Abstract
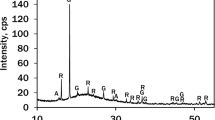
The value addition potential of ilmenite was examined using several characterization techniques. Raw ilmenite is composed of 93.65 wt.% of FeOTiO2, 3.55 wt.% of SiO2, 1.22 wt.% of Al2O3, and the remainder being other minor oxides. Ilmenite and powdered carbonized coconut shells were mixed in the weight ratio of 4:1. A separate fraction of the same mixture was added with powdered seashells in the weight ratio of 4:1:0.5. Six fractions of each mixture were distinctly milled from 1 to 6 h in a planetary ball mill. XRD spectra (broadened and diminished reflections of ilmenite) and FTIR observations (Ti―O―C bonding) of milled samples indicate the possible incorporation of carbon into the ilmenite structure. Any clues of an occurrence of new rutile peaks were not observed in XRD spectra of milled samples. Consequently, the carbothermic reduction has not been initiated during mechanical activation. In this case, samples powdered for 6 h in a mill were isothermally annealed for 2 h under normal airflow at temperatures of 800 °C, 1000 °C, and 1200 °C, respectively. According to the X-ray diffractograms, the annealing temperatures of 1000 °C and 1200 °C exhibited almost similar trends with rutile (R), pseudobrookite (PB) and elemental iron (F), and a very few ilmenite (I) peaks. Consequently, 6 h of milling and 1000 °C annealing were concluded as the optimum conditions for the carbothermic reduction. Moreover, this study indicated seashells as a potential rate raiser for the carbothermic reduction of ilmenite at 800 °C. Therefore, this process is applicable to upgrade ilmenite into a mixture of synthetic rutile and elemental iron.









Similar content being viewed by others
References
Adipuri A, Li Y, Zhang G, Ostrovski O (2011) Chlorination of reduced ilmenite concentrates and synthetic rutile. Int J Miner Process 100:166–171. https://doi.org/10.1016/j.minpro.2011.07.005
Mackey TS (1974) Acid leaching of ilmenite into synthetic rutile. Ind Eng Chem Prod Res Dev 13:9–18. https://doi.org/10.1021/i360049a003
Zhang W, Zhu Z, Cheng CY (2011) A literature review of titanium metallurgical processes. Hydrometallurgy 108:177–188. https://doi.org/10.1016/j.hydromet.2011.04.005
Mackey TS (1994) Upgrading ilmenite in to high-grade synthetic rutile. JOM 46:59–64. https://doi.org/10.1007/BF03220676
Dooley GJ (1975) Titanium production: ilmenite vs. rutile. JOM 27:8–16. https://doi.org/10.1007/BF03355886
Wang YM, Yuan ZF, Guo ZC, Tan QQ, Li ZY, Jiang WZ (2008) Reduction mechanism of natural ilmenite with graphite. Trans Nonferrous Met Soc China 18:962–968. https://doi.org/10.1016/S1003-6326(08)60166-1
Wang Y, Yuan Z (2006) Reductive kinetics of the reaction between a natural ilmenite and carbon. Int J Miner Process 81:133–140. https://doi.org/10.1016/j.minpro.2006.07.010
Perks C, Mudd G (2019) Titanium, zirconium resources and production: a state of the art literature review. Ore Geol Rev 107:629–646. https://doi.org/10.1016/j.oregeorev.2019.02.025
Chen Y, Hwang T, Marsh M, Williams JS (1997) Mechanically activated carbothermic reduction of ilmenite. Metall Mater Trans A 28:1115–1121. https://doi.org/10.1007/s11661-997-0277-1
Mahmoud MHH, Afifi AA, Ibarhim IA (2004) Reductive leaching of ilmenite ore in hydrochloric acid for preparation of synthetic rutile. Hydrometallurgy 73:99–109. https://doi.org/10.1016/j.hydromet.2003.08.001
Lee CT, Sohn HY (1989) Recovery of synthetic rutile and iron oxide from ilmenite ore by sulfation with ammonium sulphate. Ind Eng Chem Res 28:1802–1808. https://doi.org/10.1021/ie00096a011
Nurdin M, Maulidiyah WAH, Abdillah N, Wibowo D (2016) Development of extraction method and characterization of TiO2 mineral from ilmenite. Int J ChemTech Res 9:483–491
Neurgaonkar VG, Gokarn AN, Joseph K (1986) Beneficiation of ilmenite to rutile by selective chlorination in a fluidised bed. J Chem Technol Biotechnol 36:27–30. https://doi.org/10.1002/jctb.280360105
Rhee KI, Sohn HY (1990) The selective carbochlorination of iron from titanlferous magnetite ore in a fluidized bed. Metall Mater. Trans B 21:341–347. https://doi.org/10.1007/BF02664202
Shiah CD (1978) U.S. Patent No. 4,085,190. Washington, DC: U.S. Patent and Trademark Office
Wright JB, Elger GW, Tress JE, Bell HE (1985) Chlorination-grade feedstock from domestic ilmenite. MME 2:198–202. https://doi.org/10.1007/BF03402619
Robinson M, Clamp F, Mobbs DB, Pearse RV (1997) Advances in extractive metallurgy (Ed. M.J. Jones), The institution of Mining and Metallurgy, London, 89–96.
