Abstract
Ozone is a sturdy oxidant which canrub insects and microorganisms. Thus, it could be useful in eliminate the Housefly in hospitals. The purpose of test here is to determine degree of ozone exposure required to remove houseflies and reduction of pathogenic bacteria. Ozone was effective with housefly, requiring only low minutes of treatment (1 to 14 min.) at minimum concentrations of ozone gas O3 (0.125 g/m3 to 1 g/m3), the insects were eliminated the ozone. Thus, ozone exhibit potential as a fumigant for ousefly nesting materials, but moreover research is needed to evaluate its acceptability and efficacy in that field. The need for a dependable method to decontaminate housefly nesting materials as part of an overall ousefly (Musca domestica) system at hospitals sterilization is discussed. In this paper, we are using ozone gas as an alternative housefly pest and to remove house flies at hospitals, and we also illustrate data on the efficacy of ozone against the housefly (Musca domestica) and its effect on specific characteristics and properties of the treated zone application is currently attracting attention, particularly since. (a) There are no residues on the product. (b) There is no need for aeration to remove the gas. Green synthesis industrial applications and improvements in ozone technology together with new regulatory actions worldwide have emerged in last years, making it easier to use and applicable in a wide range - as the same with the pathogenic bacteria we are using ozone gas to control it, the numbers of bacterial cells remaining after ozone treatments were less than for untreated cells. The lowest dose of ozone gas for complete reduction of pathogenic bacteria was 0.125 g/m3 after 24 h of incubation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ozone is a kind of oxygen, or trioxygen, which is substantially less stable than O2 and breaks down with a half-life of 20 to 50 min at room temperature to normal O2 (O3), which is a severe oxidant. It is frequently used as a treatment for both drinking water and swimming pools because it may disinfect as well as remove tastes, odors, and color’s (Usepa 1999). It has recently been created as an agricultural fumigant; for instance, it is used to protect stored grains from rot and to get rid of insect and fungal pests (Kells et al. 2001; Allen et al. 2003; Mendez et al. 2003; Ballinger et al. 2005; Awad et al. 2022; Boopathy et al. 2022). O3 has been used successfully as a disinfectant and sanitizer to eliminate pesticides, inorganic, and organic substances as well as to control insects and microbes (Boopathy et al. 2022). So, it is a powerful oxidant and highly harmful to living things at high doses, it is effective for these uses. Thus, it must be used in a sealed fumigation chamber, past from workers, and it is acutely harmful to people as well (Fouda et al. 2022). Ozone, however, quickly breaks down into oxygen (O3), so it does not linger in the air or on wood or plastic. If ozone is produced directly from air and administered in a fumigation chamber in the hospitals and residences (Bernhardt et al. 2019), the registration procedures are quite straightforward. O3 Application has the advantage of being more environmentally friendly than conventional fungicides and insecticides. Only air and power are necessary to produce ozone at the treatment facility. O3 hence offers various safety benefits over traditional fungicides and insecticides. There are no harmful chemicals present, no residues on the products, no risk of chemical mixing, and no concerns with the disposal of old insecticides or containers (Légeron 1980; Law and Kiss 1991; Bornholdt et al. 2002; Lu et al. 2009; Rangel et al. 2021). The housefly may complete its life cycle in human and pet environments and is widespread in human activity areas such as hospitals, food markets, slaughterhouses, food centers or restaurants, poultry, and cattle farms (Francke et al. 2003; Fontes et al. 2012). Over 100 distinct bacteria capable of causing human diseases are colonized by houseflies in Central Europe (Fotedar and Roberts 1992; Falkenstein and Coogan 1997). Like Staphylococcus aureus (Gad et al. 2021), Klebsiella spp., Escherichia coli O157:H7 (Falkenstein and Coogan 1997; Hughes and Parkes 2007; Pereira et al. 2008; Rozado et al. 2008), Salmonella spp., Shigella spp. and Proteus (Jian et al. 2013). The goal of this study was to determine whether ozone can kill houseflies (Musca domestica) and, if so, to develop response curves for different ozone concentrations as well as pathogenic bacteria. The concentration of ozone required to kill bacteria has been recorded to range between 0.04 and 0.1 ppm.
