Abstract
Phytohormones promote growth in the vegetative phase, flowering and fruit development. A novel class of PGR comprises brassinosteroids (BRs) and polyamines (PAs). In fruit crops, fruit drop at the maturity stage is controlled by the external application of BRs and PAs. BRs such as brassinolide, 24-epibrassinolide and 28-homobrassinolide and PAs such as putrescine, spermidine and spermine play significant roles in plant growth and development. Brassinolide provides tolerance against biotic and abiotic stresses. 24-Epibrassinolide promotes blossoming, fruit retention, fruit set and fruit growth. 28-homobrassinolide promotes cell division and cell elongation. Putrescine enhances seed germination and adventitious root formation in seedlings. Moreover, spermidine provides tolerance against drought and salinity. Furthermore, spermine promotes flowering and fruiting and competes with ethylene precursors. It would be beneficial to apply plant growth regulators such as BRs and PAs to increase fruit yield and quality. This review discusses how phytohormone (BR and PA) application can improve the productivity, quality, physiological, biochemical and postharvest aspects of some tropical, subtropical and temperate fruits and focuses on research areas such as the mode of action and stage of application of BRs and PAs which enhance the yield and quality of these perishable fruit crops.
Article highlights
-
The newly emerged plant growth hormones brassinosteroids and polyamines are vital PGRs for the cultivation of horticulture commodities.
-
BRs and PAs are environmentally safe phytohormones that improve the yield and quality of fruits.
-
These materials are effectively utilized in both fruit production and postharvest management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Plant growth regulators (PGRs) are the class of naturally occurring organic substances. At a minimum concentration, it influences physiological processes [1]. The intricate and well-coordinated action of endogenous hormones strongly regulates the process of plant growth and development as well as the response to environmental stimuli [2]. Through their influence on different metabolic processes, plant growth hormones can increase plant productivity, quality and control the growth of individual plant parts whereas metabolism provides a plant's energy and structural components [3]. Plant hormones are synthesized in particular tissues and translocated to other cells to trigger specific responses [4]. Recently, chemical toxicity in crops has been mitigated by plant derived hormone application [5].
Plant growth hormones can be broadly classified into five groups: auxins, cytokinins, gibberellins, ethylene and abscisic acid. Auxin play a significant role in the early stages of plant growth and development and it is found in juveniles and seedlings. Concentration of auxin is more in leaf, shoots and tips of branches and low in roots [6]. During abiotic stress auxin interacts with salicylic acid and abscisic acid to regulate plant growth [7]. Gibberellins synthesized in plants itself promotes cell elongation [8] and influence the nitrogen metabolism as it improves the soil nitrogen redistribution in plants [6]. Cytokinin delay the senescence by reducing the chlrophyll breakdown [9]. A combination of Cytokinin (cell division stimulators and mitosis influencers) and auxin (for cell cycle initiation and DNA replication) are essential for plant cell division [6].The ethylene which is known as aging hormone responsible for crop maturation [6]. When the cytokinin level gets increased in the plant, the roots synthesize more ethylene and it affects negatively on plant as it causes loss of cellular membrane integrity [10]. Abscisic acid plays critical role in accelerating leaf senescence through transcriptional regulation [11]. Besides these hormones a new class of phytohormones called brassinosteroids (BRs) and polyamines (PAs) has been identified [12]. BRs are naturally occurring, non–genotoxic, non toxic, biologically safe and eco-friendly phytohormones that can be utilised for horticultural crops to obtain optimum growth, yield and quality of produce [13]. Under stress conditions, BRs increase proline, total phenols and soluble sugars in plants and reduce electrolyte leakage [14]. Extensive studies on BRs have shown that BRs cause a wide range of physiological and morphological reactions in plants, such as elongation of the stem, bending and epinasty of the leaves, induction of the ethylene biosynthetic pathway, proton pump activation, synthesis of proteins and nucleic acids, control of the assimilation and allocation of carbohydrates, seedling photomorphogenesis, vascular differentiation, flowering and fruit ripening [15]. 24-Epibrassinolide alleviates water stress, decreases malondialdehyde (MDA) levels and increases antioxidant enzymes [16]. BRs overcome environmental stress such as high temperature, chilling, drought, salinity and heavy metals in a wide range of plants [17]. There is prompt evidence that BRs function as an emerging growth regulator for plant growth through their interaction with other phytohormones and stress signaling molecules. These BR substances may either exist in free state or be conjugated with sugars or fatty acids [18]. In addtition BR regulates antioxidants, chlorophyll contents, photosynthetic efficiency and carbohydrate metabolism are regulated by BRs [19].
Among these PGRs, BRs has been shown to be highly advantageous [20]. BRs control a number of significant processes, including plant growth and architecture, photosynthesis, flowering and fruit set [21]. The impact of the essential bioactive brassinosteroids brassinolide (BL), 24-epibrassinolide (24-EBL) and 28-homobrassinolide (28-HBL) on plant growth and metabolism have been thoroughly investigated (Fig. 1). Through their effects on enzyme activities and gene expression, these enzymes contribute to the process of cell enlargement. They have also been shown to increase nodulation, nitrogen fixation and metabolite contents [22]. 24-Epibrassinolide (EBL) and 28-homobrassinolide (HBL) confer resistance to zinc stress and maintain the relative water content [23]. BRs transduce signals and regulate developmental processes in plants [24]. By interacting with other florigens, BRs aid in the promotion of flowering. The synergistic and additive effects of brassinosteroids and other hormones related to flowering have been evaluated [25]. The development of primary roots and growth are influenced by BRs [26].
Polyamines which are composed of two or more primary amino groups and variable hydrocarbon chains. The organic polycations are widely present in living creatures and are more concentrated in actively multiplicating cells. The number of amines in polyamines ranges from diamine putrescine to triamine spermidine and tetraamine spermine [27]. Putrescine increases biomass production [28]. Spermine and spermidine improve stomatal conductance, relative water content and chlorophyll content under water stress [29]. Polyamines undergo metabolic changes and signal enzyme activity [30]. Because of their influence on cell division, cell differentiation, flowering, growth, development and fruit ripening. In plants, PAs are necessary for various functions, including growth and development. PAs are responsible for plant tolerance to abiotic stresses such as salinity, water stress, heavy metal toxicity, low and high temperature stresses and biotic stresses [31]. Heavy metal stress is mediated by polyamines [32].
PAs have been shown to be antisenescent agents that delay the softening of a variety of fruits by maintaining the integrity of the cell [33]. Putrescine, spermidine and spermine are the three main types of PAs. Putrescine is categorized as a diamine, whereas the other two are thought to be higher polyamines. Numerous physiological and biochemical processes viz, cell division, cell elongation, flowering and fruit set are influenced by PAs [34] (Fig. 2).
