Abstract
This paper presents the results obtained by processing a gold and silver telluride mineral concentrate from a mine in Colombia. An acid wash, also known as acidification, was carried out, followed by oxidation with nitric acid as a pretreatment. Cyanidations of the pretreatment residues were carried out and the results were compared with results from cyanidations of base materials. The removal of carbonates in the acidification, a tellurium extraction close to 90% during the pretreatment, and a 30% improvement in the gold recovery compared to the conventional process were achieved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Gold is a metal that, thanks to its unique properties, has been widely appreciated from ancient times to the present day. Traditionally, gold ores are treated by physical and chemical processes, cyanidation being the method par excellence for more than a hundred years [1]. Cyanide has several advantages, such as low cost, high efficiency, and selectivity, compared to other compounds such as halides, thiosulfate, thiourea, and thiocyanate, which are also capable of extracting gold [2]. However, the treatment of so-called refractory gold ores using cyanidation is problematic and new solutions are needed due to the constant depletion of easy-to-treat gold ores [1, 3].
A refractory gold ore is defined as an ore with low gold recovery (less than 80%) when treated by conventional cyanidation. This refractory behavior represents a fundamental metallurgical problem, as it does not allow maximum use of natural resources [1, 4,5,6]. Although at present only a portion of global gold resources and reserves can be considered as refractory, interest in refractory gold ores has been increasing significantly over the years [1]. The main reasons for this have been that the amount of gold produced from refractory gold ores has also been continuously increasing [7] and that easy-to-treat gold deposits have been largely depleted [8]. These trends suggest that the future of the extractive metallurgy of gold will focus on the processing of refractory gold ores, raising the need to develop processes capable of more efficiently recovering gold associated with these complex ores.
Gold and gold-silver tellurides are the most common and easily found gold compouds in nature and are also a subgroup of the refractory gold ores [1]. Within the large number of minerals that make up the group of gold and gold-silver tellurides, the most economically and metallurgically feasible minerals are: calaverite [AuTe2], krennerite [(Au1-x,Agx)Te2], sylvanite [AuAgTe4], muthmannite [(Ag,Au)Te2], montbrayite [(AuSb)2Te3], petzite [Ag3AuTe2], hessite [Ag2Te] [9, 10]. These minerals exhibit a “chemical refractoriness” which consists of a blockage of the gold atoms within the chemical structure of poorly soluble minerals [1, 2, 5].
The refractory behavior generated by tellurides has been less studied than the refractoriness generated by the presence of oxygen/cyanide consuming species or by preg-robbing phenomena. However, it was reported that the formation of a passivating layer of TeO2 during the cyanidation of tellurides is possible and can be responsible for the slow dissolution of gold-bearing tellurides [10,11,12,13]. It was also noted that the dissolution of AuTe2 in alkaline cyanide solutions can be affected by the presence of a passivating layer of H2TeO3 [14]. Deschênes et al. [13] reported the formation of a tellurium hydroxide Te(OH)6 that does not affect mass transfer in the cyanidation of synthetic calaverite when high levels of oxygen and silver nitrate are used.
For the treatment of refractory gold ores, the application of pretreatments that eliminate the elements causing refractoriness has been proposed. Some of the proposed pretreatments are [15]: roasting of the whole ore or concentrate, pressure oxidation of the ore concentrate, bio-oxidation of the ore concentrate, cyanidation at high pH or pre-aeration, and leaching using chlorination. Nitric acid has been used successfully in the pretreatment of gold ores associated with sulfides such as pyrite and arsenopyrite. Some of the advantages of using nitric acid over other reagents are that nitric acid is both a strong acid and a strong oxidant, and it can be easily regenerated, reducing acid consumption [16]. So, the aim of the present work is to study a pretreatment with nitric acid as an alternative to improve the recovery of gold from ores associated with gold and gold-silver tellurides.
2 Materials and methods
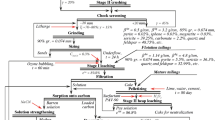
A mineral concentrate (MC) obtained by flotation in a mine located in the northwestern region of Colombia was used in this study. 10 kg of material were received in the form of a fine-grained powder with a smooth texture and light color. All reagents used in this work are chemical, analytical, or high-purity grade for metal analysis. None of the reagents used underwent further purification and deionized water was used in all tests.