Materials and methods
Ozone system
Ozone gas was obtained from a generator developed by the Center of Plasma Technology. In the gas generation process, the oxygen was used as input, passing through a dielectric barrier discharge (DBD) reactor. This type of discharge is produced by applying a discharge voltage between two coaxial electrodes, having a glass dielectric between them and a free space where the oxygen flows through.
In this free space a filament discharge is produced, where electrons are generated with enough energy to breakdown the oxygen molecules forming ozone.
The diagram of ozone generation is shown in Fig. 1. The ozone was generated using coaxial dielectric barrier discharge (DBD) technique at the Center of Plasma Technology, Faculty of Science, Al-Azhar University, Nasr City, Cairo, Egypt.
The Dielectric barrier discharge (DBD) cell was fed by oxygen gas. The concentration of the generated ozone was controlled by the discharge current, and the oxygen gas flow rate was adjusted to 0.1 L/min.
Effect of Ozone on house fly Musca domestica
Ozone was applied directly into the jars containing the adult housefly (Musca domestica). The concentration of ozone inside the jars was measured using ozone analyzer (Model H1-AFX-Instrumentation, USA) Fig. 1.
The input voltage of the AC test set (more specifically a variable high voltage transformer) was 220 V at 50 Hz. A voltage transformer was connected to two outer electrode and inner electrode, which were separated by gap. Voltages used to generate ozone were controlled through a transformer control box (Variac Variable AC Power Transformer Regulator).
Effect of ozone on pathogenic Bacteria
The initial tests with the seudomonas aeruginosa, Escherichia coli, lebsiella pneumoniae, Staphylococcus aureus, and Bacillus cereus strains demonstrated that doses greater than or equal to 0.125 g/m3, 0.25 g/m3, 0.5 g/m3 and 1 g/m3for 1 ,2, 5 and 10 min.
We used the growth measurement according to (Fontes et al. 2012). The pellets of four broth cultures (control and treated) were serially diluted with distilled water and the dilutions were made up to 10 − 7 from 10 − 5 and 10 dilution 1 ml of each suspension was spreader on nutrient agar plates Fig. 2. The plates were incubated for 37ºC at 24 h. After incubation the bacterial colonies were counted, and result was recorded. No. of cells = Organisms per millimeter / Gram of the sample = No. of colonies (Average of 3 replicates) / Amount plated X dilution.
Results and discussion
Electrical characteristic of DBD
Figure 3 shows waveforms of the applied voltage on the reactor (coaxial dielectric barrier discharge) and the associated discharge current measured in Oxygen plasma at gas flow rate of 0.1 (L/min). When the AC applied voltage on the DBD reactor reaches the onset value, the streamer discharge starts in the gap inside the reactor in the form of discrete current spikes. These spikes are related to the formation of micro discharges (filaments) of tens of nanosecond (ns) duration in the gap space (Gherardi et al. 2000). The filaments are randomly distributed over entire electrode surface. The streamers cross the discharge gap and spread on the surface of the dielectric barrier, building up surface charges, which produce electric field opposite to that of the applied voltage. After a short time (several ns), the streamer activity in that spot is extinguished, followed by streamer initiation in another location.
The peak of each individual spike is related to the number of instantaneous microfilaments that were formed at this instant, and hence a high current spike indicates that a high number of micro discharges initiated almost simultaneously.