Murty CVGK, Upadhyay R, Asokan S (2007) Electro smelting of ilmenite for production of TiO2 slag-potential of India as a global player. Proc Infacon XI, India, pp 18–21
Chen Y, Hwang T, Williams JS (1996) Ball milling induced low-temperature carbothermic reduction of ilmenite. Mater Lett 28:55–58. https://doi.org/10.1016/0167-577X(96)00026-2
Welham NJ (1996) A parametric study of the mechanically activated carbothermic reduction of ilmenite. Miner Eng 9:1189–1200. https://doi.org/10.1016/S0892-6875(96)00115-X
Setoudeh N, Saidi A, Welham NJ (2005) Effect of elemental iron on the carbothermic reduction of the anatase and rutile forms of titanium dioxide. J Alloys Compd 395:141–148. https://doi.org/10.1016/j.jallcom.2004.10.058
Urakaev FK (2011) Influence of the condition of mechanical activation of the mixture of ilmenite and carbon on the pigment preparation. Int J Miner Process 101:37–41. https://doi.org/10.1016/j.minpro.2011.07.006
Chen Y, Williams JS, Ninham B (1997) Mechanochemical reaction of ilmenite with different additives. Colloids Surf A: Physicochem Eng Asp 129–130:91–66. https://doi.org/10.1016/S0927-7757(97)00027-7
Mourao MB, Nascimento RC, Takano C (2006) Kinetic compensation effect on the carbothermic reduction of iron oxides. Can Metall Q 45:161–166. https://doi.org/10.1179/cmq.2006.45.2.161
Lv W, Lv X, Xiang J, Zhang Y, Li S, Bai C, Song B, Han K (2017) A novel process to prepare high-titanium slug by carbothermic reduction of pre-oxidized ilmenite concentrate with the addition of Na2SO4. Int J Miner Process 167:68–78. https://doi.org/10.1016/j.minpro.2017.08.004
Taylor (1976) Process for ilmenite ore reduction. United States Patent 3:966,455
Barnes C, Pickles CA (1988) A thermogravimetric study of the catalyticeffect of alkali carbonates on the reduction of ilmenite. High Temp Technol 6:195–201. https://doi.org/10.1080/02619180.1988.11753400
Mohammad WASBW, Othman NH, Ibrahim MHW, Rahim MA, Shahidan S, Rahman RA (2017) A review on seashells ash as partial cement replacement. In IOP Conference Series: Mater Sci Eng 271:012059. https://doi.org/10.1088/1757-899X/271/1/012059
Tayeh BA, Hasaniyah MW, Zeyad AM, Yusuf MO (2019) Properties of concrete containing recycled seashells as cement partial replacement: a review. J Clean Prod 237:117723. https://doi.org/10.1016/j.jclepro.2019.117723
De Alvarenga RAF, Galindro BM, de Fátima HC, Soares SR (2012) The recycling of oyster shells: An environmental analysis using life cycle assessment. J Environ Manage 106:102–109. https://doi.org/10.1016/j.jenvman.2012.04.017
Geological Survey of Ceylon (1970) Beach mineral sands and silicon sands of Ceylon-pamphlet. Geological Survey and Mines Bureau. Colombo, Sri Lanka
Herath JW (1980) Mineral resources of Sri Lanka, second revised ed., Economic Bulletin No. 2, Geological Survey Department. Colombo.
Wickremeratne WS (1986) Preliminary studies on the offshore occurrences of monazite-bearing heavy-mineral placers, southwestern Sri Lanka. Mar Geol 72:1–9. https://doi.org/10.1016/0025-3227(86)90095-2
Sri Lanka Minerals Year Book (2014) Geological Survey and Mines Bureau. Colombo, Sri Lanka. isbn:978-955-9323-75-4
Elsner H (2010) Heavy minerals of economic importance. Germany: Bundesanstalt für Geowissenschaften und Rohstoffe (BGR). Federal Institute for Geosciences and Natural Resources.