2.1 Characterization
Different physical and chemical characterization tests were carried out. Particle size analysis was performed using a Mastersizer 2000® (Malvern Instruments) with water as dispersant; gold content in the ore using both conventional fire assay and microwave acid digestion using a microwave digester Ethos UP-EASY® (Milestone) followed by elemental analysis using an ICP-OES 5100® (Agilent); chemical composition using a X-Ray fluorescence EPSILON 1® (Malvern Panalytical); mineralogy using X-Ray Diffraction Multiflex® 2 kW (Rigaku); tellurium content of the ore by microwave acid digestion using an Ethos UP-EASY® (Milestone) microwave digester and elemental analysis using ICP-OES. The nitric acid pretreatment solutions were analyzed using ICP-OES to determine tellurium concentration; cyanidation solutions were analyzed using an atomic absorption spectrometer 240AA® (Agilent) to determine gold concentration.
2.2 Acidification
The mineral concentrate (MC) was subjected to an acidification process and an acidified mineral concentrate (AMC) was obtained. The acidification process was carried out to eliminate acid consumer species present in the MC that could affect the subsequent nitric acid pretreatment. Four acidification tests were carried out to determine the most suitable acid (H2SO4 or HCl) and acid concentration needed to eliminate the calcite peaks identified in the XRD of the MC. The acidification conditions in each of the tests were:
-
0.5 M H2SO4, L/S: 4/1; 2 h; washing residue with water.
-
1.0 M H2SO4, L/S: 10/1.5; 6 h; washing the residue with a solution of HCl pH 1 and water.
-
0.5 M HCl, L/S: 4/1; 6 h; washing residue with water.
-
0.5 M HCl, L/S: 4/1; 2 h; washing residue with water.
All acidification tests were carried out in an open reactor at 21.6 °C. Agitation was provided by a mechanical stirrer coupled to a stir bar with two blades. The pH was measured with an Oakton series 10 pH meter. The acidification residues were vacuum filtered using a filtration unit (pump, flask, and ceramic funnel) and then dried in an oven at 80–70 °C for 12 h.
2.3 Pretreatments
After acidification, the filtered and dried solid residue (called acidified mineral concentrate - AMC) was pretreated with a nitric acid solution to decompose refractory minerals with the aim of improving the gold recovery in subsequent cyanidation. To determine the effect of the pretreatment variables on gold recovery in subsequent cyanidation, a \({2}_{IV}^{3}\) completely randomized factorial design was carried out with three replicates in the center to estimate the pure experimental error. Three factors were studied: A = nitric acid concentration, B = temperature, and C = stirring speed. The response variable was Y: gold recovery percentage.
The values chosen for factors A and B were taken based on the values used by other authors in works on the use of nitric acid in the pretreatment of refractory gold ores (Nitrox and Arseno/Redox process) [16,17,18]. The values for the C variable (agitation speed) were chosen in a range suitable to keep the solids in suspension and avoid splashing. The variables and levels studied are summarized in Table 1.
For the \({2}_{IV}^{3}\) factorial design and its analysis, the Minitab® 17 software was used. Table 2 shows the experiments performed according to the proposed factorial desing.
Two preliminary tests of the pretreatment were performed with the aim of having an initial panorama on the dissolution of the tellurides in nitric acid solutions. These two preliminary tests were carried out to establish the most convenient time to carry out the pretreatments established in the design of experiments described before. The two preliminary tests were performed using 1 and 2 M of HNO3, S/L ratio = 1:5, stirring speed 360 rpm at 80 °C for 6 h. Preliminary tests and all subsequent pretreatment tests were performed in round bottom flasks with two mouths closed with rubber corks (thermometer and pH probe were introduced through the rubber corks as needed). The temperature was controlled with a water bath on a CORNING® plate and the agitation was provided with a magnetic agitator controlled by the same plate. Temperature deviation did not exceed 3 °C in any of the tests. When necessary, samples of the pretreatment solution were collected using a syringe attached to a 0.22 µm microfilter and plastic hose.