Polyamines act as precursors for certain alkaloids such as pyrrolizidine, tropane and quinolizidine alkaloids and phenolamides [35]. PAs can interact with other hormones to regulate plant growth and development [36]. Fruit ripening is a primary factor influencing relationship between PAs and fruits. Both endogenous polyamine and ethylene have the same precursor, S-adenosylmethionine (SAM) during their biosynthetic pathway and there is a typical inverse relationship between these two compounds [37]. The rationale for discussing the major commercial grown fruit crops in this paper reason out its importance and fulfills the daily requirement of human intake, maintains the balanced diet and supply the rich source of vitamins and minerals.This paper summarizes the effectiveness of BR and PA in some major fruit crops as discussed below (a) Interaction effect of BR and PA with other phytohormones (b) Synthesis of brassinosteroid and polyamine in plants (c) Mechanistic insight of BR and PA (d) Role of brassinosteroids and polyamines in fruits (e) Effectiveness of BR and PA and its environmental impact and concluding that the high nutrient, mineral and antioxidant contents of fruits play vital roles in human diets. To meet the demands of human diet and other requirements, it is essential to increase the yield and productivity of fruit crops by utilizing BR and PA implication in all possible fruit crops. The aim of this review is to highlight the exogenous application of phytohormones to improve the yield and quality of commercial fruit crops; and to focus on the use of plant-derived hormones (BRs and PAs) to overcome flower and fruit drop; and enhance physiological, biochemical changes and shelf life of horticulture produce.
2 Interaction of BR, PA and phytohormones
2.1 Combined effect of brassinosteroid with other PGRs
Both BRs and auxin are involved in the development of lateral root. BRs act at the initiation site of lateral root primordia (LRP), whereas auxin synthesized at initiation and emergence of lateral root formation [38]. For an optimal root growth, a balanced concentration of BRs and auxin is recommended. BRs and auxin signaling was necessary for the gravity-induced root curvature which was mediated by endocytic PIN2 through the attenuation of differential cell elongation [39]. Exogenous treatment of IAA, GA3, or 24-EBL in white light promotes the hypocotyl elongation, while the inhibitors of GA3, IAA, and BRs of these PGRs decreased the shade-induced hypocotyl elongation [40]. High concentration of synthetic GA activates the prime BR signaling pathway to help the cell elongation [41]. BR signaling is needed for GA work to boost hypocotyl extension [42].Cytokinins (CKs) and BRs have collegial interaction to stimulate cellular division and growth in plants [43]. Interaction among CK and BRs enhances the biosynthesis of anthocyanin [44]. The ethylene production was decreased significantly in seedlings, when they were treated with a low concentration (10–100 nM) of 24-epibrassinolide (EBL). Cytokinin pathway follows the BR signaling, that ultimately antagonizes the roots directional growth by using ethylene-stimulated machinery [45]. ABA stimulates seed dormancy in maturating embryos and inhibits seed germination, whereas BRs antagonistically promote seed germination through enhancing the potential of embryo growth. BR follows similar patterns to that of ABA for the promotion of stomatal closure and inhibition of stomatal opening [46].
2.2 Combined effect of polyamine with other PGRs
The action of PAs and ABA is linked during stomatal closure due to the increased reactive oxygen species level in guard cells [47]. PAs especially Spm led to further increases in the ABA content and PAs are involved in plant tolerance and acclimation to drought by modulating the ABA biosynthesis. ABA stimulates the activities of enzymes involved in PA biosynthesis [48]. Exogenous Spd application regulate the expression of genes related to GA biosynthesis and increased the activity of GA3-oxidase and GA20-oxidase [49]. PAs play a major role in modulating ethylene production during salinity [47]. Application of exogenous PAs modulated GA and SA content in some plant species under drought conditions and increases plant tolerance to stress [50]. Kinetin and spermine alleviates the negative effects of stress by stimulating leaf area expansion, pigment production, photosynthetic activity and improve the chloroplast ultrastructure [51]. PAs probably maintain the proper ABA content in plants exposed to low-temperature stress. Higher ABA content enhanced the tolerance of chilling by inducing stomatal closure and promoting the expression of cold-induced genes [47]. The co-action of PAs and BRs lead to the enhanced expression of IAA metabolic genes and higher IAA content, probably stimulating plant growth and tolerance [52]. Combined application of BRs and PAs increased phytochelatin and photosynthetic pigment content [53].
3 Synthesis of brassinosteroid and polyamine in plants
BRs have been found in leaves, stems, roots, flowers, pollen, anthers, and seeds but the higher concentration was reported in seed, pollen and fruit. Synthesis of BR is diagrammatically represented (Fig. 3) [54].
The level of BR in the young plant tissue is 1–100 ng/g fresh weight which is more than the mature ones (0.01–0.1 ng/g fresh weight) [55]. 24-epibrassinolide (EBR) is the most commonly used BR for studying the physiological effects on plants [56]. The plant brassinosteroid receptor, BRASSINOSTEROID INSENSITIVE 1 (BRI1), undergoes constitutive cycling between the plasma membrane and the internal membranes. Dephosphorylation targets the BR-activated BRI1 for degradation, thereby inhibiting BR signaling and controlling [57].
Plants endogenously synthesize PAs [58]. Synthesis of polyamine is illustrated here (Fig. 4) [58]. PAs exert a concentration-dependent effect on cellular functions through their degradation during which H2O2 is produced. H2O2 may serve as a signalling molecule in modifying cellular processes such as growth, division, differentiation, and adaptation [59].
4 Mechanistic insight of BR and PA
At molecular level, the BR intrinsic mechanism was reported in plants that the Brassinolide signals the temperature-induced lipocalins, 11SK2 dehydrins, remorin and abscisic acid stress ripening like proteins during cold stress which reduces the fruit fluidity. Transcriptomic analysis of BR identified abiotic stress-based alteration in BR-RESPONSIVE RECEPTOR-LIKE KINASE, WARKY 33, ACID PHOSPHATASE5 and JACALIN RELATED LECTIN1–3 genes [60]. Heat shock proteins are known to be up-regulated under abiotic stress have been found to be the direct target of BZR1/BES1 [61]. LATE EMBRYOGENESIS PROTEINS provide stress resistance which is also mediated by BR signalling. BR-biosynthesis genes such as HYDROXYSTEROID DEHYDROGENASE1 and AtDWF4 results in increased abiotic stress tolerance in plants [62]. Exogenous EBL promoted the accumulation of total anthocyanins (5 mg/g) in grape berries compared to the control group (2.5 mg/L) and developed its pericarp color after 46 days treatment by up-regulating the expressions of genes VvCHI1, VvCHS3, VvF305 0H, VvDFR, and VvUFGT [63]. BR receptors detect the BR at the cell surface, triggering a series of phosphorylation events that activate the central transcription factor (TF) Brassinazole-resistant 1 (BZR1), which regulates the transcription of BR-responsive genes in the nucleus [55].
Putrescine-derived polyamines also contribute to unfolded protein response (UPR), a cellular stress response associated with the accumulation of unfolded proteins in the endoplasmic reticulum [64]. Polyamines conjugate in regulating transcription, structuring pollen cell wall, modulating cytoskeleton dynamics and affecting the levels of reactive oxygen species (ROS). PA catabolism is catalyzed by copper amine oxidases (CuAO) and flavin dependent polyamine oxidases (PAO) in plant development [65]. Exogenous PA application increased the cellular phytochelatins content [58]
5 Role of brassinosteroids and polyamines in fruits
The plant steroidal hormone, BR has a wide range of application in horticultural crops [66]. Besides, polyamines regulate plant growth and development, they are involved in tolerance to biotic and abiotic stresses [67]. The effects on fruits such as mango, banana, grape, papaya, citrus, litchi, passion fruit, apple, peach, pear, plum and strawberry fruits are briefly discussed.
