. Two anonymous reviewers are thanked for improving the manuscript.
Funding
Open Access funding provided by Colombia Consortium The authors have no financial or proprietary interests in any material discussed in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that there are no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manzano, M.R., Moreno, C.A., Melo-Cerón, C. et al. Understanding the ability of Pachycrepoideus vindemiae (Hymenoptera: Pteromalidae) to find and parasitize buried Diptera pupae in southwestern Colombia. Int J Trop Insect Sci 44, 41–51 (2024). https://doi.org/10.1007/s42690-023-01118-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-01118-z