Abstract
The increasing resistance of microbes to standard antibiotics is a major concern in the management of contagious infections and has led to an unprecedented increase in mortality rates. The identification of the microbial "superbugs" responsible for hospital-acquired pathogenic maladies has prompted a significant amount of research in recent years, with the hope of revealing potentially beneficial answers to the ensuing health challenges by leveraging on ethnomedicinal remedies. The antibacterial activity of phytophenols was demonstrated against a wide range of pathogens, including those that have developed resistance to conventional antibiotics via their aligning resistance mechanisms. Here, we have recapitulated the most important bacterial drug resistance pathways and elucidated the dynamics of the phytophenols in Thymus vulgaris, Piper nigrum, Nigella sativa, and Annona muricata in potentially hindering and disrupting the antibiotic resistance dynamics of microbial superbugs implicated in nosocomial infections. The in-vitro antibacterial activity of phytophenolic substances and their synergistic selectivity with chemotherapeutics validated an efficacy in combating antibiotic resistance mechanisms in microbial superbugs in-vitro. The mechanisms of antibiotic resistance synthesis and polyphenolic interactions with microbial cellular pathways via antioxidant properties showed promising prospects in ameliorating the public health threat posed antibiotic-resistant superbugs. The major challenge in developing new phytochemicals with public health importance for microbial "superbugs" is the replication of in-vitro explorations via in-vivo experimentation and, ultimately, human medical testing for clinical trial. However, additional research is needed to confirm the activity pathways and stability of these phytoactive compounds, as a number of these phytoactives exhibit synergistic antimicrobial effects with chemotherapeutics.
Article Highlights
-
The threat posed by antibiotic-resistant "superbugs" in healthcare settings is necessitated by high mortality owing to the public health challenges of recalcitrant microbes.
-
The synergistic effects of phytophenols from Thymus vulgaris, Piper nigrum, Nigella sativa, and Annona muricata with chemotherapeutics demonstrate efficacy in combating antibiotic resistance mechanisms in microbial superbugs in-vitro..
-
Polyphenols interact with cellular microbial pathways, particularly through their antioxidant properties, to inhibit the proliferation of antibiotic-resistant superbugs effectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Resistance to chemotherapeutic treatment or prophylaxis has been regularly observed not only in bacteria but also in fungi, viruses, protozoa, and other parasites; hence, these bacteria are referred to as “microbial superbugs” [1]. According to the WHO [2], antibiotic resistance is a major threat to world health because of the staggering number of lives it claims each year [3]. Superbug microorganisms, which have evolved to withstand antibiotics, do so through a series of physiological changes, thereby aiding in the neutralization of antibiotics in the bacterial/fungal cell wall. The drug's target site, the regulation of inflow and efflux pumps, drug-metabolizing enzymes and pathways, etc., could all be affected. To treat these MDR bacteria, therapeutic techniques that can directly target and change the pathways that contribute to their resistance need to be developed [4].
In response, the discovery of several chemical substances, also known as phytochemicals, in plants has led to the identification of better alternatives to industrially synthesized antimicrobial drugs for which superbugs have developed resistance. These findings suggest a promising future for ethno-phytophenolic compounds in the world of microbiology and pharmacology if critically explored, as they could lead to novel research in developing a new pharmaceutical approach to tackling and altering the underlying mechanisms of drug resistance targets in microbial superbugs [3, 4]. Phytochemicals, which have therapeutic potential and mediate the treatment of ailments and diseases, can be found in many different plant components, including flowers, leaves, roots, bark, stems, roots, and seeds [3, 5].
Thymus vulgaris, also known in local Nigerian dialect as Efirin-aja (Yoruba), and Nchanwu (Igbo) has been shown to be effective against a wide range of bacteria and fungi, making it a viable option for treating a number of different infectious disorders and serving a number of other uses [5]. Phytochemical screening of Piper nigrum, also known in local Nigerian dialet as Iyere (Yoruba), and Oziza (Igbo) exhibited the presence of numerous secondary metabolites in the plant that exhibit antibacterial activity over time, depending on the dose and technique of extraction utilized [6]. In addition, extracts and phytoconstituents of Annona muricata also known in local Nigerian dialet as Gishiri (Yoruba), and Ehuru or Ehiri (Igbo) and Nigella sativa also known in local Nigerian dialet as Isalube funfun (Yoruba) essential oils have been shown to possess antimicrobial activity, among many other beneficial properties [7]. The advantageous activities and variety of useful biomedical applications of A. muricata are associated with its enrichment of secondary metabolites, mainly phenolic acids, acetogenins, alkaloids, terpenoids, and flavonoids, which are inherently found in its leaves and fruits [8, 9]. Having established the ethno-medicinal potency of plant bioactive compounds, with a major focus on Piper nigrum, Annona muricata, Thymus vulgaris and Nigella sativa as substitutes for the increasing resistance of superbugs to antimicrobial pharmaceutical drugs, a paradigm shift to plant chemicals is needed to revolutionize therapeutic strategies effective at combating infectious diseases, thereby improving quality of health and increasing life expectancy. The aim of this review study is to ascertain the potential of phytophenolic derivatives as ameliorative agents against microbial superbug bacterial and fungal pathogens mainly responsible for or implicated in nosocomial infections.
