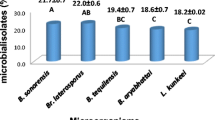
Abstract
Stonebrood is a virulent disease afflicting honey bee colonies. Bacterial strains present in the gut of the Egyptian honeybee, Apis mellifera were isolated, identified and characterized. Moreover, its inhibitory effect against the etiological agent of Stonebrood disease was determined according to their antifungal activity. Both Gram-positive and Gram-negative bacteria were observed in all seasons. The spring season was found to possesses the highest abundance and variety of these bacteria. A total of 93 bacterial isolates from honeybee gut microbiota were identified by Vitek Ms method elucidating that bacterial populations in the gut of the Egyptian honeybee consist of, Firmicutes phyla (75.2%), Bacteroidetes (3.22%), Alpha-Proteobacteria (1.07%) and Gamma-Proteobacteria (20.4%). In vitro antifungal activity was carried out to determine the ability of Cell-Free Supernatants (CFS) to inhibit two Aspergillus sp., the causal agent of the Stonebrood disease. Out of 93 isolates, three of them exhibited potent antifungal activity against Aspergillus niger and A. flavus. Most antagonistic CFS identifications were performed by 16S rRNA gene sequence analysis revealing that bacterial isolates belong to Sphingomonas paucimobilis, Staphylococcus aureus, Pseudomonas aeruginosa and Lactobacillus kunkeei. Bacterial isolates obtained in this study may be potential candidates as biological control agents against Aspergillus sp.




Similar content being viewed by others
Abbreviations
- CFS:
-
Cell-free supernatants
- PDA:
-
Potato Dextrose Agar
- MRS:
-
Man Rogosa Sharpe
- CFU:
-
Colony-Forming Unit
- MALDI-TOF:
-
Matrix Assisted Laser Desorption Ionization Time-of-Flight
- MS:
-
Mass Spectrometry
- PCR:
-
Polymerase Chain Reaction
References
Amaike S, Keller NP (2011) Aspergillus flavus. Annu Rev Phytopathol 49:107–133
Anderson KE, Carroll MJ, Sheehan T, Mott BM, Corby-Harris V (2014) Hive-stored pollen of honey bees: Many lines of evidence are consistent with pollen preservation, not nutrient conversion. Mol Ecol 23:5904–5917
Anderson KE, Sheehan TH, Mott BM, Maes P, Snyder L, Schwan MR, Walton A, Jones BM, Corby-Harris V (2013) Microbial ecology of the hive and pollination landscape: bacterial associates from floral nectar, the alimentary tract and stored food of honey bees (Apis mellifera). PloS One 8(12)
Aronstein KA, Murray KD (2010) Chalkbrood disease in honeybees. J Invertebr Pathol 103:20–29
Bhabhra R, Askew DS (2005) Thermotolerance and virulence of Aspergillus fumigatus: role of the fungal nucleolus. Med Mycol 43(sup1):87–93
Bleau N, Bouslama S, Giovenazzo P, Derome N (2020) Dynamics of the honeybee (Apis mellifera) gut microbiota throughout the overwintering period in Canada. Microorganisms 8(8):1146
Brodschneider R, Crailsheim K (2010) Nutrition and health in honeybees. Apidologie 41:278–294
Chen YP, Siede R (2007a) Honeybee viruses. Adv Virus Res 70:33–80
Chen YP, Siede R (2007b) Honeybee viruses. In: Maramorosh K, Shatkin AJ, Murphy FA (eds) Advances in virus research. Elsevier Academic Press, Amsterdam, The Netherlands, pp 34–80
Cheng HY, Ning MX, Ma WT, Chen DK (2019) Interactions between the gut microbiota and the host innate immune response against pathogens. Front Immunol 10:607
Corby-Harris V, Maes P, Anderson KE (2014) The bacterial communities associated with honey bee (Apis mellifera) foragers. PloS One 9(4):e95056
Dagenais TR, Keller NP (2009) Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev 22(3):447–465
Dillon RJ, Dillon VM (2004) The gut bacteria of insects: non-pathogenic interactions. Annu Rev Entomol 49:71–92
Engel P, Moran NA (2013) The gut microbiota of insects-diversity in structure and function. FEMS Microbiol Rev 37(5):699–735
Engel P, Martinson VG, Moran NA (2012) Functional diversity within the simple gut microbiota of the honeybee. Proc Natl Acad Sci 109(27):11002–11007
Foley K, Fazio G, Jensen AB, Hughes WO (2012) Nutritional limitation and resistance to opportunistic Aspergillus parasites in honeybee larvae. J Invert Pathol 111(1):68–73
Foley K, Fazio G, Jensen AB, Hughes WO (2014) The distribution of Aspergillus spp. opportunistic parasites in hives and their pathogenicity to honeybees. Vet Microbiol 169(3–4):203–210
Gallai N, Salles JM, Settele J, Vaissiere BE (2009) Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol Econ 68:810–821
Genersch E, Aubert M (2010) Emerging and re-emerging viruses of the honeybee (Apis mellifera L.). Vet Res 41:54
Gilliam M, Lorenz BJ, Richardson GV (1988a) Digestive enzymes and micro-organisms in honeybees, Apis mellifera: influence of streptomycin, age, season and pollen. Microbios 55(223):95–114
Gilliam M, Vandenberg JD, Morse RA, Nowogrodzki R (Eds.) (1988b) Honeybee Pests, Predators, and Diseases, Comell University Press, Ithaca, NY
Hamdi C, Balloi A, Essanaa J, Crotti E, Gonella E, Raddadi N, Ricci I, Boudabous A, Borin S, Manino A, Bandi C (2011) Gut microbiome dysbiosis and honeybee health. J Appl Entomol 135(7):524–533
