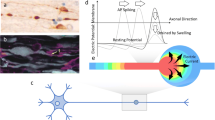
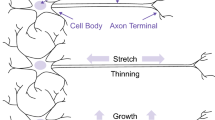
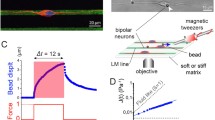
Abstract
Neurons are prone to deformations as a result of the shear effect between the grey and white matters caused by the rapid and sudden movement of the brain tissue during an event of a mechanical impact or neurodegenerative diseases. These structural damages are significantly prominent in the causation of axonal injuries which come in the form of stretching primarily and consequently followed by local swelling as a secondary effect. The microstructural alterations, which are brought about as a result of these physical distortions, have a significant impact on the electrophysiological performance of the neuron cells. The objective of our research was to examine the neural activity in response to the simultaneous impact of swelling and stretching resulting from an altered morphology referred to as varicosity. Our hypothesis postulates that the neuron is able to sustain its signal conduction capacities up to a specific threshold, following exposure to mechanical trauma, and experiences a loss of electrophysiological functionality afterwards upon reaching the critical threshold. Therefore, the aim of our study has been to examine the characteristics of action potential signals within a stretch-induced swelled segment of the axon using computational models aimed at imitating the electrical activity in neuronal cells. The simulations were structured according to the premise that the stretch-induced swelling site possesses ion channels that are vulnerable to mechanical damage, hence influencing the electrophysiology of the transmitted signals. The simulations were conducted to examine the effects of different swelling radii on mechanical strains of fluctuating magnitudes. Thus, we have obtained that the minimum threshold for the applied strain to be \(17\%\) to cause alterations in the AP signal transmission through an unmyelinated stretched-swelled axon. Notwithstanding this comprehension, further investigation has been required to examine the impact of higher magnitudes of strain rate changes on the performance of ion channels. In summary, the utilization of numerical simulations in conjunction with the models facilitated our ability to predict the damage threshold for a neuron cell by analyzing the extent of axonal electrophysiological deficits in response to the injury sustained at the cellular level.







Similar content being viewed by others
Data availability
The data supporting this study's findings can be requested from the corresponding author, [Ashfaq Adnan].
References
M.C. Dewan, A. Rattani, S. Gupta, R.E. Baticulon, Y.-C. Hung, M. Punchak, A. Agrawal, A.O. Adeleye, M.G. Shrime, A.M. Rubiano, J.V. Rosenfeld, K.B. Park, Estimating the global incidence of traumatic brain injury. J. Neurosurg. 130, 1080–1097 (2019). https://doi.org/10.3171/2017.10.JNS17352
C.S. Hill, M.P. Coleman, D.K. Menon, Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci. 39, 311–324 (2016). https://doi.org/10.1016/j.tins.2016.03.002
A.I. Maas, N. Stocchetti, R. Bullock, Moderate and severe traumatic brain injury in adults. Lancet Neurol. 7, 728–741 (2008). https://doi.org/10.1016/S1474-4422(08)70164-9
N.D. Osier, S.W. Carlson, A. DeSana, C.E. Dixon, Chronic histopathological and behavioral outcomes of experimental traumatic brain injury in adult male animals. J. Neurotrauma 32, 1861–1882 (2015). https://doi.org/10.1089/neu.2014.3680
V.E. Johnson, W. Stewart, D.H. Smith, Axonal pathology in traumatic brain injury. Exp. Neurol. 246, 35–43 (2013). https://doi.org/10.1016/j.expneurol.2012.01.013
Y. Li, C. Li, C. Gan, K. Zhao, J. Chen, J. Song, T. Lei, A. Precise, Controllable in vitro model for diffuse axonal injury through uniaxial stretch injury. Front. Neurosci. (2019). https://doi.org/10.3389/fnins.2019.01063
V. Di Pietro, A.M. Amorini, B. Tavazzi, D.A. Hovda, S. Signoretti, C.C. Giza, G. Lazzarino, R. Vagnozzi, G. Lazzarino, A. Belli, Potentially neuroprotective gene modulation in an in vitro model of mild traumatic brain injury. Mol. Cell. Biochem. 375, 185–198 (2013). https://doi.org/10.1007/s11010-012-1541-2