Multidrug-resistant (MDR) bacteria pose a significant threat to public health due to their ability to withstand multiple antibiotics, making infections caused by them challenging to treat. The rise of MDR bacteria is a global concern, with various studies highlighting the mechanisms behind their resistance and the impact on healthcare systems. González‐Villarreal et al. [10] emphasized the molecular mechanisms driving multidrug resistance in clinically relevant enteropathogenic bacteria, underscoring the severe illnesses and economic burden they impose on healthcare. This resistance is further compounded by the overuse and misuse of antibiotics, inadequate infection control measures, and global movement of people and goods, as discussed by Daoud and Dropa, [11]. The consequences of infections with antibiotic-resistant bacteria are dire, with Cassini et al. [12] estimating nearly 5 million deaths worldwide in 2019 associated with bacterial resistance, including 1.27 million directly attributable to MDR bacteria.
The statistics surrounding MDR bacteria paint a grim picture of the impact on mortality rates, particularly in hospital settings where nosocomial infections caused by antibiotic-resistant bacteria lead to high mortality rates. Huguet et al. [13] highlights that infections due to MDR bacteria are responsible for one death every 15 min in the United States, emphasizing the urgency of addressing this issue. Alkofide et al. [14] further supports this by noting that over 70% of bacteria causing hospital-acquired infections are resistant to commonly used antimicrobial agents, indicating a critical need for effective treatment strategies against MDR bacteria.
The complexity of MDR bacteria extends beyond their resistance mechanisms to the challenges they pose in clinical settings. Donkor et al. [15] found high rates of multidrug resistance among Gram-negative bacteria isolated from bloodstream infections in Ghana, with significant resistance to third-generation cephalosporins and fluoroquinolones. This observation was also supported by the need for the regulation of over-the-counter prescription of antipseudomonal and antienterococcal quinolone drugs for the management of nosocomial infections owing to the spread of QnrS and QnrB genes by horizontal gene transfer and selective pressure in clinical settings as inferred by Bayode et al. [16] who studied the multiple antibiotic resistance (MAR) index and detection of quinolone-resistant genes in the bacterial consortium of urine specimens from clinical settings in Nigeria. Owing to the current lack of clinical knowledge and self medication, this underscores the difficulty in treating infections caused by MDR bacteria, leading to poorer patient outcomes and increased healthcare costs in Nigeria. Moreover, Yan et al. [17] highlights the evolution of sophisticated resistance mechanisms by pathogens, contributing to the rapid spread of MDR bacteria and complicating treatment strategies.
The dynamics of MDR bacteria colonization and transmission are crucial aspects to consider in combating their spread. Kantele et al. [18] suggested that international travel plays a significant role in the acquisition of multidrug-resistant Gram-negative strains, emphasizing the need for global efforts to address this issue. Additionally, the role of environmental factors in the spread of MDR bacteria is evident in the study by Kwansa-Bentum et al. [19], which explores the effects of plant extracts on multidrug-resistant bacteria, highlighting the potential of alternative antimicrobial strategies. The prevalence of multidrug-resistant bacteria presents a formidable challenge to healthcare systems worldwide, with high mortality rates associated with difficult-to-treat nosocomial infections. Understanding the molecular mechanisms of resistance, addressing the global spread of MDR bacteria, and developing effective treatment strategies are essential in combating this growing public health threat.
The selection of Thymus vulgaris, Piper nigrum, Nigella sativa, and Annona muricata for the study on combating antibiotic resistance in microbial "superbugs" is a strategic choice based on their known efficacy and potential as natural remedies against nosocomial infections. Thymus vulgaris, commonly known as thyme, has been extensively studied for its antimicrobial properties. Research by Kryvtsova et al. [20] highlights the antimicrobial and antibiofilm properties of T. vulgaris essential oil against clinical isolates of opportunistic infections, showcasing its potential in combating resistant bacteria. This underscores the rationale behind including T. vulgaris in the study, as its bioactive compounds have demonstrated effectiveness against a range of microbial pathogens.