5.1 Mango
Brassinosteroids exhibited concentration-specific behavior in fruits. BRs which are applied at greater concentration (80 ng g−1) to the "Dashehari" mango (Mangifera indica Linn.) fruits resulted in slowing down of metabolic process and postponed the ethylene biosynthetic process. The fruits which are treated with BRs (80 ng g−1) increased the number of days to ripen, retained their pulp color, peel thickness and improved the sensory scores [68]. Hence, it was concluded that when the BR concentration increases, the aging process decreases and vice-versa during ripening. Additionally, when the trees at the pea stage are sprayed with 1 ppm of Br, the fruit length, fruit girth, fruit weight, fruit volume, mesocarp-pulp and pulp: peel ratio increased [69]. The combined effect of 1.0 ppm 28-homobrassinolide and shoot thinning (keeping one shoot in the newly formed vegetative shoot cluster) during the post-monsoon period promoted improved growth, flowering, and fruit set in Kesar mangoes [70]. However, mango inflorescence treated with 0.01 or 0.1 µM 7,8-dihydro-8α-20-hydroxyecdysone (DHECD; a brassinosteroid mimic) and 24-epibrassinolide (a natural brassinosteroid) during the large bud swell stage decreased the percentage of malformed inflorescences, promoted the growth of inflorescences, increased the number of flowers per inflorescence and improved the pollen viability and germination [71]. Application of epibrassinolide (Epi-BL) as a postharvest dip at concentration of 45 and 60 ng g−1, accelerated the color of the fruit, delayed the onset of the climacteric peak of ethylene production and respiration rate [72] and facilitates the development of skin color in "Kensington Pride" mango fruits [73].
Putrescine (PUT) sprayed at 50 ppm to 32-year-old Alphonso mango trees significantly impacted the morpho-physiological characteristics such as increased fruit count per tree and fruit yield [74]. However, the Dashehari mango fruits treated with 1.0 mM putrescine resulted in low physiological weight loss, extended the storage time without causing any symptoms of chilling injury [75]. The uniformly matured Langra mangoes dipped in putrescine (2.0 mmol l−1) solution, air-dried under shade and kept for four weeks at 13 °C with 90–95% relative humidity (RH), results in a high palatability rating, an optimal balance of acidity, TSS, low percentage of physiological weight loss and spoiling [76]. The “ Nam Dok Mai No.4 " mangoes dipped in 2 mmol l−1 PUT resulted in greater fruit hardness, titratable acidity (TA), total antioxidant content and activities of certain enzymes such as catalase (CAT), ascorbate peroxidase (APX), superoxide dismutase (SOD), glutathione reductase (GR) and guaiacol peroxidase (GPOX) during fruit storage[77]. Similarly, mango cv. Samar Bahisht Chausa fruits were exposed to different PUT concentrations (0.0, 0.1, 1.0, and 2.0 mM) and allowed to ripen at 32 ± 2 ͦ C for 7 days, after which they were stored at 11 ± 1 ͦ C for a maximum of 28 days after which the levels of cell wall degrading enzymes such as exopolygalacturonase (exo-PG), pectin esterase (PE) and antioxidative enzymes such as SOD, peroxidase (POX), endo-polygalacturonase (endo-PG) and CAT decreased the total phenolic and antioxidant contents and decreased the SSC and SSC:TA during ripening and cold storage [78]. Two self-incompatible mango cultivars, Langra and Dashehari panicles sprayed with putrescine (10–4 M) and spermine (10–4 M) promoted fruit set in 'Langra' (when applied at full bloom) and 'Dashehari' (when applied before anthesis) [79]. Compared with putrescine or spermidine, spermine increased fruit retention in both cultivars (Fig. 5). On postharvest dipping of Kensington Pride mango fruits at concentration of 0.01, 0.5, and 1.0 mM delay the development of mean fruit softness and visual color, decreased weight loss, respiration rate and ethylene production during storage [80]. The same variety of fruits sprayed with PAs (putrescine, spermine and spermidine − 0.01, 0.1, and 1 mM) increased the pulp total carotenoids, decreased the ascorbic acid content and resulted in high fruit retention, greater fruit yield, enhanced fruit quality and low acid content of the ripe fruit was observed [81]. However, the dipping of mangoes in putrescine (0.5, 1 and 2 mM) for 30 min retained the quality attributes such as firmness, skin color, total soluble solids (TSS), titratable acidity (TA), pH, ascorbic acid, polyphenol oxidase (PPO), and polygalacturonase (PG) enzyme activity [82].
5.2 Banana
At the nursery stage, the banana (Musa spp.) cv. Berangan plants treated with BR at a concentration of 6.88 g l−1 increases the growth attributes such as the pseudostem diameter and plant height [83]. Bunches sprayed with brassinosteroid @ 2 ml l−1 improves the yield parameters such as bunch size, bunch weight, finger size, finger weight and third hand weight [84]. Combined application of sulfate of potash (SOP) at 2% and BR at 2 ppm results in increased fruit yield [85].
The uniformly matured unripe banana cv. Grand Naine dipped in 5 µM spermine reduces the physiological weight loss, fruit softening, color change, reduces the consumption of organic acids, respiration rate, improves ascorbic acid and titratable acidity [86]. The AAA group of ‘Hom Thong’ banana plants immersed in 0.5 mM PUT or SPM for 5 min improves the postharvest quality of the fruit and reduced the browning symptoms [87].
5.3 Grapes
Flame seedless grape (Vitis vinifera L.) vines sprayed with 0.5 and 1.0 mg l−1 of BR increased the cluster weight, berry softening, maintained the external color, stabilized the anthocyanins, total phenols and decreased the rate of degradation [88]. Several studies have shown that the compounds 22S-homobrassinolide and 23S-homobrassinolide sprayed on 'Alphonse Lavallée' vines enhanced the tensile strength and length of the clusters on the pedicel [89]. Double dip of seedless grape variety Tas-A-Ganesh at BR 0.4 ppm promotes the greatest bunch weight, berry weight, berry volume and number of berries per bunch [90]. BR application at 0.4 mg l−1 on Cabernet Sauvignon berry cluster increases the soluble sugars and total anthocyanins [91] (Fig. 6).
The combined application of 2.0 g of Put and 4.0 mM SA enhances the overall yield and quality of the clusters in terms of weight, length, width, diameter, firmness, volume of 100 berries, increased the percentage of marketable clusters and prolonged the postharvest life of the clusters [92]. Berries of "Shahroudi" grape cluster treated with 1 mM PUT decreases the browning, berry shattering, cracking, weight loss, and decay incidence. The berries treated with PUT (1 mM and 2 mM) reduces the quercetin 3-galactoside content and increases the total phenolic content, catechin content, total quercetin content and antioxidant activity [93]. Flame seedless grape vines treated with putrescine and spermidine at concentration of 0.5 mM effectively inhibits the pectin methyl esterase activity, decreased electrolyte leakage, preserved anthocyanin content, berry firmness, peel color and extended the shelf life [94].
5.4 Papaya
Preharvest spray of brassinosteroid (0.1%) on the papaya (Carica papaya) cultivar Red Lady reduces the disease symptoms [95]. Combined application of 300 ppm salicylic acid and 0.1% brassinosteroid improve the TSS, carotene content and a longer shelf life period upto 9 days under ambient storage. The combined application of potassium sulfate (2%), zinc (0.5%), boron (0.1%,) and brassinosteroids (2 ppm) on TNAU papaya CO8 resulted in high fruit yield [96].