A simple method for obtaining the consumed power is using the discharge Lissajous figures, obtained when plotting transported electric charge Q through the discharge as a function of the applied periodical voltage (Francke et al. 2003; Nersisyan and Graham 2004). The charge Q is delivered from the voltage drop across a measuring capacitor of 3.35µF. The average electric energy dissipated in a discharge cycle, is simply the area of the characteristic Lissajous figure, which in most cases is nearly a parallelogram (Falkenstein and Coogan 1997). Lissajous diagrams have been plotted at KVs applied voltages where the voltage is difference between the two electrodes has been measured as a function of the charge on the electrodes. Figure 4 shows Lissajous diagrams at 3.6, 3.8, 4, 4.4 KVs measured for Oxygen plasma at gas flow rate of 0.1 (L/min), the area of parallelogram increases with the increase in applied voltage because; the energy dissipated in a discharge cycle is proportional to the area of the parallelogram. The dissipated power has been calculated by multiplying the area of the parallelogram by the frequency of the used AC power supply (50 HZ). The change of the average dissipated power, calculated from the Lissajous figures with the applied voltage is shown in (Table 1). As can be seen from this table, the average dissipated power increases with the increase in applied voltage but remains relatively low even at a higher applied voltage. This result may be referred to the characterized filamentary discharge behavior where, the time of the filament is very short (few tens of nanoseconds).
It is noticed that the area of parallelogram increases with the increase in applied voltage because; the energy dissipated in a discharge cycle is proportional to the area of the parallelogram. The dissipated power has been calculated by multiplying the area of the parallelogram by the frequency of the used AC power supply (50 Hz). The change of the average dissipated power calculated from the Lissajous figures with the applied voltage is shown in Table 2.
Power measurement method
The power was analyzed following the original work of (Manley 1943), who has utilized voltage–charge Lissajous figures to characterize the average consumed power through the discharge (Fotedar and Roberts 1992; Gherardi et al. 2000; Francke et al. 2003; Gad et al. 2021). The charge–voltage characteristic plot was revealed in Fig. 2. The two values of effective discharge capacitance are indicated by obtaining of two distinct slopes of the Q–V plot. The reactor (coaxial dielectric barrier discharge) power formula was shown in Eq. (1), where is the total power Pel related to the operating frequency (f), the peak voltage Vmax and the minimum discharge voltage Vmin at which micro-discharges are monitored in the discharge gap with the capacitances of the dielectric CD and the gas gap Cg. From this so-called Lissajous Figure the minimum external voltage Vmin at which the ignition occurs, the electric energy consumed per voltage cycle Eel and the electric power Pel can be estimated by the following relations (Ballinger et al. 2005):
In this study, the consumed power was determined to be 26 W at applied voltage of 11.2 kV treatments was applied in case of Oxygen gas.
As can be seen from this table, the average dissipated power decreases with the increase in applied voltage but remains relatively low even at higher applied voltage. This result may be referred to the characterized filamentary discharge behavior where, the time of the filament is very short (few tens of nanoseconds).
Flies provide opportunity to study the effects of ozone, “They have a high metabolic rate, a short life span (about 30 days), and an extensive tracheal-tubular respiratory system that directly distributes inhaled substances to all tissues in the body. Their entire body is respiratory, Ozone-caused tissue damage even at low concentrations (Falkenstein and Coogan 1997; Kogelschatz et al. 1997; Nersisyan and Graham 2004). If the insect respiratory system was the major entry route of O3 into the insect body (Kogelschatz et al. 1997), increased respiration rate with an increase of temperature might result in more mortality because the increased gas exchange would increase the amount of O3 inside insect bodies (Janeco et al. 2011; Biganzoli et al. 2014).