Piper nigrum, black pepper, is another plant species chosen for its potential antimicrobial properties. While not as extensively studied as T. vulgaris, black pepper has shown promise in antimicrobial applications. Kováčová et al. [21] demonstrated the antibacterial activity of silver nanoparticles synthesized using extracts from P. nigrum, indicating its potential as an antibacterial agent. This evidence supports the inclusion of P. nigrum in the study, as it offers a unique source of phytochemicals that may aid in combating antibiotic resistance in microbial superbugs.
Nigella sativa, black seed, has a long history of medicinal use and is renowned for its diverse therapeutic properties. Studies by Khalil et al. [22] and Mekky, [23] emphasized the medicinal value of N. sativa, showcasing its potential in various applications, including food science and antimicrobial activity. The bioactive components of N. sativa, such as thymoquinone, have been attributed to its antimicrobial effects, making it a valuable candidate for combating antibiotic resistance [24]. The inclusion of N. sativa in the study aligns with its reputation as a potent natural remedy with broad-spectrum antimicrobial properties. The study by Abdulrahman et al. [25] on the effects of N. sativa in COVID-19 patients highlights the importance of exploring traditional remedies for modern healthcare challenges.
Annona muricata, commonly known as soursop, is the fourth plant species chosen for its potential in managing nosocomial infections. Studies on A. muricata have revealed the presence of novel antineoplastic metabolites in its extracts, highlighting its potential as a source of potent antioxidant and therapeutic agents Gavamukulya et al. [26]. The antibacterial activity of A. muricata extracts against biofilm-forming MRSA further underscores its efficacy in combating antibiotic-resistant bacteria [27].
The selection of Thymus vulgaris, Piper nigrum, Nigella sativa, and Annona muricata for the study on combating antibiotic resistance in microbial "superbugs" is grounded in their historical uses, documented antimicrobial properties, and potential synergistic effects with conventional treatments. By elucidating the mechanisms of action of phytophenols from these plant species, the study aims to provide insights into novel strategies for managing nosocomial infections and addressing the escalating threat of antibiotic-resistant superbugs.
The utilization of natural secondary metabolites from Nigella sativa, Piper nigrum, Thymus vulgaris, and Annona muricata for medicinal and remedial applications in managing nosocomial diseases is crucial due to their therapeutic properties and historical significance in traditional medicine. These plant species have been extensively studied for their bioactive compounds, which exhibit a wide range of pharmacological activities beneficial for combating nosocomial infections.
Similarly, research on N. sativa has demonstrated its antimicrobial properties, making it a valuable candidate for managing bacterial infections. The antibacterial activity of N. sativa leaves' extracts against a variety of bacteria highlights its potential in addressing common bacterial diseases [28]. Moreover, the hypoglycemic, hypolipidemic, and antioxidant activities of Nigella sativa make it a promising natural remedy for conditions like diabetes [29]. These findings emphasized the importance of exploring the therapeutic potential of Nigella sativa in combating nosocomial infections.
Piper nigrum, known for its antimicrobial properties, offers another valuable source of secondary metabolites for medicinal applications. The antibacterial activity of silver nanoparticles synthesized using P. nigrum extracts showcases its potential as an antibacterial agent. The modulatory effect of P. nigrum extracts on selected antibiotics against biofilm-forming MRSA further highlights its role in enhancing the efficacy of conventional antibiotics [27]. These studies support the preference for utilizing P. nigrum in the management of nosocomial infections.
Thymus vulgaris, commonly known as thyme, is renowned for its antimicrobial properties, making it a valuable resource for combating infectious diseases. Research on T. vulgaris essential oil has demonstrated its antimicrobial and antibiofilm properties against clinical isolates, indicating its potential in addressing opportunistic infections. The inclusion of T. vulgaris in medicinal formulations can provide synergistic effects with conventional treatments, enhancing the efficacy of antimicrobial therapies as reported by Tacconelli et al. [30].
The preference for utilizing natural secondary metabolites from Nigella sativa, Piper nigrum, Thymus vulgaris, and Annona muricata in medicinal and remedial applications for nosocomial diseases' management stems from their well-documented pharmacological activities, including antimicrobial, antioxidant, and therapeutic properties. These plant species offer a rich source of bioactive compounds that can aid in combating antibiotic resistance and addressing the challenges posed by nosocomial infections, making them valuable assets in the field of natural medicine.
