Abstract
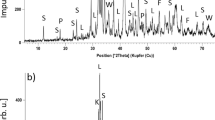
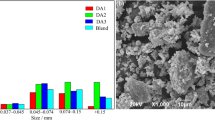
In this study, a mixture rich in K, Na, and Pb, composed of undesired elements rich iron ore A, sinter dust, and blast furnace dust (ore-dust mix,), is sintered along with the regular iron ore. The K, Na, and Pb compounds within the ore-dust mix are identified as alkali chlorides, alkali aluminosilicates, PbO, and PbSO4 using X-ray diffraction. The primary objective is to investigate the impact of three distinct sintering technologies: regular (pre-reduction) sintering, double-layer sintering, and hearth-layer sintering, on the removal degrees of K, Na, and Pb. In both the regular and double-layer sintering processes, the K, Na, and Pb contents within the blend of raw materials was measured approximately 0.430 wt%, 0.105 wt%, and 0.033 wt%, respectively. Moreover, the ratio of ore-dust mix to the regular ore was 0.54. In the regular sinter pot test, sinter feed was uniformly placed in the pot. In the run of the regular sinter pot test with the optimal coke breeze ratio of 20 wt%, the removal degrees of K, Na, and Pb were 79.5%, 67.5% and 92.7%, respectively. In comparison, the double-layer sintering technology resulted in a slight increase in the removal degrees for alkali metals and a similar removal degree for Pb, while utilizing a reduced coke breeze ratio of only 8.10 wt%. The removal mechanism of the hearth-layer and double-layer sintering processes are similar. However, the coke breeze ratio decreases to 6.63 wt% as the fraction of ore-dust mix within the blend of raw materials is reduced to 7 wt% during the hearth-layer sintering process.
Graphical Abstract







Similar content being viewed by others
References
Angalakuditi VB, Appala A, Singh N, et al (2023) A process intensification approach to improve productivity, quality, and reducing emissions in the iron ore sintering process. J Sustain Metall 9:73–80. https://doi.org/10.1007/s40831-023-00660-x
Acharyulu AVB, Sudhakar K, Ramarao G, et al (2021) Thermodynamic and mineralogical aspects of injecting LPG, coke oven gas, and oxygen into goethitic iron ore sintering process. J Sustain Metall 7:136–150. https://doi.org/10.1007/s40831-020-00327-x
Li S, Chang J, Chu M, et al (2022) A blast furnace coke ratio prediction model based on fuzzy cluster and grid search optimized support vector regression. Appl Intell 52:13533–13542. https://doi.org/10.1007/s10489-022-03234-8
Xu R, Xu L, Xu B (2017) Assessing CO2 emissions in China?s iron and steel industry: evidence from quantile regression approach. J Clean Prod 152:259–270. https://doi.org/10.1016/j.jclepro.2017.03.142
Li C, Yan H, Zhang W, Xue Z (2021) Fundamental study on the re-utilization of waste magnesia-carbon bricks as MgO additive for iron ore sintering. J Sustai Metall 7:597–609. https://doi.org/10.1007/s40831-021-00366-y
Lanzerstorfer C, Bamberger-Strassmayr B, Pilz K (2015) Recycling of blast furnace dust in the iron ore sintering process: investigation of coke breeze substitution and the influence on offgas emissions. ISIJ Int 55:758–764. https://doi.org/10.2355/isijinternational.55.758
Pal J (2019) Innovative development on agglomeration of iron ore fines and iron oxide wastes. Miner Process Extr Metall Rev 40:248–264. https://doi.org/10.1080/08827508.2018.1518222
Lanzerstorfer C (2015) Application of air classification for improved recycling of sinter plant dust. Resour Conserv Recycl 94:66–71. https://doi.org/10.1016/j.resconrec.2014.11.013
Wang Y, Gan M, Fan X, et al (2022) Phase transformation and enhanced Zn removal technology during the iron ore sintering process. JOM 75(2):310–320. https://doi.org/10.1016/j.resconrec.2014.11.013https://doi.org/10.1007/S11837-022-05603-7
Chen YB, Deng Y, Liu R, et al (2022) Optimization of alkali metals discharge performance of blast furnace slag and its extreme value model. High Temp Mater Process 41:306–314. https://doi.org/10.1515/htmp-2022-0013
Chen S, Wang W, Xu R, et al (2020) Influence of multiple factors on the alkali metal enrichment in blast furnaces by a thermodynamic model. Ironmak Steelmak 47:219–227. https://doi.org/10.1080/03019233.2019.1709700
Hamann C, Spanka M, Stolle D, et al (2021) Recycling of blast-furnace sludge by thermochemical treatment with spent iron(II) chloride solution from steel pickling. J Hazard Mater 402:123511. https://doi.org/10.1016/j.jhazmat.2020.123511
Fredman TP (2002) Accretions in the blast furnace stack - background factors. Can Metall Q 41:475–486. https://doi.org/10.1179/cmq.2002.41.4.475
Biswas Anilk (1981) Principles of blast furnace ironmaking: Theory and Pratice, 1st ed. Cootha publishing house, Brisbane, Australia
Ahindra Ghosh, Chatterjee A (2008) Ironmaking and Steelmaking: Theory and practice. PHI Learning Pvt. Ltd.