5.5 Citrus
The Satsuma mandarin (Citrus unshiu) fruits treated with BR (5 mg l−1) reduces the disease incidence to 4% [97]. The fruits treated with BR at 1.5 ppm, minimize the physiological disorders (chilling injury), peel, pulp lipid peroxidation and hydrogen peroxide content and extended the storage life upto five months at 3 °C [98]. Acid lime (Citrus aurantifolia Swingle) cv. Phule Sharbati fruits sprayed at BR 15 ppm enhances the individual fruit weight and yield [99].
Lemon (Citrus limon (L.) Burm.) trees sprayed with 1 mM putrescine exhibits greater level of fruit firmness and reduces the weight loss [100]. External application of PAs accelerate the root colonization and improves the mycorrhizal growth in plants [101]. The productivity and quality of Valencia orange is enhanced by the postharvest dip of 10–3 mM PUT [102].
5.6 Litchi
The "nuomoci" pericarp structure of litchi (Litchi chinensis Sonn.) which is a loose attachment of the pulp indicates that the structure was identical to that of the leaf and easily led to cracking of the fruit [103]. To overcome this disorder, a plant derived brassinosteroid (0.5, 0.75 or 1.0 mg l−1) is sprayed on litchi trees prior to anthesis, it enhances calcium content, enzyme activity and reduces fruit cracking [104]. The maximum fruit yield and weight is noticed when sprayed at 1 mg l−1 of brassinolide [105].
5.7 Passion fruit
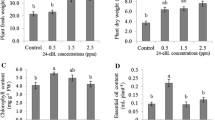
Yellow passion fruit (Passiflora edulis f. flavicarpa Deg.). vines sprayed with BR at 0.1 mg/L. The results indicates that vines sprayed at the 3rd week (BR-3) after the first flower appearance is the most promising treatment as they produced a high number of fruits per plant with increased soluble solid content and yield by 65% [106]. Vines treated with brassinosteroid did not show any significant difference in height, leaf area, fresh or dry mass due to water stress condition [107].
5.8 Apple
One-year-old grafted apple (Malus domestica Borkh.) plants of Super Chief and Red Chief varieties underwent morphological and physio-biochemical changes during water stress. Under this condition, foliar spray is given at 0.05 and 0.1 ppm concentration of BR. Trees treated with BR at 0.05 ppm exhibits protection from the harmful effect of water stress; increases the osmo-regulating substance such as total soluble sugars (TSS), free amino acids, antioxidant enzyme catalase and improves the drought tolerance [108]. Moreover, the combined application of NAA and 28-homocastasterone (28-HCSs increases the TSS, color attributes (C and h) of the skin, total antioxidant activity of the skin, flesh and peroxidase activity of galaxy apples [109].
Low chilling variety like 'Anna' apples treated with polyamines α-difluoromethylarginine (DFMA) or α-difluoromethylornithine (DFMO) promotes bud break after the required chilling time is completed [110]. The postharvest dip of ‘Golden Delicious’ and ‘McIntosh’ apples in PUT solution increases the fruit firmness and softens the fruit by cell wall rigidification [111]. The antagonistic effect of polyamines and ethylene in 1-MCP treated apple plants reported in early days but disappear in later days due to the polyamine homeostasis mechanism [112]. 0.25 mM of spermidine has a significant effect on the yield, weight, number of fruits and fruit set of red delicious apples [113].
5.9 Peach
Peach (Prunus persica (L.) Batsch) cv. Babygold 6 treated with putrescine (1 mM) improves fruit firmness, minimizes the respiration rate and ethylene emission [114]. The combined effect of ultrasonic therapy (32 kHz for 10 min) and putrescine (1 mM) in peach results in improved fruit firmness, reduced fruit acidity, respiration rate and delays the ripening process [115]. Peach fruit maturation and ripening are influenced by polyamine application [116].
5.10 Plum
The infiltration of putrescine into plum (Prunus domestica) fruits led to a reduction of mechanical damage, increased firmness, inhibits ethylene and CO2 production [117]. Plums treated with PUT (0.1, 1.0 or 2.0 mmol l−1) improves the fruit firmness, titratable acidity (TA), delayed ethylene production, respiration, decreased ascorbic acid, total carotenoid, soluble solid content (SSC) and total antioxidant levels. PUT application to plum cv. Amber Jewel and Angelino fruits delays the fruit ripening process [118].
5.11 Pear
Treatment of ‘Nanguo’ pears (Pyrus spp.) with PA reduces the browning symptoms [119]. Pear trees (Prunus persica L. Batsch cv. Stark Red Gold) treated with putrescine (5, 10, and 20 mM), spermidine (0.5, 1 and 2 mM) and aminoethoxyvinylglycine (AVG; 0.32, 0.64, and 1.28 mM) decreases the ethylene production, postpones the loss of firmness and maintains the titratable acidity [120]. Pear trees sprayed with Spd (0.05 mM) and Put (0.25 mM) results in a significant increase in fruit set [121].
5.12 Strawberry
BR positively influence the number of leaves, petiole length and crown count of Miyoshi and Enrai of day-neutral strawberry (Fragaria ananassa Duch.) fruits, decreased the total leaf area, increased the dry weight of leaves, petioles, crowns, total sugars, total number of marketable berries and total yield per plant [122]. The yield and proline content of Albion and Sweet Ann is increased with response to BR treatment [123].
The shelf life of Strawberry cv. Selva fruits is greatly extended to 12 and 14 days when dipped in 0.3, 0.5, 1 or 2 mM of putrescine for five minutes [124]. The fruits immersed in 2 mM of putrescine improves the quality traits, firmness, reduces the titratable acidity, vitamin C and antioxidants [125]. The cultivars Paros and Selva fruits treated with SPD and PUT at different concentration of 0.5, 1 and 1.5 mM results in a greater number of flowers, fruits per tree and a reduced number of runners [126].
5.13 Pomegranate
The foliar application of 28-Homobrassinolide (HBL) influence the growth of floral parts in bisexual flowers of pomegranate [127]. Spraying of 24-Epi-brassinolide (EBR) increases the efficiency of Ca and B in the aril, reduces the browning disorder, improves the pollen function, total soluble solids (TSS), total anthocyanin, phenolic and flavonoid content in ‘Rabab’ pomegranate [128, 129].
6 Effectiveness of BR and PA and its environmental impact
BR-induced increased stress toleration is strongly related to the BR-induced recovery in CO2 absorption, photoprotection antioxidant potential (enzymatic and non-enzymatic), redox homeostasis, ROS scavenging, defensive reaction, autophagy, secondary metabolism, and the ability for detoxification [130]. BR increases crop yield and show anti-stress effects on several plants at very low doses. Besides this, they are easily metabolized. In order to make them cost-effective many types of BR analogues such as BB6 and MH5, DI-31 (BB16) and DI-100 have been prepared. Exogenous application of Put resulted lower membrane injury and malondialdehyde (MDA) content, enhanced antioxidant and proline levels. Spd Effectively regulates the transcription of Calvin cycle genes in order to improve and regulate the defense response. PAs effectively remove excessive ROS, in turn reducing cell damage, thus enhancing stress tolerance [131]. Antioxidants (ascorbate and glutathione) and PAs (PUT, SPD, and SPM) has effectively alleviated cadmium stress in plants [132]. The influence of brassinosteroids and polyamines on fruits are illustrated here (Table 1).