1.1 Pharmacological potential and biochemical derivatives of Nigella sativa, Piper nigrum, Thymus vulgaris, and Annona muricata
The use of biochemical derivatives of medicinal plants has considerably improved drug research and infectious disease management. Currently, 60% of all antibacterial and anticancer drugs on the market are derived from plants [31]. Nigella sativa has been used for millennia in traditional Arabic medicine and is one of the first known herbal remedies. The seeds of N. sativa contain oil, protein, carbohydrates, fiber, and saponins. The essential oil of Nigella sativa contains nigellone, thymoquinone, and thymohydroquinone; the fixed oil contains arachidonic, linoleic, oleic, almitoleic, palmitic, stearic, myristic, sterole, and eicosadenoic acids [32]. In addition to its culinary use, Piper nigrum is widely used in traditional medicine across a number of nations. Several nations typically cultivate Thymus vulgaris L. for commercial use to harvest its dried leaves, plant extracts, plant oil, and oleoresins [33]. Due to its broad aromaticity, Thymus vulgaris L. is utilized commercially as a flavoring ingredient in the food industry [34]. Along with its application for flowering and decorative purposes, it is also employed for the preservation of meat, poultry, and fish [35]; [36]. Furthermore, A. muricata is a plethora of medicinal plants that have been widely studied in recent years due to its pharmacological potential. In folklore treatment, various A. muricata plant parts have been used as potential cures for a number of ailments, such as arthritis, and they have been reported to be useful for treating diabetes, inflammation, spasm, and cancer [37]; [8].
1.2 Antimicrobial, anti-inflammatory, and immunomodulatory properties of Nigella sativa, Piper nigrum, Thymus vulgaris and Annona muricata
Nigella sativa has been used for a variety of ailments in traditional Arabic herbal medicine, including pyrexia, conjunctivitis, dermatitis, internal bleeding, and gastrointestinal disorders [38]. The potential of its crude leaf extracts to boost the activity of intrinsic enzymes with antioxidant properties, such as glutathione peroxidase and catalase, can be attributed to some of these effects. Additionally, the oil also alters B-cell-dependent immunological pathways, influences the production of prostaglandins, cytokines, and chemokines, and scavenges free radicals [39].
Many diseases have been treated or managed using various P. nigrum components, including flowers, seeds, fruits, and leaves [40]. The Asia region has the most reports on the traditional use of P. nigrum. Additionally, menstrual abnormalities such as menorrhagia, oligomenorrhea, hypomenorrhea, and dysmenorrhea are among the maladies that P. nigrum has been applied for treatment [40]. In addition to being used to treat fever, jaundice, and snake bites, Piper nigrum has also been used to treat skin conditions such as scabies, itch, bed sores, and boils [40]. Babayemi et al. [41] also confirmed the potency of phytophenolic compounds comprising phytol, octadecanoic acid, and n-hexadecanoic acid elucidated via chromatographic studies due to inherent inhibitory upshot on nosocomial wound MDR bacterial pathogens including Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. Thymus vulgaris, due to its wide range of pharmacological qualities, has a variety of ethno-medicinal uses. The plant is largely used for its healing and disinfecting properties on wounds [33]. Ancient Europeans employed the antibacterial properties of this plant's aerial parts for fumigation, skin, and respiratory ailments [5]. Satyal et al. [42] has demonstrated the antibacterial activity of T. vulgaris for virulent, common MDR strains of bacteria and fungal pathogens. Benameur et al. [43] suggested that plants can reduce bacterial adsorption and the formation of biofilm matrices. Furthermore, Thymus vulgaris L. is thought to possess antibacterial, astringent, carminative, tonic, and anthelminthic properties. Due to its ability to combat intestinal diseases and infestations caused by ascarids, hookworms, fungi, yeasts, and bacteria, the plant has become quite well known [5]. Additionally, thyme can also be used to manage dermatological conditions such as rheumatoid arthritis, sciatica, greasy skin, dermatitis, and bug bites [5]. According to Tian et al. [44], Thymus vulgaris L. alleviates the neurological problems caused by bites and stings. The red variety of Thymus vulgaris L. oil and T. vulgaris L. oil is used to cure bodily aches and skin conditions. Recent research on anti-inflammatory agents has shown that Thymus vulgaris L. is effective at reducing oxidative stress and cell-mediated immunity [45].
Annona muricata, popularly known as soursop, is a plant fruit found in tropical and subtropical parts of the world. In Africa, India, and tropical America, A. muricata has been widely used customarily against an assortment of human illnesses and diseases. Over the years, the leaves, fruits, seeds, and bark of A. muricata have been used in traditional medicines in various cultures and from various Nations [46]. As a consequence, it has become widely known and used for hypoglycemic, hypotensive, and hypolipidemic treatments [47,48,49]. Takahashi et al. [50] previously reported the antimicrobial properties of A. muricata, although its mechanism of action is not fully understood. Thus, the plant fruit A. muricata has attracted increased amounts of attention due to its potential to treat viral infections, and new therapeutic agents against microbial activity are becoming a focus of research [51]. Nícolas et al. [52] have demonstrated that A. muricata possesses a broad spectrum of antibacterial activity against both gram-negative and gram-positive bacteria, and bacterial membranes have been identified as the primary targets of A. muricata. To further corroborate the antimicrobial properties of A. muricata, as reported by Campos et al. [9], Takahashi et al. [50] reported significant antifungal activity against fluconazole-, itraconazole-, and anidulafungin-resistant C. albicans.