R. Shi, J. Whitebone, Conduction deficits and membrane disruption of spinal cord axons as a function of magnitude and rate of strain. J. Neurophysiol. 95, 3384–3390 (2006). https://doi.org/10.1152/jn.00350.2005
D.W. Simon, M.J. McGeachy, H. Baylr, R.S.B. Clark, D.J. Loane, P.M. Kochanek, The far-reaching scope of neuroinflammation after traumatic brain injury. Nat. Rev. Neurol. 13, 171–191 (2017). https://doi.org/10.1038/nrneurol.2017.13
D.J. DiSabato, N. Quan, J.P. Godbout, Neuroinflammation: the devil is in the details. J. Neurochem. 139, 136–153 (2016). https://doi.org/10.1111/jnc.13607
J. Wang, R.J. Hamm, J.T. Povlishock, Traumatic axonal injury in the optic nerve: evidence for axonal swelling, disconnection, dieback, and reorganization. J. Neurotrauma 28, 1185–1198 (2011). https://doi.org/10.1089/neu.2011.1756
K.V. Kolaric, G. Thomson, J.M. Edgar, A.M. Brown, Focal axonal swellings and associated ultrastructural changes attenuate conduction velocity in central nervous system axons: a computer modeling study. Physiol. Rep. (2013). https://doi.org/10.1002/phy2.59
C. Gu, Rapid and reversible development of axonal varicosities: a new form of neural plasticity. Front. Mol. Neurosci. (2021). https://doi.org/10.3389/fnmol.2021.610857
C. Sun, L. Qi, Y. Cheng, Y. Zhao, C. Gu, Immediate induction of varicosities by transverse compression but not uniaxial stretch in axon mechanosensation. Acta Neuropathol. Commun. 10, 7 (2022). https://doi.org/10.1186/s40478-022-01309-8
W.L. Maxwell, A. Domleo, G. McColl, S.S. Jafari, D.I. Graham, Post-acute alterations in the axonal cytoskeleton after traumatic axonal injury. J. Neurotrauma 20, 151–168 (2003). https://doi.org/10.1089/08977150360547071
A.S. Cohen, B.J. Pfister, E. Schwarzbach, M. Sean Grady, P.B. Goforth, L.S. Satin, Injury-induced alterations in CNS electrophysiology. In: pp. 143–169 (2007). https://doi.org/10.1016/S0079-6123(06)61010-8.
D.M. Geddes, R.S. Cargill, M.C. LaPlaca, Mechanical stretch to neurons results in a strain rate and magnitude-dependent increase in plasma membrane permeability. J. Neurotrauma 20, 1039–1049 (2003). https://doi.org/10.1089/089771503770195885
M.C. LaPlaca, C.M. Simon, G.R. Prado, D.K. Cullen, CNS injury biomechanics and experimental models. In: pp. 13–26, (2007). https://doi.org/10.1016/S0079-6123(06)61002-9.
J.A. Wang, W. Lin, T. Morris, U. Banderali, P.F. Juranka, C.E. Morris, Membrane trauma and Na + leak from Nav1.6 channels. Am. J. Physiol.-Cell Physiol. 297, C823–C834 (2009). https://doi.org/10.1152/ajpcell.00505.2008
W.L. Maxwell, Histopathological changes at central nodes of Ranvier after stretch-injury. Microsc. Res. Tech. 34, 522–535 (1996). https://doi.org/10.1002/(SICI)1097-0029(19960815)34:6%3c522::AID-JEMT4%3e3.0.CO;2-L
J. Engelbrecht, T. Peets, K. Tamm, M. Laasmaa, M. Vendelin, On modelling of physical effects accompanying the propagation of action potentials in nerve fibres, (2016). physics.bio-ph.
A. Jérusalem, J.A. García-Grajales, A. Merchán-Pérez, J.M. Peña, A computational model coupling mechanics and electrophysiology in spinal cord injury. Biomech. Model. Mechanobiol. 13, 883–896 (2014). https://doi.org/10.1007/s10237-013-0543-7
H. Chen, D. Garcia-Gonzalez, A. Jérusalem, Computational model of the mechanoelectrophysiological coupling in axons with application to neuromodulation. Phys. Rev. E 99, 032406 (2019). https://doi.org/10.1103/PhysRevE.99.032406
M.J. Sætra, G.T. Einevoll, G. Halnes, An electrodiffusive neuron-extracellular-glia model for exploring the genesis of slow potentials in the brain. PLoS Comput. Biol. 17, e1008143 (2021). https://doi.org/10.1371/journal.pcbi.1008143
B. Lu, Y.C. Zhou, G.A. Huber, S.D. Bond, M.J. Holst, J.A. McCammon, Electrodiffusion: a continuum modeling framework for biomolecular systems with realistic spatiotemporal resolution. J. Chem. Phys. (2007). https://doi.org/10.1063/1.2775933
J. Pods, J. Schönke, P. Bastian, Electrodiffusion models of neurons and extracellular space using the poisson-nernst-planck equations—numerical simulation of the intra- and extracellular potential for an axon model. Biophys. J. 105, 242–254 (2013). https://doi.org/10.1016/j.bpj.2013.05.041