Hashem AH, Suleiman WB, Abu-elreesh G, Shehabeldine AM, Khalil AMA (2020) Sustainable lipid production from oleaginous fungus Syncephalastrum racemosum using synthetic and watermelon peel waste media. Bioresour Technol Rep 12:100569
Jeyaprakash A, Hoy MA, Allsopp MH (2003) Bacterial diversity in worker adults of Apis mellifera capensis and Apis mellifera scutellata (Insecta: Hymenoptera) assessed using 16S rRNA sequences. J Invertebr Pathol 84(2):96–103
Kešnerová L, Emery O, Troilo M, Liberti J, Erkosar B, Engel P (2020) Gut microbiota structure differs between honeybees in winter and summer. ISME J 14(3):801–814
Khan S, Somerville D, Frese M, Nayudu M (2020a) Environmental gut bacteria in European honey bees (Apis mellifera) from Australia and their relationship to the chalkbrood disease. Plos One 15(8)
Khan KA, Al-Ghamdi AA, Ghramh HA, Ansari MJ, Ali H, Alamri SA, Hafeez M (2020b) Structural diversity and functional variability of gut microbial communities associated with honey bees. Microb Pathog 138:103793
Killer J, Mrázek J, Bunešová V, Havlík J, Koppová I (2013) Pseudoscardovia suis gen. nov., sp. nov., a new member of the family Bifidobacteriaceae isolated from the digestive tract of wild pigs (Sus scrofa). Syst Appl Microbiol 36:11–16
Lamei S, Stephan JG, Nilson B, Sieuwerts S, Riesbeck K, de Miranda JR, Forsgren E et al (2019) Feeding Honeybee Colonies with Honeybee-Specific Lactic Acid Bacteria (Hbs-LAB) Does Not Affect Colony-Level Hbs-LAB Composition or Paenibacillus larvae Spore Levels, Although American Foulbrood Affected Colonies Harbor a More Diverse Hbs-LAB Community. Microb Ecol 79:743–755
Latgé JP (1999) Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12(2):310–350
Mattila HR, Rios D, Walker-Sperling VE, Roeselers G, Newton IL (2012) Characterization of the active microbiotas associated with honey bees reveals healthier and broader communities when colonies are genetically diverse. PLoS One 7(3)
Moran NA, Hansen AK, Powell JE, Sabree ZL (2012) Distinctive gut microbiota of honeybees assessed using deep sampling from individual worker bees. PloS One 7(4)
Morse RA, Calderone NW (2000) The value of honeybee pollination in the United States. Bee Culture 128(18):1–5
Miller DL, Smith EA, Newton IL (2020) A bacterial symbiont protects honey bees from fungal disease. Mbio 12(3):e00503-e521
Oda T, Imai K, Shinbara H, Sakita M (2004) Changes in muscular blood flow induced by acupuncture in rat ischemic hindlimb. JJSAM 54(2):163–178
Ptaszyńska AA, Borsuk G, Zdybicka-Barabas A, Cytryńska M, Małek W (2016) Are commercial probiotics and prebiotics effective in the treatment and prevention of honeybee nosemosis? Parasitol Res 115(1):397–406
Romero S, Nastasa A, Chapman A, Kwong WK, Foster LJ (2019) The honey bee gut microbiota: strategies for study and characterization. Insect Mol Biol 28(4):455–472
Shoreit MN, Bagy MMK (1995) Mycoflora associated with stonebrood disease in honeybee colonies in Egypt. Microbiol Res 150(2):207–211
Subotic S, Boddicker AM, Nguyen VM, Rivers J, Briles CE, Mosier AC (2019) Honey bee microbiome associated with different hive and sample types over a honey production season. PloS One 14(11)
UNEP (2010) UNEP Emerging Issues: Global honey bee colony disorder and other threats to insect pollinators. (www.unep.org/dewa/Portals/67/pdf/Global_bee_colony_disorder_and_threats_insect_pollinators.pdf)
Van Engelsdorp D, Meixner MD (2010) A historical view of managed bee populations in Europe and the United States and the factors that may affect them. J Invertebr Pathol 103:S80–S95
VanGordon J, Clay B (2017) U.S. Patent No. 9,536,726. Washington, DC: U.S. Patent and Trademark Office
Vieco-Saiz N, Belguesmia Y, Raspoet R, Auclair E, Gancel F, Kempf I, Drider D (2019) Benefits and inputs from lactic acid bacteria and their bacteriocins as alternatives to antibiotic growth promoters during food-animal production. Front Microbiol 10:57
Vojvodic S, Jensen AB, James RR, Boomsma JJ, Eilenberg J (2011) Temperature dependent virulence of obligate and facultative fungal pathogens of honeybee brood. Vet Microbiol 149(1–2):200–205
Wang H, Yan Y, Wang J, Zhang H, Qi W (2012) Production and characterization of antifungal compounds produced by Lactobacillus plantarum IMAU10014. PloS One 7(1)
Funding
This study did not receive any specific funding.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: AIH, AMS, AHH. Performed the experiments: AIH, AMS. Data analysis: AIH, AMS. Writing – original draft: AIH, AMS. Writing–review & editing: AIH, AMS, AHH.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing of interests
We are declaring that there aren’t any potential competing interests and all authors read and agreed the publication rules.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Shehabeldine, A.M., Hashem, A.H. & Hasaballah, A.I. Antagonistic effect of gut microbiota of the Egyptian honeybees, Apis mellifera L. against the etiological agent of Stonebrood disease. Int J Trop Insect Sci 42, 1357–1366 (2022). https://doi.org/10.1007/s42690-021-00654-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42690-021-00654-w