2.4 Cyanidation
The MC and AMC were subjected to cyanidation tests (base cyanidations) to determine the gold recovery obtained without any pretreatment, as well as to corroborate the refractory behavior of the MC and the AMC. The base cyanidations were performed at pH = 11, S/L ratio = 1:3, and stirring speed = 420 rpm, for 24 h with NaCN concentrations of 1 g/L. The cyanide concentration was determined through titration using silver nitrate at defined time intervals (1, 2, 4, 6, 8, and 24 hours). When it was necessary, lime and cyanide were added to maintain the desired concentrations. Aliquots were taken with the help of a syringe. Cyanidation tests were performed in 250 mL beakers at room temperature. The agitation was provided by a mechanical agitation bank with 6-blade impellers. All cyanidations of the pretreatment residues were carried out under the same conditions as base cyanidations.
3 Results and Discussion
3.1 Characterization
Fig. 1 displays the particle size distributions obtained in the granulometric analysis of MC and AMC. The results show that the acidification with hydrochloric acid solution reduced the particle size of the MC. This occurs because during acidification some minerals are dissolved (mainly carbonates). Both materials (MC and AMC) had a fine grain size, with up to 80% of the particles having a grain size smaller than 53 µm. Therefore, no additional size reduction was needed.
The analyzed gold content for the MC was 24.8 g/t by fire assay and 23.4 g/t by acid digestion coupled with ICP-OES. For subsequent gold recovery calculations, the gold grade determined by fire assay was used. A tellurium concentration of 66.17 ppm was obtained for the MC.
The results of the XRF analysis of the MC are presented in Table 3. These results shown that the MC sample was mainly composed of aluminosilicates, and it had a low content of sulfides. The presence of elements associated with tellurides such as tellurium, lead, silver, and gold was also confirmed.
3.2 Acidification
The diffractograms of the acidification residues obtained by XRD (Fig. 2 and Fig. 1S) showed that in all four acidification tests, the calcite peaks appearing in the diffractogram of the MC were eliminated. However, the formation of a hydrated gypsum phase was detected in the two acidifications where sulfuric acid was used. The presence of a hydrated gypsum phase explains the increase in mass obtained after the acidifications with sulfuric acid solutions. Since gypsum is an acid consumer under the proposed pretreatment conditions, sulfuric acid was no loger considered to be a suitable reagent for the acidification of the MC.
The diffractograms of the residues from the HCl acidification tests showed that calcite dissolution was achieved and that no new calcium phases were formed regardless of the acidification time (6 or 2 h). A mass loss between 7 and 9% was registered after HCl acidifications tests. This is in accordance with the dissolution of the calcite detected by semi-quantitative analysis of XRD data and by mineralogical reconstruction from XRF data.
The decomposition of the carbonates in the MC material during the acidification stage contributed to the nitric acid being more available to oxidize tellurides and sulfides during the pretreatment. In order to maintain a more stable nitric acid concentration throughout the duration of the pretreatment tests, it was decided to produce the AMC by acidifying the MC with a 0.5 M HCl solution for 2 h. With the acidification conditions chosen, there was no loss of gold into the acidification solution and the tellurium detected in the HCl solution was less than 5.0% of the tellurium in the MC sample.
3.3 Base cyanidations
The base cyanidations of MC confirmed the refractory behavior of the ore associated with gold and gold-silver tellurides. In a conventional cyanidation process applied to MC, a maximum gold recovery of 58.4% after 24 h was reached, which is considered refractory (see Fig. 3). In the case of the AMC, after 24 h, the maximum gold recovery was only 45.2%, which indicates two things: first, the mineral still has a refractory behavior after being acidified; and second, the acidification stage alone has a negative effect on the gold recovery. This diminution in the gold recovery after acidifying with a diluted solution of hydrochloric acid could be explained by the formation of species that negatively affect the cyanidation.
It should be noted that, although the gold recovery in the cyanidation of the MC material was higher than the gold recovery achieved in the cyanidation of the AMC, the MC could not be fed directly to the pretreatment reactor without being previously subjected to an acidification step. There are two main reasons for this: first, the violent effervescence reaction that occurs when the carbonates in the MC come into contact with the nitric acid solution, making it dangerous and unfeasible to control the pretreatment conditions; and second, due to its carbonates content, the MC could consume part of the nitric acid used during pretreatments (up to 32% when 1 M HNO3 solution is used), which could affect the dissolution of gold-tellurides.