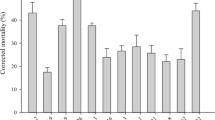
The effects of ozone treatments with concentrations of 0.125, 0.25, 0.5, and 1.0 g/m3 on the mortality of housefly (Musca domestica) adults are presented in Table 2. The results revealed that the mortality of housefly was increased with increasing the concentration and the time of exposure of insects to ozone from 10 to 100%. The highest adult mortality percent (100%) was induced with the concentration 0.125 g/m3 at 14 min, this percent decreased to 90.0% after 11 min and gradually decreased till reached the lowest value 10.0% at the lowest time 1.0 min. Meanwhile, at the concentration 0.25 g/m3 the highest mortality percent (100%) was recorded after 9 min and the lowest mortality percent was (30.0%) after one minute. Also, complete adult mortality was caused after 7 min at the concentration 0.5 g/m3, this mortality percent decreased to 50.0% after one minute. At the highest concentration 1.0 g/m3, the mortality percent was 80.0 and 100.0% after 1.0 and 3.0 min respectively Fig. 5. The effects of ozone treatments at concentrations of 0.125, 0.25, 0.5, and 1.0 g/m3 on the reduction of pathogenic bacteria after 24 h of incubation were recorded in Table 3. The results revealed that the reduction of pathogenic bacteria was increased with increasing the concentration and time of ozone from 10 to 100%. Complete reduction of pseudomonas aeruginosa growth was caused at dose 0.25 g/m3 after 10 min of ozonation. Also, complete reduction of Escherichia coli growth was caused at 0.125 g/m3 after 10 min of ozonation. Klebsiella pneumoniae, the complete reduction proceeds after 10, 5 and 2 min with ozone dose 0.125, and 1.0 g/m3 respectively. The optimum ozone dose for complete reduction of Staphylococcus aureus and Bacillus cereus was caused at 0.25, 0.5 and 1.0 g/m3 after 10, 5 and 2 min respectively.
Scanning electron micrographs of the head of housefly (Musca domestica) showing different between treated and untreated house fly and showing damage of tissues of the insect head, antenna and organs due to the exposed to ozone, which caused an explosion in the insects’ organs in the treated house flies
References
Allen B, Wu J, Doan H (2003) Inactivation of fungi associated with barley grain by gaseous ozone. J Environ Sci Health B 38:617–630
Awad MA, Eid AM, Elsheikh TMY, Al-Faifi ZE, Saad N, Sultan MH, Selim S, Al-Khalaf AA, Fouda A (2022) Mycosynthesis, characterization, and mosquitocidal activity of silver nanoparticles fabricated by aspergillus niger strain. J Fungi (Basel) 8
Ballinger CA, Cueto R, Squadrito G, Coffin JF, Velsor LW, Pryor WA, Postlethwait EM (2005) Antioxidant-mediated augmentation of ozone-induced membrane oxidation. Free Radic Biol Med 38:515–526
Bernhardt V, Finkelmeier F, Verhoff MA, Amendt J (2019) Myiasis in humans-a global case report evaluation and literature analysis. Parasitol Res 118:389–397
Biganzoli I, Barni R, Gurioli A, Pertile R, Riccardi C (Year) Experimental investigation of Lissajous figure shapes in planar and surface dielectric barrier discharges. In: Journal of Physics: Conference Series: IOP Publishing. 012039
Boopathy B, Rajan A, Radhakrishnan M (2022) Ozone: an alternative fumigant in controlling the stored product insects and pests: a status report. Ozone Sci Eng 44:79–95
Bornholdt J, Dybdahl M, Vogel U, Hansen M, Loft S, Wallin H (2002) Inhalation of ozone induces DNA strand breaks and inflammation in mice. Mutat Res 520:63–71
Falkenstein Z, Coogan JJ (1997) Microdischarge behaviour in the silent discharge of nitrogen-oxygen and water-air mixtures. J Phys D 30:817
Fontes B, Cattani Heimbecker AM, De Souza Brito G, Costa SF, Van Der Heijden IM, Levin AS, Rasslan S (2012) Effect of low-dose gaseous ozone on pathogenic bacteria. BMC Infect Dis 12:358