7 Conclusion and future trends
The overall objective of this review was to provide a scientific framework for identifying how PGRs can enhance plant productivity and quality. Several studies in the scientific literature have documented the benefits of phytohormones (brassinosteroids and polyamines) on physiological and biochemical changes and the storage life of fruits. Hence, these materials can be utilized to increase the growth, yield and quality of produce because they are a natural, nontoxic, nongenotoxic, biosafe and eco-friendly phytohormones. In particular, epibrassinolide and spermine promote fruit set and retention and improves the productivity of fruit crops. Furthermore, additional studies are required as BR also plays a major role in plant protection during various stress condition. Therefore, it can easily and efficiently replace different pesticides and fungicides which will safeguard the environment. The optimal time and dosage of application of BR and PA for each of the horticultural crops have to be standardized.
Data availability
No datasets were generated or analysed during the current study.
Abbreviations
- PGRs:
-
Plant growth regulators
- BRs:
-
Brassinosteroids
- PAs:
-
Polyamines
- MDA:
-
Malondialdehyde
- BL:
-
Brassinolide
- 24-EBL:
-
24-Epibrassinolide
- 28-HBL:
-
28-Homobrassinolide
- DHECD:
-
7,8-Dihydro-8α-20-hydroxyecdysone
- Epi-BL:
-
Epibrassinolide
- SOP:
-
Sulfate of potash
- TSS:
-
Total soluble solids
- 28-HCS:
-
28-Homocastasterone
- PUT:
-
Putrescine
- SPM:
-
Spermine
- SPD:
-
Spermidine
- RH:
-
Relative humidity
- TA:
-
Titratable Acidity
- CAT:
-
Catalase
- APX:
-
Ascorbate peroxidase
- SOD:
-
Superoxide dismutase
- GR:
-
Glutathione reductase
- GPOX:
-
Guaiacol peroxidase
- exo-PG:
-
Exopolygalacturonase
- PE:
-
Pectin esterase
- EGase:
-
Endo1, 4-d-glucanase
- POX:
-
Peroxidase
- endo-PG:
-
Endo-polygalacturonase
- SSC:
-
Soluble solids content
- FBD:
-
Flower bud differentiation
- GP:
-
Grown panicles
- FB:
-
Full bloom
- IFS:
-
Initial fruit set
- PPO:
-
Polyphenol oxidase
- PG:
-
Polygalacturonase
- SA:
-
Salicylic acid
- DFMA:
-
α-Difluoromethylarginine
- DFMO:
-
α-Difluoromethylornithine
- 1-MCP:
-
1- Methylocyclopropane
- AVG:
-
Aminoethoxyvinylglycine
- SAMDC:
-
SAM decarboxylase
- MGBG:
-
Methylglyoxyl-bis guanylhydrazone
- dSAM:
-
Decarboxylated S-adenosylmethionine
- DFMO:
-
Difluoromethylornithine
- ODC:
-
Ornithine decarboxylase
- ADC:
-
Arginine decarboxylase
- DFMA:
-
Difluoromethylarginine
- AVG:
-
Aminoethoxyvinylglycine
- ACC:
-
1-Aminocyclopropane-1-carboxylate
References
Meena NK et al., Effects of brassinosteroids application on quality and storage of fruits. Trends & prospects in post harvest management of horticultural crops. Today & Tomorrow’s Printers and Publishers, Delhi, 2018: p. 65–79.
Davies PJ. Plant hormones and their role in plant growth and development. Berlin: Springer Science Business Media; 2012.
Kagale S, et al. Brassinosteroid confers tolerance in Arabidopsis thaliana and Brassica napus to a range of abiotic stresses. Planta. 2007;225:353–64.
Bhatla SC, Lal MA. Plant growth regulators: an overview. Plant Physiol Dev Metabol. 2023. https://doi.org/10.1007/978-981-99-5736-1_14.
Jan S, et al. Plant growth regulators: a sustainable approach to combat pesticide toxicity. 3 Biotech. 2020;10:1–11.
Farman S, Mushtaq A, Azeem MW. Plant growth regulators (PGRs) and their applications: a review. Int J Chem Biochem Sci. 2019;15:94–103.
Park C-M. Auxin homeostasis in plant stress adaptation response. Plant Signal Behav. 2007;2(4):306–7.
Iqbal M, Ashraf M. Gibberellic acid mediated induction of salt tolerance in wheat plants: Growth, ionic partitioning, photosynthesis, yield and hormonal homeostasis. Environ Exp Bot. 2013;86:76–85.
Schäfer M, et al. The role of cis-zeatin-type cytokinins in plant growth regulation and mediating responses to environmental interactions. J Exp Bot. 2015;66(16):4873–84.
Schaller GE. Ethylene and the regulation of plant development. BMC Biol. 2012;10:1–3.
Chen K, et al. Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol. 2020;62(1):25–54.
Rao SSR, et al. Brassinosteroids–a new class of phytohormones. Cur Sci. 2002;82:1239–45.
Canales E, et al. ‘Candidatus Liberibacter asiaticus’, causal agent of citrus Huanglongbing, is reduced by treatment with Brassinosteroids. PLoS ONE. 2016;11(1): e0146223.
Nawaz F, et al. Understanding brassinosteroid-regulated mechanisms to improve stress tolerance in plants: a critical review. Environ Sci Pollut Res. 2017;24:15959–75.
Sasse J, Physiological actions of brassinosteroids: an update. 2003. 22: p. 276–288.
Damghan I. Exogenous application of brassinosteroid alleviates drought-induced oxidative stress in Lycopersicon esculentum L. Gen Appl Plant Physiol. 2009;35(1–2):22–34.
Ahammed J, G., et al. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Cur Prot Pep Sci. 2015;16(5):462–73.
Hayat S, Ahmad A, Fariduddin Q. Brassinosteroids: a regulator of 21st century in brassinosteroids: bioactivity and crop productivity. Berlin: Springer; 2003.
Anwar A, et al. The physiological and molecular mechanism of brassinosteroid in response to stress: a review. Biol Res. 2018. https://doi.org/10.1186/s40659-018-0195-2.
Ali B. Practical applications of brassinosteroids in horticulture—some field perspectives. Sci Hortic. 2017;225:15–21.
Tang J, Han Z, Chai J. Q&A: what are brassinosteroids and how do they act in plants? BMC Biol. 2016;14:1–5.
Vardhini BV, Anjum NA. Brassinosteroids make plant life easier under abiotic stresses mainly by modulating major components of antioxidant defense system. Front Environ Sci. 2015;2:67.
Ramakrishna B, Rao SSR. Foliar application of brassinosteroids alleviates adverse effects of zinc toxicity in radish (Raphanus sativus L.) plants. Protoplasma. 2015;252:665–77.
Li J, Chory J. Brassinosteroid actions in plants. J Exp Bot. 1999;50(332):275–82.
Khripach VA, Zhabinskii V, de Groot AE. Brassinosteroids: a new class of plant hormones. Cambridge: Academic Press; 1998.
Planas-Riverola A, et al. Brassinosteroid signaling in plant development and adaptation to stress. Development. 2019;146(5):151894.
Takahashi T, Kakehi J-I. Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann Bot. 2010;105(1):1–6.