3.4 Pretreatment
3.4.1 Time effect
Fig. 4 shows the recovery tellurium through the test carried out to determine the appropriate pretreatment time. It is observed that the dissolution of tellurides occurs rapidly during the first 15 min, reaching a tellurium recovery greater than 50%. It is also observed that for the first 60 min, the recovery of tellurium is fast and then it remains approximately constant. Tellurium recovery is 78.05% in 60 min and after 6 h is close to 80%. Thus, considering that after 60 min there is no considerable change in the tellurium recovery, 1 h was chosen as the duration of the pretreatment tests.
3.4.2 Effect of the nitric acid concentration during the pretreatment
The tellurium recovery as a function of the concentration of nitric acid in the pretreatment is shown in Fig. 5. It could be noted that tellurium recovery is proportional to acid concentration, and a recovery close to 90% was reached when 3 M solution of nitric acid was used.
3.4.3 Cyanidations
Several cyanidations were carried out to determine the effect of the pretreatment on gold recovery. The pretreatments were done at concentrations of 1 and 3 M HNO3, temperatures of 20 and 80 °C and, stirring speeds of 700 and 1100 rpm. Fig. 6 shows that the gold recovery attains values up to 86%, which represents an increase close to 30 and 40% compared to what was obtained in the cyanidation of MC and AMC materials without any pretreatment.
3.5 Statistical analysis of the experiment
The results of the experimental tests carried out to study the effect of the pretreatment variables on the gold recovery during the cyanidation of the pretreatment residues are presented in Table 4. A maximum gold recovery of 86.46% was achieved when more acid, temperature, and agitation was used. This improvement represents an increase of 28.01% and 41.21% when compared with MC and AMC base cyanidations respectively. In all pretreatment test more gold was recovered compared to AMC base cyanidation results.
From normal probability plot, (Fig. 7), it is concluded that the main effects A (concentration of nitric acid), B (temperature) and, C (stirring speed) are significant on the gold recovery in the cyanidation of the AMC after the pretreatment. It is also observed that the temperature has the greatest effect on the recovery of gold, and the stirring speed has the least effect. The three factors studied in the pretreatment have a significant effect on the recovery of gold in the cyanidation process, see Fig. 8.
In Table 5 is shown the analysis of variance (ANOVA) for the initial model, considering α = 0.05. It can be seen that only the main effects A, B and C are significant. A coefficient of determination equal to 98.36% was obtained, indicating that the considered factors contribute satisfactorily to the study of the gold recovery during the cyanidation of the solid pretreatment residues.
By sending the interactions to error, the best ANOVA was obtained (Table 6). An adjusted coefficient of determination equal to 93.48% was obtained, so it can be concluded that the factors considered contribute satisfactorily to the study. The following first-order regression model was then obtained for the uncoded variables:
In this case, from the Table 6, a P-Value of 0.606 is observed for the lack of fit, which allows us to conclude that the first-order model statistically represents the results.
The residuals were analyzed and found to be independent and to follow a normal distribution with zero mean and constant variance. These results confirmed the ANOVA assumptions and the validity of the statistical analysis, as established by the methodology [19].
4 Conclusions
A mineral concentrate containing aluminosilicates, carbonates, sulfides, and gold-silver tellurides was processed. Through a conventional cyanidation test, it was determined that the mineral was refractory, since only a 58% gold recovery was achieved. Elimination of the carbonates was achieved using an acidification stage with dilute HCl solution. Although acidification reduced acid consumption during the pretreatment, it also had a negative effect on gold recovery.
During the pretreatment of the acidified mineral (AMC), a tellurium recovery close to 90% was achieved after one hour. Tellurium extraction was found to be positively dependent on nitric acid concentration. After the pretreatment, a gold recovery of 86% was achieved by cyanidation, which represents an improvement of more than 30% compared to conventional cyanidation of the MC. More studies to improve the gold recovery are necessary since the ore continues to show a slight and moderate refractory behavior in most of the pretreatment conditions.
The experiment design showed that the three studied pretreatment variables (acid concentration, temperature and, stirring speed) have a positive effect on gold recovery during the subsequent cyanidation of pretreatment residues. A first order regression model with a coefficient of determination equal to 93.48% was established for gold recovery in the experimental region studied.
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information Files.