Y.T. Wu, K. Gilpin, A. Adnan, Effects of focal axonal swelling level on the action potential signal transmission. J. Comput. Neurosci. 48, 253–263 (2020). https://doi.org/10.1007/s10827-020-00750-9
P.-A. Boucher, B. Joós, C.E. Morris, Coupled left-shift of Nav channels: modeling the Na+-loading and dysfunctional excitability of damaged axons. J. Comput. Neurosci. 33, 301–319 (2012). https://doi.org/10.1007/s10827-012-0387-7
B.C. Carter, B.P. Bean, Incomplete inactivation and rapid recovery of voltage-dependent sodium channels during high-frequency firing in cerebellar purkinje neurons. J. Neurophysiol. 105, 860–871 (2011). https://doi.org/10.1152/jn.01056.2010
D. Purves, G.J. Augustine, D. Fitzpatrick, L.C. Katz, A.-S. LaMantia, J.O. McNamara, S.M. Williams, Neuroscience, 2nd edition, Sinauer Associates, Sunderland (MA), 2001. https://www.ncbi.nlm.nih.gov/books/NBK10799/. Accessed 14 Mar 2024
N. Eijkelkamp, J.E. Linley, M.D. Baker, M.S. Minett, R. Cregg, R. Werdehausen, F. Rugiero, J.N. Wood, Neurological perspectives on voltage-gated sodium channels. Brain 135, 2585–2612 (2012). https://doi.org/10.1093/brain/aws225
D. Sterratt, B. Graham, A. Gillies, D. Willshaw, Principles of Computational Modelling in Neuroscience (Cambridge University Press, 2011). https://doi.org/10.1017/CBO9780511975899
A.L. Hodgkin, A.F. Huxley, A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952). https://doi.org/10.1113/jphysiol.1952.sp004764
M. Pospischil, M. Toledo-Rodriguez, C. Monier, Z. Piwkowska, T. Bal, Y. Frégnac, H. Markram, A. Destexhe, Minimal Hodgkin-Huxley type models for different classes of cortical and thalamic neurons. Biol. Cybern. 99, 427–441 (2008). https://doi.org/10.1007/s00422-008-0263-8
D. Lang-Ouellette, K.M. Gruver, A. Smith-Dijak, F.G.C. Blot, C.A. Stewart, P. de Vanssay, C.H. de Blavous, C.V. Li, C. Eitrem, P.L. Rosen, M. Faust, A.J.W. Schonewille, Purkinje cell axonal swellings enhance action potential fidelity and cerebellar function. Nat. Commun. 12, 4129 (2021). https://doi.org/10.1038/s41467-021-24390-4
E.J. López-Sánchez, J.M. Romero, H. Yépez-Martínez, Fractional cable equation for general geometry: a model of axons with swellings and anomalous diffusion. Phys. Rev. E 96, 032411 (2017). https://doi.org/10.1103/PhysRevE.96.032411
S.R. Williams, G.J. Stuarty, Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J. Physiol. 521, 467–482 (1999). https://doi.org/10.1111/j.1469-7793.1999.00467.x
M.I. Khan, F. Hasan, K.A.H. Al Mahmud, A. Adnan, Recent computational approaches on mechanical behavior of axonal cytoskeletal components of neuron: a brief review. Multiscale Sci. Eng. 2, 199–213 (2020). https://doi.org/10.1007/s42493-020-00043-4
Z. Chai, S. Gu, G. Lykotrafitis, Dynamics of the axon plasma membrane skeleton. Soft Matter 19, 2514–2528 (2023). https://doi.org/10.1039/D2SM01602H
Y. Wei, G. Ullah, S.J. Schiff, Unification of neuronal spikes, seizures, and spreading depression. J. Neurosci. 34, 11733–11743 (2014). https://doi.org/10.1523/JNEUROSCI.0516-14.2014
Acknowledgements
This material is based upon research supported by the PANTHER program (URL: https://www.panther.engr.wisc.edu/; Director: Dr. Christian Franck, University of Wisconsin – Madison) through the U.S. Office of Naval Research (ONR) (award number: N00014-21-1-2855(0000001556); Program Director: Dr. Timothy Bentley). The authors would like to acknowledge the High-Performance Computing Modernization Program (HPCMP) by the U.S. Department of Defense (DOD) for providing the computational resources which contributed to achieving the outcomes reported in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no financial and personal conflict of interest in this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rifat, M.N.I., Adnan, A. Axonal Varicosity Leading to Combined Effect of Stretching and Swelling on Action Potential Transmission: A Computational Study. Multiscale Sci. Eng. (2024). https://doi.org/10.1007/s42493-024-00112-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42493-024-00112-y