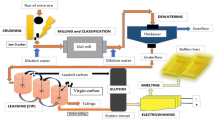
Abstract
As a result of the rise in global demand for pure copper, there is urgent need to consider the processing of low-grade chalcopyrite ores. Ammoniacal leaching solutions have been reported as an effective and efficient leachant for the dissolution of chalcopyrite. This work reports different parameters that influence the leaching kinetics of a Nigerian chalcopyrite-rich siderite ore obtained from Wase Local Government Area, Plateau State, Nigeria, and assayed as 11.30 wt% of Cu. At the optimum leaching conditions (0.4 mol/L NH3-(NH4)2SO4 leachant concentration; 75 °C reaction temperature; 45 μm particle size), the extent of chalcopyrite ore dissolution reached 87.4% within 120 min. The dissolution rate was affirmed to conform to the diffusion control mechanism with the activation energy, Ea, of 38.84 kJ/mol and a reaction order of 0.661 for the leachant concentration being established. The leaching mechanism was affirmed by characterizing the raw ore and the leached product by XRD and SEM-EDS analysis. Ascertaining optimal leaching conditions for copper using alkaline leachant is advantageous from the environment and economic points of view.











Similar content being viewed by others
References
Baba AA, Ayinla IK, Adekola FA, Bale RB, Ghosh MK, Alabi AGF, Sheik AR, Folorunso IO (2013) Hydrometallurgical application for treating a Nigerian chalcopyrite ore in chloride medium: part I. Dissolution kinetics assessment. International Journal of Minerals, Metallurgy, and Materials 20(11):1021
Van Schalkwayk RF, Eksteen JJ, Peterson J, Thyse EL, Akdogan G (2011) An experimental evaluation of the leaching kinetics of PGM – containing Ni-Cu-Fe-S Pierce Smith converter matte, under atmospheric leach conditions. Minerals Engineering 24:524
Wang S (2005) Copper leaching from chalcopyrite concentrates. Journal of Metallurgy:48–51
Haver FP, Wong MM (1971) Recovery of copper, iron and sulfur from chalcopyrite concentrate using a ferric chloride leach. Metals 23(2):25–29
Antonijevic MM, Jankovic ZD, Dimitrijevic MD (2004) Kinetics of chalcopyrite dissolution by hydrogen peroxide in sulphuric acid. Hydrometallurgy 71:329–334
Havlik T, Skrobian M, Balaz P, Kammel R (1995) Leaching of chalcopyrite concentrate with ferric chloride. International Journal of Mineral Processing 43:1
Dutrizac J (1981) The dissolution of chalcopyrite in ferric sulfate and ferric chloride media. Metallurgical Transactions B 12B:371
Velasquez-Yevenes L, Nicol M, Miki H (2010) The dissolution of chalcopyrite in chloride solutions: part I. The effect of solution potential. Hydrometallurgy 103:108–113
Nicol M, Miki H, Velasquez-Yevenes L (2010) The dissolution of chalcopyrite in chloride solutions: part 3. Mechanism. Hydrometallurgy 103:86–95
Romero R, Mazuelos A, Palencia I, Carranza F (2003) Copper recovery from chalcopyrite concentrates by the BRISA process. Hydrometallurgy 70:205–215
Gok O, Anderson CG (2013) Dissolution of low-grade chalcopyrite concentrate in acidified nitrite electrolyte. Hydrometallurgy 134:134–135
Watling H (2013) Chalcopyrite hydrometallurgy at atmospheric pressure: 1. Review of acid sulfate, sulfate-chloride and sulfate-nitrate process options. Hydrometallurgy 140:163–180
Castellon CI, Hernandez PC, Velasquez-Yevenes L, Taboada ME (2020) An alternative process for leaching chalcopyrite concentrate in nitrate-acid-seawater media with oxidant recovery. Metals 10:518
Kimball BE, Rimstidt JD, Brantley SL (2010) Chalcopyrite dissolution rate laws. Applied Geochemistry 25(7):973–983
Klauber C (2008) A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. International Journal of Mineral Processing 86(1 – 4):1–17
Torres CM et al (2015) The effect of seawater-based media on copper dissolution from low-grade copper ore. Minerals Engineering 71:139–145
Ma Y, Yang Y, Fan R, Gao X, Zheng L, Chen M (2021) Chalcopyrite leaching in ammonium chloride solutions under ambient conditions: insight into the dissolution mechanism by XANES, Raman spectroscopy and electrochemical studies. Minerals Engineering 170:107063
Meng X, Han KN (1996) The principles and applications of ammonia leaching of metals – a review. Mineral Processing and Extractive Metullargy Review 16(1):23–61
Aghazedeh S, Abdollahi H, Gharabaghi M, Mirmohammadi M (2021) Selective leaching of antimony from tetrahedrite rich concentrate using alkaline sulfide solution with experimental and design: optimization and kinetic studies. Journal of the Taiwan Institute of Chemical Engineers 119:298–312
Sokic MD, Markovic B, Zivkovic D (2009) Kinetics of chalcopyrite leaching by sodium nitrate in sulphuric acid. Hydrometallurgy 95:273–279
Raji MA, Baba AA, Ibraheem L, Alabi AGF, Ghosh MK, Bale RB (2020) Preparation of industrial alumina from a biotite-rich kaolinite (BRK) ore by Cyanex®272. Indian Chemical Engineer 63(3):231–239
Raji MA, Baba AA, Bale RB, Alabi AGF, Ghosh MK (2020) Removal of iron impurities from a Nigerian biotite-rich kaolinite ore by a sulphuric acid solution. Journal of Chemical Technology and Metallurgy 55(6):2128–2135
Tanda BC, Eksteen JJ, Oraby EA, O’Connor GM (2019) The kinetics of chalcopyrite leaching in alkaline glycine/glycinate solutions. Minerals Engineering 135:118–128
Baba AA, Ashaolu OH, Gatta NF, Raji MA, Aremu AS, Okeola FO, Bello SO (2022) Purification of a pargasite rich ganophyllite-oligoclase ore by oxalic acid solution: statistics for industrial considerations. Journal of Chemical Technology and Metallurgy 57(1):166–175
Chindo SY, Omoniyi KI, Raji MA (2022) Chalcopyrite leaching in ammonia-ammonium chloride solutions: insight into the dissolution kinetic studies. Journal of Sustainable Materials Processing and Management 2(2):90–97
Yin S, Wang L, Wu A, Kabwe E, Chen X, Yan R (2018) Copper recycle from sulfide tailings using combined leaching of ammonia solution and alkaline bacteria. Journal of Cleaner Production. https://doi.org/10.1016/j.jclepro.2018.04.116
Aydogan S, Ucar G, Canbazoglu M (2006) Dissolution kinetics of chalcopyrite in acidic potassium dichromate solution. Hydrometallurgy 81:45–51
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chindo, S.Y., Omoniyi, K.I., Agbaji, E.B. et al. Leaching of Copper from a Nigerian Chalcopyrite-Rich Siderite Ore by Ammonia-Ammonium Sulphate Solutions. Mining, Metallurgy & Exploration 40, 161–169 (2023). https://doi.org/10.1007/s42461-022-00728-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-022-00728-4