1.3 Microbial inhibition profile of Thymus vulgaris, Piper nigrum, Nigella sativa, and Annona muricata on nosocomial infection-causing bacteria, fungi and viruses
Nigella sativa, commonly known as Black Cumin, has been extensively studied for its antibacterial properties against various microbial pathogens. Research by Tiji et al. [53] highlighted the antimicrobial activity of Moroccan Nigella sativa extracts, showing MIC values ranging from micrograms to milligrams per milliliter against different pathogens. Specifically, the MIC for Staphylococcus aureus was found to be 0.96 mg/mL, for Bacillus cereus 0.5 mg/mL, and for Escherichia coli 0.68 mg/mL. This indicates the potency of Nigella sativa in inhibiting the growth of these bacteria at relatively low concentrations.
Denizkara et al. [54] provides information on determining MIC and MBC values for antimicrobial agents. In this context, the MIC and MBC values of purified aqueous extract against Staphylococcus aureus bacteria were reported as 35.15 and 23.39 µg/mL, respectively. This finding aligns with the concept of assessing the inhibitory and bactericidal concentrations of compounds, similar to the approach taken with Piper nigrum by Sanad et al. [55] who discussed the use of ciprofloxacin as a reference to determine MIC and MBC values against selected strains. The MIC/MBC values of ciprofloxacin were reported as 2.9/5.9 μM. This observation highlights the importance of understanding the potency of antimicrobial agents through MIC and MBC assessments, which can be applied to evaluate the efficacy of compounds from Piper nigrum.
Sirimongkolvorakul and Jasancheun, [56] presents the MIC and MBC values of Piper nigrum ethanol extract against Staphylococcus aureus, with MIC and MBC reported as 0.13 and 0.52 mg/mL, respectively. This inference aligns with the concept of determining the inhibitory and bactericidal concentrations of natural extracts, similar to the evaluation of compounds from Piper nigrum.
Thyme essential oil inhibited the adherence of Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus saprophyticus in the presence of sucrose, with minimum adherence inhibitory concentrations ranging from 0.78–3.12 μL/mL as reported by Gedikoğlu et al. [57]. Thyme essential oil showed potent inhibitory activity against methicillin-resistant Staphylococcus aureus (MRSA) strains when combined with the antimicrobial clindamycin, with MIC ranges between 125–500 μg/mL [58]. Mohsenipour and Hassanshahian, [59] reported inhibitory effect of Thymus vulgaris essential oil extracts on bacteria with MIC values ranging from 64 to 512 μg/mL against the planktonic form and biofilm structures of human pathogenic bacteria comprising Klebsiella pneumoniae, Pseudomonas aeruginosa, and Staphylococcus saprophyticus. In a study by Jalal et al. [60], biosynthesized silver nanoparticles showed promising MIC values ranging from 156.25 to 1,250 µg/mL against various Candida species. Although this study did not specifically involve Annona muricata, it sheds light on the efficacy of silver nanoparticles against fungal pathogens, including Candida species, which are relevant in the context of antifungal activity. Additionally, the MBC values for MDR Pseudomonas aeruginosa strains were reported by Murad, [61], with the bacterial strain showing an MBC of 0.5 mg/mL and the MDR strain exhibiting an MBC of 1 mg/mL. This information underscores the challenge posed by MDR strains and the need for effective antimicrobial agents like Annona muricata to address such infections. The study by Gavamukulya et al. [26] have explored the antineoplastic and cytotoxic activities of Annona muricata extracts, indicating the presence of compounds with potential antiviral properties. These findings lay the groundwork for investigating the effectiveness of Annona muricata against viruses responsible for nosocomial infections, highlighting its broad-spectrum therapeutic potential.
Moreover, the study by Bitar et al. [62] delves into the anti-inflammatory effects of Annona muricata through the inactivation of the NALP3 inflammasome. This mechanism of action suggests that Annona muricata may possess immunomodulatory properties that could be beneficial in combating viral infections by regulating the inflammatory response, which is crucial in managing nosocomial viral outbreaks. Furthermore, the research by Vignera et al. [63] on the use of ellagic acid and Annona muricata to improve semen quality in men with high-risk papillomavirus infection hints at the potential antiviral effects of Annona muricata. This study underscores the ability of compounds derived from Annona muricata to modulate viral infections, indicating a promising avenue for exploring its antiviral activity against nosocomial pathogens.
