Abstract
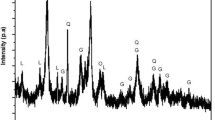
The process of selectively reducing limonite ore involves adding 10 wt% sodium sulfide and using anthracite as a reducing agent in varying amounts (5, 10, 15, and 20 wt%). The research aims to optimize the extraction process by studying how factors like reduction temperature, holding time, and reducing agent dosage affect on iron and nickel content and recovery. The ideal conditions identified are a temperature of 1150 °C, a 10 wt% additive, and a corresponding 10 wt% reducing agent amount, with a crucial 60-min reduction process. X-ray diffraction (XRD) results show dominant phases like iron-nickel (FeNi), iron sulfide (FeS), fayalite (Fe2SiO4), and wustite (FeO) under these conditions, indicating complex chemical interactions. Impressive X-ray fluorescence (XRF) test results precisely measure a nickel component with a 3.03 wt% and a recovery rate of 89.32%, highlighting the process’s effectiveness in extracting potential from limonitic nickel ore. The resulting ferronickel alloy has a controlled particle size of 29.23 µm. The study emphasizes the influence of sodium sulfide and anthracite dosage on the selective reduction of limonite ore.









Similar content being viewed by others
References
Li YJ, Yu HC, Wang DQ, Yin WX, Bai YS (2010) The current status of laterite ore resources and its processing technology. Jinshu Kuangshan/Metal Mine 11:5–9
Rao MJ, Li GH, Jiang T, Luo J, Zhang YB, Fan XH (2013) Carbothermic reduction of nickeliferous laterite ores for nickel pig iron production in China: a review. JOM 65(11):1573–1583. https://doi.org/10.1007/s11837-013-0760-7
Wang CY, Yin F, Chen YQ, Wang Z, Wang J (2008) Worldwide processing technologies and progress of nickel laterites. Chinese J Nonferrous Metals 18(S1):1–8
Dalvi AD, Bacon WG, Osborne RC (2004) The past and the future of nickel laterites. PDAC 2004 Int. Conf. Trade Show and Investors Exchange, Toronto, 7–10 March, 1–27
Bahfie F, Manaf A, Astuti W, Nurjaman F, Prasetyo E, Sumardi S (2022) Characterization of thermal upgrading of nickel from limonite by selective reduction. Izvestiya Ferrous Metallurgy 65(7):471–478. https://doi.org/10.17073/0368-0797-2022-7-471-478. Бaxфи Ф., Maнaф A., Acтyти B., Hypджaмaн Ф., Пpaceтиo Э., Cyмapди C. Tepмичecкoe oбoгaщeниe никeля из лимoнитa мeтoдoм ceлeктивнoгo вoccтaнoвлeния. Извecтия вyзoв. Чepнaя мeтaллypгия. 2022; 65(7): 471–478. https://doi.org/10.17073/0368-0797-2022-7-471-478
Bahfie F, Manaf A, Astuti W, Nurjaman F (2020) Studies on reduction characteristics of limonite and effect of sodium sulphate on the selective reduction to nickel. J Inst Eng (India): Series D 102(1):149–157. https://doi.org/10.1007/s40033-020-00240-3
Bahfie F, Manaf A, Astuti W, Nurjaman F, Prasetyo E (2022) Studies of carbon percentage variation and mixing saprolitelimonite in selective reduction. Mater Today: Proc 62:4156–4160. https://doi.org/10.1016/j.matpr.2022.04.679
Bahfie F, Murti DU, Nuryaman A, Astuti W, Nurjaman F, Prasetyo E, Sudibyo S, Susanti D (2022) Kinetic properties of nickel leaching by ANOVA method. Prog Phys Metals 23(3):476–88. https://doi.org/10.15407/ufm.23.03.476
Nurjaman F, Handoko AS, Bahfie F, Astuti W, Suharno B (2021) Effect of modified basicity in selective reduction process of limonitic nickel ore. J Market Res 15:6476–6490. https://doi.org/10.1016/j.jmrt.2021.11.052
Li GH, Shi TM, Rao MJ, Jiang T, Zhang YB (2012) Beneficiation of nickeliferous laterite by reduction roasting in the presence of sodium sulphate. Miner Eng 32:119–126. https://doi.org/10.1016/j.mineng.2012.03.012
Kim J, Dodbiba G, Tanno H, Okaya K, Matsuo S, FujitaT, (2010) Calcination of low-grade laterite for concentration of Ni by magnetic separation. Miner Eng 23:282–288. https://doi.org/10.1016/j.mineng.2010.01.005
Zheng GL, Zhu DQ, Pan J, Li QH, An YM, Zhu JH, Liu ZH (2014) Pilot scale test of producing nickel concentrate from low-grade saprolitic laterite by direct reduction-magnetic separation. J Central South Univ 21:1771–1777. https://doi.org/10.1007/s11771-014-2123-0
Li B, Wang H, Wei YG (2011) The reduction of nickel from lowgrade nickel laterite ore using a solid-state deoxidisation method. Miner Eng 24:1556–1562. https://doi.org/10.1016/j.mineng.2011.08.006