Amalan K, Ratnayake AS, Ratnayake NP, Weththasinghe SM, Dushyantha N, Lakmali N, Premasiri R (2018) Influence of nearshore sediment dynamics on the distribution of heavy mineral placer deposits in Sri Lanka. Environ Earth Sci 77:737. https://doi.org/10.1007/s12665-018-7914-4
Shahien MG, Khedr MMH, Maurice AE, Farghali AA, Ali RAM (2015) Synthesis of high purity rutile nanoparticles from medium-grade Egyptian natural ilmenite. Beni-Suef University, J Basic Appl 4:207–213. https://doi.org/10.1016/j.bjbas.2015.05.013
Gammage RB, Glasson DR (1979) Wear of mill components during ball milling of calcium carbonate. J Colloid Interface Sci 71:522–525. https://doi.org/10.1016/0021-9797(79)90326-6
Kaczmarek WA, Calka A, Ninham BW (1994) Evaluation of Fe contamination in ball milling of nonmagnetic materials by VSM. Physica Status Solidi A 141:123–126
Yadav TP, Yadav RM, Singh DP (2012) Mechanical milling: a top-down approach for the synthesis of nanomaterials and nanocomposites. Nanosci Nanotechnol 2:22–48
Dworkin JP, Adelman LA, Ajluni T, Andronikov AV, Aponte JC, Bartels AE, Burton AS (2018) OSIRIS-REx contamination control strategy and implementation. Space Sci Rev 214:19. https://doi.org/10.1007/s11214017-0439-4
Li C, Liang B, Guo LH, Wu ZB (2006) Effect of mechanical activation on the dissolution of Panzhihua ilmenite. Miner Eng 19:1430–1438. https://doi.org/10.1016/j.mineng.2006.02.005
Tromans D, Meech JA (2001) Enhanced dissolution of minerals: stored energy, amorphism and mechanical activation. Miner Eng 14:1359–1377. https://doi.org/10.1016/S0892-6875(01)00151-0
Tao T, Chen QY, Hu HP, Yin ZL, Chen Y (2012) TiO2 nanoparticles prepared by hydrochloric acid leaching of mechanically activated and carbothermic reduced ilmenite. Trans Nonferrous Met Soc China 22:1232–1238. https://doi.org/10.1016/S1003-6326(11)61310-1
Welham NJ, Williams JS (1999) Carbothermic reduction of ilmenite (FeTiO3) and rutile (TiO2). Metall Mater Trans B 30:1075–1081. https://doi.org/10.1007/s11663-999-0113-7
Chen G, Song Z, Chen J, Peng J, Srinivasakannan C (2013) Evaluation of the reducing product of carbonthermal reduction of ilmenite ores. J Alloys Compd 577:610–614. https://doi.org/10.1016/j.jallcom.2013.06.038
Harizanov O, Harizanov A, Ivanava T (2004) Formation and characterisation of sol gel barium titanate. Mat Sci Eng B 106:191–195. https://doi.org/10.1016/j.mseb.2003.09.014
Ashiri R (2013) Detailed FT-IR spectroscopy characterisation and thermal analysis of synthesis of barium titanate nanoscale particles through a newly developed process. Vib Spectrosc 66:24–29. https://doi.org/10.1016/j.vlbspec.2013.02.001
Ghamsari MS, Bahramian A (2008) High transparent sol-gel derived nanostructured TiO. Mater Lett 62:361–364. https://doi.org/10.1016/j.matlet.2007.05.053
Al-Amin M, Dey SC, Rashid TU, Ashaduzzaman M, Shamsuddin SM (2016) Solar assisted photocatalytic degradation of reactive azo dyes in presence of anatase titanium dioxide. Int J Latest Res Eng Technol 2:14–21
Ahangaran F, Hassanzadeh A, Nouri S (2013) Surface modification of Fe2O4@SiO2 microsphere by silane coupling agent. Int Nano Lett 3:23. https://doi.org/10.1186/2228-5326-3-23
Razavi R, Hosseini SMA, Ranjbar M (2014) Production of nanosized synthetic rutile from ilmenite concentrate by sonochemical HCl and H2SO4 leaching. Iran J Chem Chem Eng 33:29–36
Siddick SZ, Lai CW, Juan JC (2018) An investigation of the dye-sensitized solar cell performance using graphene-titania (TrGO) photoanode with conventional dye and natural green chlorophyll dye. Mater Sci Semicond Process 74:267–276. https://doi.org/10.1016/j.mssp.2017.10.046
Sasikumar C, Rao DS, Srikanth S, Ravikumar B, Mukhopadhyay NK, Mehrotra SP (2004) Effect of mechanical activation on the kinetics of sulphuric acid leaching of beach sand ilmenite from Orissa, India. Hydrometallurgy 75:189–204. https://doi.org/10.1016/j.hydromet.2004.08.001