References
Marsden JO, House CL (2006) The chemistry of gold extraction. Society for Mining, Metallurgy, and Exploration, USA
Asamoah RK, Amankwah RK, Addai-Mensah J (2014) Cyanidation of Refractory Gold Ores: A Review. 3rd UMaT Biennial Int Min Mineral Conf 204–12. https://doi.org/10.13140/2.1.4772.6407
Vaughan JP (2004) The process mineralogy of gold: The classification of ore types. JOM 56:46–48. https://doi.org/10.1007/s11837-004-0092-8
Komnitsas C, Pooley FD (1989) Mineralogical characteristics and treatment of refractory gold ores. Miner Eng 2:449–457. https://doi.org/10.1016/0892-6875(89)90080-0
Yannopoulos JC (1991) The Extractive Metallurgy of Gold. First. Boston, MA: Springer US. https://doi.org/10.1007/978-1-4684-8425-0
La Brooy SR, Linge HG, Walker GS (1994) Review of gold extraction from ores. Miner Eng 7:1213–1241. https://doi.org/10.1016/0892-6875(94)90114-7
Bas AD, Ghali E, Choi Y (2017) A review on electrochemical dissolution and passivation of gold during cyanidation in presence of sulphides and oxides. Hydrometallurgy 172:30–44. https://doi.org/10.1016/j.hydromet.2017.06.021
Tan H, Feng D, Lukey GC, van Deventer JSJ (2005) The behaviour of carbonaceous matter in cyanide leaching of gold. Hydrometallurgy 78:226–235. https://doi.org/10.1016/j.hydromet.2005.03.001
Zhao J, Pring A (2019) Mineral transformations in gold–(Silver) tellurides in the presence of fluids: nature and experiment. Minerals 9:167. https://doi.org/10.3390/min9030167
Dyer LG, Sauber M, Dixon DG, Asselin E (2017) On the refractory nature of precious metal tellurides. Hydrometallurgy 169:488–495. https://doi.org/10.1016/j.hydromet.2017.03.009
Jackman IR, Sarbutt K (1990) The recovery of gold from a telluride concentrate. Proceedings of Randol Gold Forum ’90, Colorado, p 55
Ellis S (2005) Treatment of gold-telluride ores. Dev Mineral Process 15:973–984. https://doi.org/10.1016/S0167-4528(05)15039-X
Deschênes G, Pratt A, Fulton M, Guo H (2006) Kinetics and mechanism of leaching synthetic calaverite in cyanide solutions. Mining, Metall Explor 23:133–138. https://doi.org/10.1007/BF03403200
Korolev I, Altinkaya P, Haapalainen M, Kolehmainen E, Yliniemi K, Lundström M (2022) Electro-hydrometallurgical chloride process for selective gold recovery from refractory telluride gold ores: A mini-pilot study. Chem Eng J 429:132283. https://doi.org/10.1016/j.cej.2021.132283
Acar S (2016) Process development metallurgical studies for gold cyanidation process. Mineral Metall Process 33:161–71. https://doi.org/10.19150/mmp.6837
Zárate-Gutiérrez R, Lapidus GT, Morales RD (2012) Aqueous oxidation of galena and pyrite with nitric acid at moderate temperatures. Hydrometallurgy 115–116:57–63. https://doi.org/10.1016/j.hydromet.2011.12.010
Beattie MJ V, Raudsepp R, Ismay A (1989) Arseno/redox process for refractory gold ores. In: Proceedings of the International Symposium on Processing Complex Ores, Halifax, pp 431–439
Van Weert G, Fair KJ, Schneider JE (1986) Prochem’s NITROX process. CIM Bulletin 79:84–85
Montgomery DC (2013) Design and analysis of experiments (8th ed.). John Wiley & Sons, New York
Acknowledgements
The authors thank Dr. David Dixon, Dr. Edouard Asselin, and Dr. Baseer of the University of British Columbia for their valuable collaboration during the experimental work.
Data from this manuscript had been presented in the Symposium in Honor of Patrick R. Taylor at the MINEXCHANGE 2023 SME Annual Conference & Expo, held in Denver, CO, February 26-March 1, 2023.
Funding
Open Access funding provided by Colombia Consortium.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Argumedo-Jimenez, C., Chaverra, D.E. & Restrepo-Baena, O.J. Nitric Acid Pretreatment Applied to a Refractory Gold-Tellurides Ore. Mining, Metallurgy & Exploration 40, 2051–2058 (2023). https://doi.org/10.1007/s42461-023-00817-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-023-00817-y