1.4 Evaluation of the antibacterial potential of Nigella sativa and Annona muricata against Salmonella serotypes, MRSA, VRSA, and other multidrug-resistant pathogens
Well diffusion tests and tube dilution methods were used to examine the antibacterial activity of N. sativa oil and crude extracts against the growth of six Salmonella serotypes, namely, Salmonella paratyphi, S. typhi, S. enteritidis, S. typhimurium, S. Heidelberg, and S. agona [64, 65]. Using drug sensitivity testing, it was determined that the synthetic antibiotics ceftriaxone and ciprofloxacin were ineffective against all of the Salmonella strains examined. The oil extract made only 90% of the resistant Salmonella strains vulnerable to treatment, whereas the crude extract made all of them susceptible. In another study, the well diffusion technique was used to investigate the effects of various doses of black cumin seed oil on MRSA strains that had been obtained from diabetic wounds. Each strain had been in contact with the oil for a different amount of time [65]. Another in vitro investigation revealed that 819.2 g/ml N. sativa ethanol extract was necessary to kill vancomycin-resistant Staphylococcus aureus (VRSA). The agar-well diffusion method was utilized to obtain all five clinical isolates. The average inhibitory zone for all five VRSA isolates was 22.2 mm when treated with an ethanol extract at 20 mg/ml [66].
Solomon-Wisdom et al. [67] reported the antimicrobial potential of soursop against gram-positive and gram-negative bacteria, which was comparable to that of the standard antibiotic streptomycin. The bioactivity of soursop was dependent on the kind of extract used. Additionally, Mutakin et al. [68] reported that combining an ethanol extract and antibiotic treatment increased the potency of antibiotics against multidrug-resistant strains of E. coli and S. aureus. Further investigations by Nicolas et al. [52], such as bacterial killing assays, bacterial abundance, and membrane viability analysis via fluorescent probes, as well as nucleotide leakage and outer membrane permeability assays, demonstrated the bioactivity of Annona muricata against both Gram-negative and Gram-positive bacteria. The minimal inhibitory concentration (MIC) assay showed that E. faecalis, S. typhimurium and S. aureus were the most susceptible bacteria to A. muricata, while the minimum bactericidal concentration (MBC) was shown for S. typhimurium, S. aureus and P. aeruginosa; in this order, these bacteria species were the most susceptible to soursop [52]. Gavamukulya et al. [37] suggested that the probable mechanism was due to the synergistic action of Annona muricata phytoactives, such as alkaloids, flavonoids, and polyphenols. This submission was corroborated by Campos et al. [9], as their investigation through UPLC-ESI–MS/MS also revealed the presence of similar phytochemicals, suggesting their synergetic action in nucleotide leakage and that bacterial membranes are primary targets of this extract.
1.5 Evaluation of the antifungal potential of Thymus vulgaris and Annona muricata against Candida, Cryptococcus, Aspergillus, and other pathogenic fungi
Essential thyme oil has been proven to be effective against a wide variety of fungal species, including Sclerotinia spp., Botrytis spp., Phytophthora spp., Pythium spp., Fusarium spp., Alternaria spp., and Cladobotryum spp. The sensitivity of these fungi was studied using a circumferential growth inhibition assay, and the results showed that the ED50 values for all investigated species ranged from 9.3 to 18.0%, with inhibitory values ranging from 13.9 to 41.4 mm at a 5% dosage (p ≤ 0.05) [69]. Additionally, a total of 76 C. difficile and 183 C. albicans clinical isolates were analyzed in this study. For each of the isolates, researchers determined an MIC and MFC ranging from 0.04 to 22.9 mg/mL. Thyme oil slowed fungal growth early in the time-kill test. Thyme oil plus sorbitol, a low-dose osmoprotectant (MIC = 0.08 mg/ml; p ≤ 0.05), had a synergistic effect on fungal development [70]. The antifungal effects of thymol at 64 mg/l and 128 mg/ml were significantly greater than those of conventional antibiotics (p ≤ 0.05). Thymol increases pmk-1 and sec-1 gene expression to enhance the p38 MAPK biophysical pathway; this route inhibits C. albicans proliferation [71]. Scalas et al. [72] investigated fluconazole (FLC), itraconazole (ITC), and voriconazole (VRC) for the treatment of Cryptococcus neoformans. The MIC and MFC ranged from 56 to 1.12 mg/ml VRC. However, at doses between 0.02 and 0.08 mg/ml, thymol had significant effects (p ≤ 0.05) in a single antifungal test conducted by Scalas et al. [72]. Evidence shows that thyme oil inhibits fungal development in both the vapor and liquid phases. The mycelial growth of Aspergillus flavus was completely inhibited at doses of 20 and 400 g/ml. In addition, 10 g/ml thyme oil reduced aflatoxin production by 97.0% in the liquid phase and 56.4% in the vapor phase (p ≤ 0.05) as observed by Pinto et al. [73]. A number of studies have shown the fungicidal potency of soursop against Candida species [74, 75]. However, the mechanism of action, especially for multidrug-resistant strains, has yet to be established. Recently, Campos et al. [9] evaluated the effect of the ethanol extract of A. muricata on multidrug-resistant C. albicans. The MIC revealed the fungistatic effect of A. muricata against C. albicans, which was found to be resistant to fluconazole and itraconazole. Annona muricata reduced fungal growth by 58% and cell density by 65%. Additionally, the study further revealed that after treatment with the ethanol extract, soursop affects the fungal plasma membrane and cell wall, which significantly affects and reduces the viability of the cell. Phyto-constituents such as alkaloids, flavonoids, acetogenins, phenolic acids, benzofurans, and disaccharides were detected by UFLC-QTOF-MS as major bioactive components [9].