Gupta SK, Rajakumar V, Grieveson P (1989) The influence of weathering on the reduction of ilmenite with carbon. Metall Mater Trans B 20:735–745. https://doi.org/10.1007/BF02655932
Wouterlood HJ (2007) The reduction of ilmenite with carbon. J Chem Technol Biotechnol 29:603–618. https://doi.org/10.1002/jctb.503291002
Ismail MGMU, Amarasekara J, Kumarasinghe JSN (1983) The upgrading of ilmenite from Sri Lanka by the oxidation-reduction-leach process. Int J Miner Process 10:161–164
Rao YK, Adjorlolo A, Haberman JH (1982) On the mechanism of catalysis of the Boudouard reaction by alkali-metal compounds. Carbon 20:207–212. https://doi.org/10.1016/0008-6223(82)90022-7
Spiro CL, Mckee DW, Kosky PG, Lamby EJ (1984) Observation of alkali catalyst particles during gasification of carbonaceous materials in CO2 and steam. Fuel 63:686–691. https://doi.org/10.1016/0016-2361(84)90167-4
Nishiyama Y (1991) Catalytic gasification of coals-features and possibilities. Fuel Process Technol 29:31–42. https://doi.org/10.1016/0378-3820(91)90015-5
Wang Y, Jin W, Huang T, Zhu L, Wu C, Yu G (2013) Characteristics of alkali and alkaline-earth metals for the catalytic gasification of coal char in a fixed-bed reactor. Energy Technol 1:544–550. https://doi.org/10.1002/ente.201300071
Liu Y, Guan Y, Zhang K (2017) CO2 gasification performance and alkali/alkaline earth metals catalytic mechanism of Zhundong coal char. Korean J Chem Eng 35:859–866. https://doi.org/10.1007/s11814-017-0357-x
Spiro CL, McKee DW, Kosky PG, Lamby EJ, Maylotte DH (1983) Significant parameters in the catalysed CO2 gasification of coal chars. Fuel 62:323–330. https://doi.org/10.1016/0016-2361(83)90090-X
Sekiya T, Yagisawa T, Kamiya N, Mulmi DD, Kurita S, Murakami Y, Kodaira T (2004) Defects in anatase TiO2 single crystal controlled by heat treatments. J Phys Soc Japan 73:703–710. https://doi.org/10.1143/JPSJ.73.703
Ren R, Yang Z, Shaw LL (2000) Polymorphic transformation and powder characteristics of TiO2 during high energy milling. J Mater Sci 35:6015–6026. https://doi.org/10.1023/A:1026751017284
Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile transformation. J Mater Sci 46:855–874. https://doi.org/10.1007/s10853-010-5113-0
Zhao Y, Shadman F (1991) Reduction of ilmenite with hydrogen. Ind Eng Chem Res 30:2080–2087. https://doi.org/10.1021/ie00057a005
Acknowledgements
We would like to thank Lanka Mineral Sands Limited, Pulmoddai for providing ilmenite samples and necessary information. We also wish to extend our gratitude to M.D. Nilantha, Sandun Wijerama, and Pradeep Ranathunga for assisting in geochemical laboratory work at Uva Wellassa University, Sri Lanka.
Funding
The authors would like to acknowledge the financial assistance for this study by an Accelerating Higher Education Expansion and Development (AHEAD) Development Oriented Research (DOR) grant funded by the World Bank to the corresponding author.
Author information
Authors and Affiliations
Contributions
Amila Sandaruwan Ratnayake obtained the research grant for this project, conceived of the presented idea, designed the theory of the project, verified the analytical methods, and supervised other authors. T. Dilmi U. Wijewardhana, and H.C.S. Subasinghe carried out the experiments, performed the experimental calculations, produced maps, and interpreted the results. All authors analyzed and discussed results, provided critical feedback, and contributed to the final manuscript. All authors wrote, read, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wijewardhana, T.D.U., Subasinghe, H.C.S. & Ratnayake, A.S. Value Addition to Ilmenite Using Carbonized Waste Coconut Shells: a Mechanochemical Approach Aided with Powdered Seashells as a Rate Raiser. Mining, Metallurgy & Exploration 38, 1573–1587 (2021). https://doi.org/10.1007/s42461-021-00420-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42461-021-00420-z