Pál M, et al. Role of polyamines in plant growth regulation of Rht wheat mutants. Plant Physiol Biochem. 2019;137:189–202.
Hassan F, Ali E, Alamer K. Exogenous application of polyamines alleviates water stress-induced oxidative stress of Rosa damascena Miller var trigintipetala Dieck. South Afr J Botany. 2018;116:96–102.
Podlešáková K, et al. Phytohormones and polyamines regulate plant stress responses by altering GABA pathway. New Biotechnol. 2019;48:53–65.
Todorova D, et al. Polyamines–possibilities for application to increase plant tolerance and adaptation capacity to stress. Genet Plant Physiol. 2015;5(2):123–44.
Spormann S, et al. Polyamines as key regulatory players in plants under metal stress—a way for an enhanced tolerance. Ann Appl Biol. 2021;178(2):209–26.
Khan AS, Singh Z, Abbasi NA. Pre-storage putrescine application suppresses ethylene biosynthesis and retards fruit softening during low temperature storage in ‘Angelino’plum. Postharvest Biol Technol. 2007;46(1):36–46.
Evans PT, Malmberg RL. Do polyamines have roles in plant development? Annu Rev Plant Biol. 1989;40(1):235–69.
Mustafavi SH, et al. Polyamines and their possible mechanisms involved in plant physiological processes and elicitation of secondary metabolites. Acta Physiol Plant. 2018;40:1–19.
Tyagi A, et al. Revisiting the role of polyamines in plant growth and abiotic stress resilience: mechanisms, crosstalk, and future perspectives. J Plant Growth Regul. 2023;42(8):5074–98.
Martinez-Romero D, et al. Effects of postharvest putrescine treatment on extending shelf life and reducing mechanical damage in apricot. J Food Sci. 2002;67(5):1706–12.
Bashri G, et al. Interplay of brassinosteroids and auxin for understanding of signaling pathway in brassinosteroids signalling: intervention with phytohormones and their relationship in plant adaptation to abiotic stresses. Singapore: Springer; 2022.
Retzer K, et al. Brassinosteroid signaling delimits root gravitropism via sorting of the Arabidopsis PIN2 auxin transporter. Nat Commun. 2019;10(1):5516.
Jiang H, et al. Gibberellins modulate shade-induced soybean hypocotyl elongation downstream of the mutual promotion of auxin and brassinosteroids. Plant Physiol Biochem. 2020;150:209–21.
Tong H, et al. Brassinosteroid regulates cell elongation by modulating gibberellin metabolism in rice. Plant Cell. 2014;26(11):4376–93.
Bai M-Y, et al. Brassinosteroid, gibberellin and phytochrome impinge on a common transcription module in Arabidopsis. Nat Cell Biol. 2012;14(8):810–7.
Yasin NA, et al. Cross talk between brassinosteroids and cytokinins in relation to plant growth and developments in brassinosteroids signalling: intervention with phytohormones and their relationship in plant adaptation to abiotic stresses. Singapore: Springer; 2022.
Hao J, Yin Y, Fei S-Z. Brassinosteroid signaling network: implications on yield and stress tolerance. Plant Cell Rep. 2013;32:1017–30.
Shahzadi I, et al. Brassinosteroid and ethylene-mediated cross talk in plant growth and development in brassinosteroids signalling: intervention with phytohormones and their relationship in plant adaptation to abiotic stresses. Singapore: Springer; 2022.
Mubeen S, et al. Brassinosteroids cross talk with ABA under stress condition, in brassinosteroids signalling: intervention with phytohormones and their relationship in plant adaptation to abiotic stresses. Singapore: Springer; 2022.
Napieraj N, Janicka M, Reda M. Interactions of polyamines and phytohormones in plant response to abiotic stress. Plants. 2023;12(5):1159.
Liu Y, et al. Effect of polyamines on the grain filling of wheat under drought stress. Plant Physiol Biochem. 2016;100:113–29.
Wang Y, et al. Gibberellin mediates spermidine-induced salt tolerance and the expression of GT-3b in cucumber. Plant Physiol Biochem. 2020;152:147–56.
Krishnan S, Merewitz EB. Polyamine application effects on gibberellic acid content in creeping bentgrass during drought stress. J Am Soc Hortic Sci. 2017;142(2):135–42.
Aldesuquy H, Baka Z, Mickky B. Kinetin and spermine mediated induction of salt tolerance in wheat plants: Leaf area, photosynthesis and chloroplast ultrastructure of flag leaf at ear emergence. Egyp J Basic Appl Sci. 2014;1(2):77–87.
Choudhary SP, et al. Chromium stress mitigation by polyamine-brassinosteroid application involves phytohormonal and physiological strategies in Raphanus sativus L. PLoS ONE. 2012;7(3): e33210.
Choudhary SP, et al. Interaction of brassinosteroids and polyamines enhances copper stress tolerance in Raphanus sativus. J Exp Bot. 2012;63(15):5659–75.
Baghel M, et al. Pleiotropic influences of brassinosteroids on fruit crops: a review. Plant Growth Regul. 2019;87:375–88.
Manghwar H, et al. Brassinosteroids (BRs) role in plant development and coping with different stresses. Int J Mol Sci. 2022;23(3):1012.
Fedina E, et al. Brassinosteroid-induced changes of lipid composition in leaves of Pisum sativum L. during senescence. Steroids. 2017;117:25–8.
Wu G, et al. Methylation of a phosphatase specifies dephosphorylation and degradation of activated brassinosteroid receptors. Sci Sign. 2011;4(172):29–29.
Hasanuzzaman M, et al. Polyamine action under metal/metalloid stress: regulation of biosynthesis, metabolism, and molecular interactions. Int J Mol Sci. 2019;20(13):3215.
Benkő P, Gémes K, Fehér A. Polyamine oxidase-generated reactive oxygen species in plant development and adaptation: the polyamine oxidase—NADPH oxidase nexus. Antioxidants. 2022;11(12):2488.
Divi UK, Rahman T, Krishna P. Gene expression and functional analyses in brassinosteroid-mediated stress tolerance. Plant Biotechnol J. 2016;14(1):419–32.
Shigeta T, et al. Molecular evidence of the involvement of heat shock protein 90 in brassinosteroid signaling in Arabidopsis T87 cultured cells. Plant Cell Rep. 2014;33:499–510.
Hafeez MB, et al. Brassinosteroids: molecular and physiological responses in plant growth and abiotic stresses. Plant Stress. 2021;2: 100029.
Zhou Y, et al. Exogenous 24-epibrassinolide interacts with light to regulate anthocyanin and proanthocyanidin biosynthesis in cabernet sauvignon (Vitis vinifera L.). Molecules. 2018;23(1):93.
Aloisi I, et al. Polyamines in pollen: from microsporogenesis to fertilization. Front Plant Sci. 2016;7:155.
Masson PH, Takahashi T, Angelini R. Molecular mechanisms underlying polyamine functions in plants. Front Media SA. 2017. https://doi.org/10.3389/fpls.2017.00014.
Khripach V, Zhabinskii V, de Groot A. Twenty years of brassinosteroids: steroidal plant hormones warrant better crops for the XXI century. Ann Bot. 2000;86(3):441–7.
Takahashi T. Plant polyamines. Plants. 2020. https://doi.org/10.3390/plants9040511.