1.6 Evaluation of the antiviral potential of Thymus vulgaris and Annona muricata against Influenza Virus, HIV-1, Dengue Virus, Herpes Simplex Virus, and SARS-CoV-2
Thyme oil, in both its liquid and vapor forms, has been found to be effective against the influenza virus. While the vapor phase showed only weak activity, the liquid phase completely inhibited viral growth at a concentration of 3.1 g/ml, far outperforming the control (canola oil) [40]. Researchers have also examined the effects of hemaglutinin on two main surface proteins, neuraminidase (NA) and hemagglutinin (HA), and found that HA was significantly suppressed. Additionally, a TC50 value of 14.34 l/ml (p ≤ 0.05) indicated that 50% of the culture was lost [72]. The antiviral activities of P-cymene, terpinen-4-ol, terpineol, terpinen, thymol, citral, and 1, 8-cineole were investigated. The noncytotoxic dose of citral ranged from 20 g/ml to 1250 g/ml in RC-37 kidney cells. The IC50 of 1, 8-cineole was 1200 g/mL. Thyme oil inhibited viral replication by > 96% (p ≤ 0.05) compared to that of other monoterpenes [76]. Feriotto et al. [77] studied the Tat protein, which helps transcribe the viral genome of Thymus vulgaris L. Essential oil works with other substances; long terminal repeat (LTR) transcription of HIV-1 requires the Tat protein and the Tat/TAR-RNA complex [77]. With respect to the electrochemical impedance shift assay, this molecule revealed a significant inhibitory potential (3–6 g/ml) compared to that of the control (EMIE). This dynamic was replicated in a study investigating antiviral activity against Tat-induced HIV-1 LTR transcription; the results showed 50% inhibitory activity (RT50 = 0.83 g/ml), which is a significant modulatory potential that reduces viral expression by 52% (p ≤ 0.05) [77]. Posttreatment, an aqueous extract of soursop (A. muricata) showed antiviral activity against dengue virus type 2, with 0.20 mg/mL being the EC50 value for A. muricata tested against DENV-2. Additionally, the selectivity index (SI) of soursop, an antiviral compound, was greater than 12.5 [78], suggesting that soursop is a potential antiviral agent because any antimicrobial compound with an SI greater than 10 (SI > 10) could be established as a potential antiviral agent. In another study by Balderrama-Carmona et al. [79], bacteriophage surrogates were used to evaluate the antiviral effect of an acidified ethanol extract (AEE) of A. muricata. This antiviral evaluation revealed a dose-dependent effect of soursop. An increase in the contact time and dose of the AEE extract led to a decrease in viral replication. The greatest reduction in phage yield was reported at a concentration of 1 mg/ml AEE and with a contact time greater than 30 min, with values of 7 and 4 log10 PFU/ml for Av-05 and Av-08, respectively. A similar concentration of 1 mg/mL was reported by Padmaa et al. [80] for the MIC of the ethanol extract of soursop against HSV-1. In the 1990s, Padmaa et al. [80] investigated the effects of soursop on herpes simplex virus-1 (HSV-1). This in vitro investigation using standard strains and wild clinical isolates revealed the inhibition of the cytopathic effect of HSV-1 on Vero cells, suggesting the potential of A. muricata for preventing HSV-1 infection.
More than 200 bioactive compounds have been identified, isolated, and studied from A. muricata; these principal compounds are phenols, alkaloids, acetogenins, and flavonoids [81], which have been reported for their antiviral activity. Rutin, a major component of soursop (Annona muricata), reportedly interacts with multiple viral proteins. Current in silico and in vivo investigations have reported that rutin, a major bioactive compound, inhibits the replication of SARS-CoV and SARS-CoV-2 (COVID-19). Angiotensin-converting enzyme 2 (ACE 2) is the receptor that binds to the SARS-CoV-2 glycoprotein and can cause membrane fusion and viral infection. However, Balderrama-Carmona et al. [48] reported that rutin potentially inhibits ACE2, which further suppresses the entry of SARS-CoV-2. Elmi et al. [81] reported that polyphenol bioactive compounds interfere with HIV-1 replication at an early stage of the virus. In essence, these bioactive compounds inhibit virus entry into host cells, thus reducing viral RNA levels, which indicates the virucidal activity of polyphenols found in plants.