Cameron I, Sukhram M, Lefebvre K, Davenport W (2019) Blast furnace ironmaking: Analysis, control, and optimization. Elsevier.
Fernández-González D, Ruiz-Bustinza I, Mochón J, et al (2017) Iron ore sintering: quality indices. Miner Process Extr Metall Rev 38:254–264. https://doi.org/10.1080/08827508.2017.1323744
Ji Z, Gan M, Fan X, et al (2017) Characteristics of PM2.5 from iron ore sintering process: influences of raw materials and controlling methods. J Clean Prod 148:12–22. https://doi.org/10.1016/j.jclepro.2017.01.103
Nakano M, Hosotani Y, Kasai E (2005) Observation of behavior of dioxins and some relating elements in iron ore sintering bed by quenching pot test. ISIJ Int 45:609–617. https://doi.org/10.2355/isijinternational.45.609
Tang H, Zhao L, Sun W, et al (2016) Surface characteristics and wettability enhancement of respirable sintering dust by nonionic surfactant. Colloids Surf A Physicochem Eng Asp 509:323–333. https://doi.org/10.1016/j.colsurfa.2016.09.041
Lanzerstorfer C (2019) Potential of industrial de-dusting residues as a source of potassium for fertilizer production ? A mini review. Resour Conserv Recycl 143:68–76. https://doi.org/10.1016/j.resconrec.2018.12.013
Long H, Chen K, Xu C, et al (2021) Efficient recycling of silver and copper from sintering dust by chlorination roasting process. Arab J Sci Eng 46:6663–6672. https://doi.org/10.1007/s13369-020-05291-y
Yu Y, Wang Z, Wei H, et al (2019) Separation and recovery of potassium chloride from sinter dust of a steel plant. Ironmak Steelmak 46:193–198. https://doi.org/10.1080/03019233.2017.1364037
Binnemans K, Jones PT, Manjón Fernández Á, Masaguer Torres V (2020) Hydrometallurgical processes for the recovery of metals from steel industry by-products: A critical review. Springer International Publishing
Bajamundi CJE, Vainikka P, Hedman M, et al (2014) Towards controlling PCDD/F production in a multi-fuel fired BFB boiler using two sulfur addition strategies. Part I: experimental campaign and results. Fuel 134:677–687. https://doi.org/10.1016/j.fuel.2014.05.034
Stjernberg J, Ion JC, Antti ML, et al (2012) Extended studies of degradation mechanisms in the refractory lining of a rotary kiln for iron ore pellet production. J Eur Ceram Soc 32:1519–1528. https://doi.org/10.1016/j.jeurceramsoc.2012.01.012
Lipin VA, Kazakov VG (2016) Ways to improve of aluminum content raw material treatment by sintering method. In: Light Metals 2016. Springer, pp19–22.
Fan X, Wang Y, Gan M, et al (2019) Thermodynamic analysis and reaction behaviors of alkali metal elements during iron ore sintering. J Iron Steel Res Int 26:558–566. https://doi.org/10.1007/S42243-018-0077-4/FIGURES/16
Das B, Prakash S, Reddy PSR, Misra VN (2007) An overview of utilization of slag and sludge from steel industries. Resour Conserv Recycl 50:40–57. https://doi.org/10.1016/j.resconrec.2006.05.008
Long H, Li H, Ma P, et al (2021) Effectiveness of thermal treatment on Pb recovery and Cl removal from sintering dust. J Hazard Mater 403:123595. https://doi.org/10.1016/j.jhazmat.2020.123595
Wang Y, Lv W, Fan X, Gan M (2021) Thermodynamic simulation and transformation behaviours of lead during iron ore sintering. ISIJ Int 61:2731–2736. https://doi.org/10.2355/ISIJINTERNATIONAL.ISIJINT-2021-227
Acknowledgements
The research was financially supported by the National Natural Science Foundation of China (51974371 and U1660206), the National Key R&D Program of China (No. 2018YFC1900605) and the commercialization of scientific and research findings program of Hunan province (No. 2020GK4055).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
The contributing editor for this article was Nikhil Dhawan.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Y., Gan, M., Fan, X. et al. Enhanced Removal of Potassium, Sodium, and Lead During the Iron Ore Sintering Process. J. Sustain. Metall. (2024). https://doi.org/10.1007/s40831-024-00788-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40831-024-00788-4