Liang W, Wang H, Fu JG, He ZX (2011) High recovery of ferronickel from low grade nickel laterite ore. J Central South Univ (Sci Technol) 42(8):2173–2177
Li GH, Rao MJ, Jiang T, Huang QQ, Tang TM, Zhang YB (2011) Innovative process for preparing ferronickel materials from laterite ore by reduction roasting-magnetic separation. Chinese J Nonferrous Metals 21(12):3137–3142
Huang DH, Zhang JL, Lin CC, Mao R (2011) Production of ferro-nickel granules from nickel laterite ore/coal composite briquettes by direct reduction. Chinese J Eng 33(12):1442–1447. https://doi.org/10.13374/j.issn1001-053x.2011.12.015
Cao ZC, Sun TC, Yang HF, Wang JJ, Wu XD (2010) Recovery of iron and nickel from nickel laterite ore by direct reduction roasting and magnetic separation. Chinese J Eng 32(6):708–712. https://doi.org/10.13374/j.issn1001-053x.2010.06.004
Sun TC, Ji YN, Jiang M (2011) Influence mechanism of different types of coal on selective nickel reduction in nickel laterite reduction roasting. Chinese J Eng 33(10):1197–1203. https://doi.org/10.13374/j.issn1001-053x.2011.10.015
Jiang M, Sun TC, Liu ZG, Kou J, Liu N, Cao YY, Zhang SY (2012) Effects of coal types and additives on selective direct reduction of nickel laterite. Mining Metall Eng 32(5):77–81
Li GH, Rao MJ, Jiang T, Shi TM, Huang QQ (2012) Reduction roasting-magnetic separation mechanisms of nickelferous laterite ore in presence of sodium salts. Chinese J Nonferrous Metals 22(1):274–278
Sun TC, Jiang M, Liu ZG, Liu N, Zhang SY, Kou J, Xu CY (2013) Research on the effect of additive on selective reduction of the laterite ores with low nickel and high iron content. Zhongguo Kuangye Daxue Xuebao/J China Univ Mining Technol 42(5):838–844
Zhu DQ, Cui Y, Vining K, Hapugoda S, Douglas J, Pan J, Zheng GL (2012) Upgrading low nickel content laterite ores using selective reduction followed by magnetic separation. Int J Miner Process 106–109:1–7. https://doi.org/10.1016/j.minpro.2012.01.003
Jiang M, Sun TC, Liu ZG, Kou J, Liu N, Zhang SY (2013) Mechanism of sodium sulfate in promoting selective reduction of nickel laterite ore during reduction roasting process. Int J Miner Process 123:32–38. https://doi.org/10.1016/j.minpro.2013.04.005
Valix M, Cheung WH (2002) Effect of sulphur on the mineral phases of laterite ores at high temperature reduction. Miner Eng 15:523–530. https://doi.org/10.1016/S0892-6875(02)00069-9
Lu J, Liu SJ, Shangguan J, Du WG, Pan F, Yang S (2013) The effect of sodium sulphate on the hydrogen reduction process of nickel laterite ore. Miner Eng 49:154–164. https://doi.org/10.1016/j.mineng.2013.05.023
Ali A, Zafar H, Zia M, ul Haq I, Phull AR, Ali JS, Hussain A (2016) Synthesis, characterization, applications, and challenges of iron oxide nanoparticles. Nanotechnol Sci Appl 2016(9): 49–67. https://doi.org/10.2147/NSA.S99986
Julien P, Bergthorson JM (2017) Enabling the metal fuel economy: green recycling of metal fuels. Sustain Energy Fuels 3:1–11. https://doi.org/10.1039/C7SE00004A
Bahfie F, Manaf A, Astuti W, Nurjaman F, Suharto S, Herlina U, Adi WA, Manawan M (2023) Composition of tailings after selective reduction of laterite. Izvestiya. Ferrous Metall 66(1):127–132. https://doi.org/10.17073/0368-0797-2023-1-127-132
Liu M, Xuawei (2014) Novel production process of ferronickel nugget from nickel laterite by semi-molten state reduction. ISIJ Int 54(8):1749–1754
Acknowledgements
We would like to say thanks to the Indonesia Endowment Fund for Education Agency (LPDP) and National Research and Innovation Agency (BRIN) for funding this research through the RIIM Batch-4 program with contract number B-3842/II.7.5/FR.06.00/11/2023 and B-3855/III.10/FR.06.00/11/2023.
Funding
Indonesia Endowment Fund for Education Agency (LPDP) and the National Research and Innovation Agency (BRIN) for funding this research through RIIM Batch-4 program with contract number B-3842/II.7.5/FR.06.00/11/2023 and B-3855/III.10/FR.06.00/11/2023.
Author information
Authors and Affiliations
Contributions
All authors have done this research together.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahfie, F., Triapriani, Y., Shofi, A. et al. The Effect of Sodium Sulfide and Anthracite Dosage on Selective Reduction of Limonite. Mining, Metallurgy & Exploration (2024). https://doi.org/10.1007/s42461-024-00972-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42461-024-00972-w