Meena NK, et al. Exogenous application of brassinolide affects ripening, phenotypic traits, jelly seed and sensory properties of ‘Dashehari’mango at ambient storage. Ann Plant Soil Res. 2023;25(2):205–10.
Patel MK, Panda C, Senapati S. Influence of new generation PGRs on physical parameter of mango (Mangifera indica L.) cv. Dashehari. 2021;10(10):299–302.
Ramani M, et al. Effect of 28-homobrassinolode and shoot thinning on vegetative growth, flowering and yield of mango cv. Kesar. 2014;5(17):7097–9.
Tepkaew, T., et al., Exogenous brassinosteroids regulate mango fruit set through inflorescence development and pollen fertility. 2022.
Zaharah SS, et al. Role of brassinosteroids, ethylene, abscisic acid, and indole-3-acetic acid in mango fruit ripening. J Plant Growth Reg. 2012;31:363–72.
Zaharah, S. and Z. Singh. Role of brassinosteroids in mango fruit ripening. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 934. 2010.
Burondkar, M., B. Jadhav, and M. Chetti. Post-flowering morpho-physiological behavior of alphonso mango as influenced by plant growth regulators, polyamine and nutrients under rainfed conditions. In VIII International Mango Symposium 820. 2006.
Bhat A, et al. Effect of polyamines on shelf life and chilling injury of mango cv. Dashehari. 2014;9:1097–100.
Jawandha S, et al. Effect of post-harvest treatments of putrescine on storage of mango cv. Langra. Afr J Agricu Res. 2012;7(48):6432–6.
Wannabussapawich B, Seraypheap K. Effects of putrescine treatment on the quality attributes and antioxidant activities of ‘Nam Dok Mai No. 4’mango fruit during storage. Sci Horticu. 2018;233:22–8.
Razzaq K, et al. Role of putrescine in regulating fruit softening and antioxidative enzyme systems in ‘Samar Bahisht Chaunsa’mango. Postharvest Biol Technol. 2014;96:23–32.
Singh Z, Singh L. Increased fruit set and retention in mango with exogenous application of polyamines. J Horticu Sci. 1995;70(2):271–7.
Malik A, Zora S. Pre-storage application of polyamines improves shelf-life and fruit quality of mango. J Hortic Sci Biotechnol. 2005;80(3):363–9.
Malik AU, Singh Z. Improved fruit retention, yield and fruit quality in mango with exogenous application of polyamines. Sci Hortic. 2006;110(2):167–74.
Hosseini M, Zahedi S, Fakhar Z. Pre-storage putrescine treatment maintains quality and prolongs postharvest life of Musa acuminata L. Adv Horticu Sci. 2016;30(3):159–64.
Zakaria MAT, et al. Morphological and physiological changes of banana (Musa acuminata cv. Berangan) to brassinolide at nursery stage. J Trop Plant Physiol. 2018;10(1):36–45.
Rajan R, et al. Effect of post shooting bunch spray of chemicals on bunch characters and yield of banana (Musa paradisiaca L.) cv. Grand Naine. Int J Curr Microbiol App Sci. 2017;6(8):2471–5.
Damodhar V, Waghmare G, Nainwad R. Different application methods of nutrients and plant growth regulators enhances yield and yield parameters of banana cv. grand Naine. J Pharmacognosy Phytochem. 2019;8(5):1559–62.
Archana T, Suresh G. Putrescine and spermine affects the postharvest storage potential of banana Cv. grand naine. Int J Curr Microbiol Appl Sci. 2019;8:3127–37.
Nilprapruck P, Meetum P, Chanthasa C. Role of exogenous putrescine and spermine applications for improving banana fruit quality of banana cv hom thong at low temperature storage. Sci Eng Health Stud. 2017. https://doi.org/10.14456/sustj.2017.11.
Champa WH, et al. Brassinosteroids improve quality of table grapes (Vitis vinifera L.) cv. flame seedless. Trop Agricu Res. 2015. https://doi.org/10.4038/tar.v26i2.8099.
IŞÇI, B. and Z.J.V. Gökbayrak, Influence of brassinosteroids on fruit yield and quality of table grape'Alphonse Lavallée'. 2015. 54: p. 17–19.
Bhat Z, et al. New generation growth regulators—brassinosteroids and CPPU improve bunch and berry characteristics in ‘Tas-A-Ganesh’grape. Int J Fruit Sci. 2011;11(4):309–15.
Luan L-Y, et al. Brassinosteroids regulate anthocyanin biosynthesis in the ripening of grape berries. South African J Enol Viticu. 2013;34(2):196–203.
Bassiony S, et al. Effect of foliar application of putrescine and salicylic acid on yield, fruit quality and storability of. J Plant Prod. 2018;9(12):1203–14.
Shiri MA, et al. Effect of postharvest putrescine application and chitosan coating on maintaining quality of table grape cv.“Shahroudi” during long-term storage. J Food Proc Preser. 2013;37(5):999–1007.
Champa WH, et al. Postharvest treatment of polyamines maintains quality and extends shelf-life of table grapes (Vitis vinifera L.) cv. Flame Seedless. Postharvest Biol Technol. 2014;91:57–63.
Kavya K, et al. Role of pre-harvest application of elicitors and bio-formulations on physical, physiological quality and disease index of papaya (Carica papaya L.) var. red lady fruits during storage. J Exp Agricu Int. 2022;46(7):686–94.
Manju V, Kumar S. A dynamic response of potassium and micro nutrients combined with brassinosteroids-a steroidal plant hormone, on accumulation of sugars in papaya cv TNAU Papaya CO-8. Int J Agri Sci Res. 2015;5(6):277–82.
Zhu F, et al. Effects of exogenous 24-epibrassinolide treatment on postharvest quality and resistance of Satsuma mandarin (Citrus unshiu). Postharvest Biol Technol. 2015;100:8–15.
Ghorbani B, Pakkish Z. Brassinosteroid enhances cold stress tolerance of Washington navel orange (Citrus sinensis L.) fruit by regulating antioxidant enzymes during storage. Agricu Consp Sci. 2014;79(2):109–14.
Yamini, A., et al., Effect of Foliar Application of Growth Regulators and Nutrients on Fruit Retention and Yield of acid Lime (Citrus aurantifolia Swingle).
Valero D, et al. Influence of postharvest treatment with putrescine and calcium on endogenous polyamines, firmness, and abscisic acid in lemon (Citrus lemon L. Burm cv. Verna). J Agricu Food Chem. 1998;46(6):2102–9.
Wu Q, et al. Root morphological modification of mycorrhizal citrus (Citrus tangerine) seedlings after application with exogenous polyamines. J Animal Plant Sci. 2011;21(1):20–5.
Abo-El-Ez A, et al. Effect of foliar application with multi-micronutrients and polyamines on productivity and Storeability of Valencia oranges. J Plant Prod. 2018;9(10):785–97.
Lin LJSES. Effect of mineral nutrient on fruit cracking rate of Litchi Chinensis Sonn. Soil Environ Sci. 2001;10:55–6.
Peng J, Tang X, Feng H. Effects of brassinolide on the physiological properties of litchi pericarp (Litchi chinensis cv. nuomoci). Sci horticul. 2004;101(4):407–16.
Ghosh T, et al. Role of brassinolide in fruit growth, development, quality and cracking of litchi cv. bombai grown in new alluvial zone of West Bengal. Int J Bio-Res Stress Manage. 2022;13(5):507–12.
de Menezes G, et al. Brassinosteroid analogue effects on the yield of yellow passion fruit plants (Passiflora edulis f flavicarpa). Sci Horticult. 2006;110(3):235–40.