Fotedar R, Roberts JM (1992) Cell cycle regulated phosphorylation of RPA-32 occurs within the replication initiation complex. Embo j 11:2177–2187
Fouda A, Awad MA, Al-Faifi ZE, Gad ME, Al-Khalaf AA, Yahya R, Hamza MF (2022) Aspergillus flavus-mediated green synthesis of silver nanoparticles and evaluation of their antibacterial, anti-candida, acaricides, and photocatalytic activities. Catalysts [Online] 12
Francke KP, Rudolph R, Miessner H (2003) Design and operating characteristics of a simple and reliable DBD reactor for use with atmospheric air. Plasma Chem Plasma Process 23:47–57
Gad HA, Abo Laban GF, Metwaly KH, Al-Anany FS, Abdelgaleil SaM (2021) Efficacy of ozone for Callosobruchus maculatus and Callosobruchus chinensis control in cowpea seeds and its impact on seed quality. J Stored Prod Res 92:101786
Gherardi N, Gouda G, Gat E, Ricard A, Massines F (2000) Transition from glow silent discharge to micro-discharges in nitrogen gas. Plasma Sources Sci Technol 9:340
Hughes EL, Parkes KR (2007) Work hours and well-being: the roles of work-time control and work–family interference. Work Stress 21:264–278
Janeco A, Pinhão N, Branco J, Ferreira A (2011) Measurement of Plasma power consumption in dielectric barrier discharges. ITN – Nuclear and Technological Institute, Estrada Nacional 10, 2686 – 953 Sacavém, Portugal
Jian F, Jayas DS, White ND (2013) Can ozone be a new control strategy for pests of stored grain? Agricultural Res 2:1–8
Kells SA, Mason LJ, Maier DE, Woloshuk CP (2001) Efficacy and fumigation characteristics of ozone in stored maize. J Stored Prod Res 37:371–382
Kogelschatz U, Eliasson B, Egli W (1997) Dielectric-barrier discharges. Principle and applications. Le J de Phys IV 7:C4–47
Law S, Kiss E (1991) Instrumentation for ozone-based insect control in agriculture. AGRIS
Légeron JP (1980) Comparative study of ozonation conditions in wastewater tertiary treatment. Ozone Sci Eng 2:123–137
Lu B, Ren Y, Du YZ, Fu Y, Gu J (2009) Effect of ozone on respiration of adult Sitophilus oryzae (L.), Tribolium castaneum (Herbst) and Rhyzopertha dominica (F). J Insect Physiol 55:885–889
Manley T (1943) The electric characteristics of the ozonator discharge. Trans Electrochem Soc 84:83
Mendez F, Maier DE, Mason LJ, Woloshuk CP (2003) Penetration of ozone into columns of stored grains and effects on chemical composition and processing performance. J Stored Prod Res 39:33–44
Nersisyan G, Graham W (2004) Characterization of a dielectric barrier discharge operating in an open reactor with flowing helium. Plasma Sources Sci Technol 13:582
Pereira ADM, Faroni LRDA, Sousa AHD, Urruchi WI, Paes JL (2008) Influência da temperatura da massa de grãos sobre a toxicidade do ozônio a Tribolium castaneum. Revista Brasileira de Engenharia Agrícola e Ambiental 12:493–497
Rangel K, Cabral FO, Lechuga GC, Carvalho J, Villas-Bôas MHS, Midlej V, De-Simone SG (2021) Detrimental effect of ozone on pathogenic bacteria. Microorganisms 10
Rozado AF, Faroni LR, Urruchi WM, Guedes RN, Paes JL (2008) Ozone application against Sitophilus zeamais and Tribolium castaneum on stored maize. Revista Brasileira de Engenharia Agrícola e Ambiental 12:282–285
Usepa J (1999) Alternative disinfectants and oxidants guidance manual. United States Environmental Protection Agency (USEPA)
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This study was no funded.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Metwally, A.M., Salem, S.S., Hammad, K.M. et al. Novel control of house fly Musca domestica and bacterial isolates by ozone gas. Int J Trop Insect Sci 43, 761–767 (2023). https://doi.org/10.1007/s42690-023-00984-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-023-00984-x