Jiménez-Bohórquez EF, Díaz-Arias MA, Balaguera-López HE. Exogenous brassinosteroids application in purple passion fruit plants grafted onto a sweet calabash passion fruit rootstock and under water stress. Revista Colombiana de Ciencias Hortícolas. 2024;18(1):e16514–e16514.
Kumari S, Thakur A. Morphological and physio-biochemical changes in response to foliar application of Brassinosteroid and water stress in apple plants under pot culture study. Int J Bio-Res Stress Manage. 2019;10(1):39–45.
Ramos ÂP, et al. Effects of an auxin and a brassinosteroid on physical, chemical and biochemical attributes of ‘Galaxy’apples. Ciência Rural. 2019. https://doi.org/10.1590/0103-8478cr20180311.
Wang SY, Faust M. Changes in Polyamine content during dormancy in flower buds ofAnna’Apple. J Am Soc Hortic Sci. 1994;119(1):70–3.
Kramer GF, Wang CY, Conway WS. Inhibition of softening by polyamine application inGolden Delicious’ andMcIntosh’apples. J Am Soc Hortic Sci. 1991;116(5):813–7.
Pang XM, et al. Interrelationship between polyamine and ethylene in 1-methylcyclopropene treated apple fruits after harvest. Physiol Plant. 2006;128(2):351–9.
Sayyad-Amin P, Davarynejad G, Abedy B. The application of polyamines and self-incompatibility control substance on yield indices and qualitative traits of apple cv ‘Red Delicious.’ Acta Scientiarum Biological Sciences. 2018;40:1–8.
Martínez-Romero D, et al. Exogenous polyamines and gibberellic acid effects on peach (Prunus persica L.) storability improvement. J Food Sci. 2000;65(2):288–94.
Bal E. Effects of exogenous polyamine and ultrasound treatment to improve peach storability. Chilean J Agricu Res. 2013;73(4):435–40.
Wang W, et al. Polyamine oxidase (PAO)–mediated polyamine catabolism plays potential roles in peach (Prunus persica L.) fruit development and ripening. Tree Gen Genomes. 2021;17:1–15.
Pérez-Vicente A, et al. Role of polyamines in extending shelf life and the reduction of mechanical damage during plum (Prunus salicina Lindl.) storage. Postharvest Biol Technol. 2002;25(1):25–32.
Khan A, Singh Z. Pre-harvest application of putrescine influences Japanese plum fruit ripening and quality. Food Sci Technol Int. 2010;16(1):53–64.
Li J, et al. Polyamine treatment ameliorates pericarp browning in cold-stored ‘Nanguo’pears by protecting mitochondrial structure and function. Postharvest Biol Technol. 2021;178: 111553.
Torrigiani P, et al. Pre-harvest polyamine and aminoethoxyvinylglycine (AVG) applications modulate fruit ripening in Stark Red Gold nectarines (Prunus persica L. Batsch). Postharvest Biol Technol. 2004;33(3):293–308.
Sayyad-Amin P, Davarynejad G, Abedi B. Effects of preharvest application of chemical compounds on pear (Pyrus communis cv.‘Shekari’) fruit traits. Erwerbs-obstbau. 2022;64(4):657–62.
Pipattanawong N, et al. Effects of brassinosteroid on vegetative and reproductive growth in two day-neutral strawberries. J Japan Soc Horticult Sci. 1996;65(3):651–4.
Balcı G, Keles H, Aras S. Comparison of different cover materials and external brassinosteroid application in strawberry growing in temperate climate conditions. Erwerbs-obstbau. 2023;65(4):1027–33.
Khosroshahi MRZ, Esna-Ashari M, Ershadi A. Effect of exogenous putrescine on post-harvest life of strawberry (Fragaria ananassa Duch.) fruit, cultivar Selva. Sci Horticult. 2007;114(1):27–32.
Mortazavi, S., et al. The effects of polyamines and UV-C irradiation on postharvest quality of strawberry fruit. in VII International Strawberry Symposium 1049. 2012.
Movahed, N., et al. Effects of polyamines on vegetative characteristics, growth, flowering and yield of strawberry ('PAROS'AND'SELVA'). In xxviii international horticultural congress on science and horticulture for people (ihc2010): International Symposium on 926. 2010.
Gokbayrak Z, and H. Engin, Influence of plant growth regulators on sex differentiation and floral characteristics of pomegranate flowers: a case of brassinosteroids. Acta Societatis Botanicorum Poloniae, 2018. 87(3).
Tadayon MS, Hosseini SM. 24-Epibrassinolie enhances the effect of calcium and boron on amelioration of aril browning disorder in pomegranate (Punica granatum cv.‘Rabab’). J Soil Sci Plant Nut. 2021;21(2):1679–88.
Rajendra B, et al. Scientific study of brassinosteroids on production of fruit crops: a review. Int J Plant Soil Sci. 2024;36(6):288–303.
Dehghanian Z, et al. Impact of abiotic stress on plant brassinosteroids. Clim Change Microbiome Susten Ecosph. 2021. https://doi.org/10.1007/978-3-030-76863-8_14.
Shao J, et al. Versatile roles of polyamines in improving abiotic stress tolerance of plants. Front Plant Sci. 2022;13:1003155.
Alzahrani Y, Rady MM. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol Environ Saf. 2019;182: 109378.
Malik A, Zora S. Biotechnology Pre-storage application of polyamines improves shelf-life and fruit quality of mango. J Horticult Sci Biotechnol. 2005;80(3):363–9.
Raeisi M, Samani RB, Honarvar M. Application of exogenous spermidine treatment for reducing of chilling on fruit quality and quantity of Valencia orange var. olinda. Int J Farm Allied Sci. 2013;2:1292–7.
Kaplan U, Gökbayrak Z. Effect of 22 (S), 23 (S)-homobrassinolide on adventitious root formation in grape rootstocks. S Afr J Enol Vitic. 2012;33(2):253–6.
Champa WH, et al. Postharvest treatment of polyamines maintains quality and extends shelf-life of table grapes (Vitis vinifera L.) cv. Flame Seedless. 2014;91:57–63.
Kumari S, Thakur S. Management. Morphol Physio-Biochem Chang Res Foliar Appl Brassinosteroid Water Stress Apple Plants Under Pot Cult Study. 2019;10(1):39–45.
Acknowledgements
The support and guidance of all the peer-reviewed manuscripts by the reviewers are very much appreciated.
Funding
No funding was received.
Author information
Authors and Affiliations
Contributions
M. Ashtalakshmi, S. Saraswathy: Writing of original draft and conceptualization. M. Ashtalakshmi, S. Saraswathy, S. Muthulakshmi: Revision of draft, inclusion of tables and figures, proofreading. M. Ashtalakshmi, S. Saraswathy, S. Muthulakshmi, K. Venkatesan, T. Anitha: Revision, formatting and Supervision. All the authors read and approved the final version of the manuscript.All authors contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Ashtalakshmi, M., Saraswathy, S., Muthulakshmi, S. et al. A review on exploring the efficiency of plant hormones on fruitfulness of perishables. Discov Appl Sci 6, 494 (2024). https://doi.org/10.1007/s42452-024-06201-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06201-9