2 Resistance mechanisms and connections in microbial superbugs responsible for nosocomial infections
Nosocomial, or hospital-acquired, infections caused by multidrug-resistant (MDR) "superbug" pathogens have developed various resistance mechanisms that allow them to evade the effects of commonly used antibiotics. Understanding the resistance mechanisms and connections within microbial superbugs responsible for nosocomial infections requires an exploration of the pathways through which these pathogens evade conventional treatments. The emergence of multidrug-resistant Gram-negative bacteria (MDR-GNB) poses a significant challenge in healthcare settings, as these pathogens exhibit resistance to vital antibiotics, including broad-spectrum penicillins, fluoroquinolones, aminoglycosides, and β-lactams as reported by Ahmed et al. [82]. The resistance mechanisms employed by these superbugs often involve intricate genetic adaptations that confer the ability to withstand the effects of multiple antimicrobial agents, leading to treatment failures and increased mortality rates.
One of the key players in nosocomial infections is Acinetobacter baumannii, a pathogen known for its carbapenem resistance. Carbapenem-resistant Acinetobacter baumannii (CRAB) strains have become increasingly prevalent in healthcare settings, posing a significant threat to patient safety [83]. The resistance mechanisms in Acinetobacter baumannii often involve the production of carbapenemases, enzymes that hydrolyze carbapenem antibiotics, rendering them ineffective against the bacteria. This resistance mechanism highlights the adaptability of superbugs in evading the effects of potent antibiotics, leading to persistent infections and treatment challenges.
Moreover, the use of antimicrobial peptides and the CRISPR/Cas9 system has been proposed as a potential therapeutic approach against multidrug-resistant pathogens. Antimicrobial peptides offer a novel strategy to combat nosocomial infections by targeting bacterial membranes and disrupting essential cellular functions [84]. The CRISPR/Cas9 system, known for its gene-editing capabilities, provides a promising avenue for developing targeted therapies against multidrug-resistant microbes, offering precision in combating resistant strains [84].
In the context of nosocomial infections, the presence of methicillin-resistant Staphylococcus aureus (MRSA) poses a significant threat to patient safety. MRSA contamination in healthcare settings, particularly on frequently touched objects as formites, contributes to the spread of infections and challenges infection control measures [85]. The ability of MRSA to persist on surfaces for extended periods underscores the importance of stringent hygiene practices and effective disinfection strategies to prevent nosocomial transmission.
The prevalence of nosocomial infections caused by multidrug-resistant organisms highlights the urgent need for novel antimicrobial strategies. Nanomaterials, such as silver nanoparticles, have shown promise as multimodal photothermal agents against superbugs, offering a potential solution to combat antibiotic resistance [86]. The unique properties of nanomaterials enable targeted antimicrobial effects, providing a versatile approach to addressing nosocomial infections caused by multidrug-resistant pathogens.
2.1 Resistant mechanisms of key MDR bacteria-causing nosocomial infections
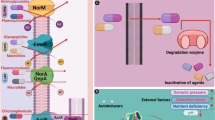
The resistance mechanisms and connections within microbial superbugs responsible for nosocomial infections are multifaceted and dynamic, requiring a comprehensive understanding of the genetic, biochemical, and environmental factors that contribute to antimicrobial resistance. By exploring novel therapeutic approaches, such as antimicrobial peptides, gene-editing technologies, and nanomaterial-based strategies, researchers aim to develop innovative solutions to combat the growing threat of multidrug-resistant superbugs in healthcare settings. The key resistance mechanisms and the associated "superbug" pathogens responsible for nosocomial infections are summarized in Table 1.
2.1.1 Enzymatic inactivation by nosocomial bacterial pathogens
Certain bacteria, such as Enterobacteriaceae and Pseudomonas aeruginosa, Klebsiella pneumoniae, Escherichia coli produce enzymes that can chemically modify and inactivate antibiotics. Examples include β-lactamases that hydrolyze β-lactam antibiotics like penicillins and carbapenems [87]; [88]; [89]. Aminoglycoside-modifying enzymes that acetylate, phosphorylate or adenylate aminoglycoside antibiotics comprising Streptomycin, Kanamycin, Gentamicin, Tobramycin, Netilmicin, Amikacin, Arbekacin and Plazomicin [90].
The presence of additional classes of antibiotics, such as aminoglycosides, reveals a distinct resistance mechanism. The biosynthetic pathway has ceased because the modified products lack the ability to bind to ribosomes and thus have a much lower affinity for RNA [33]; [91]. Acetyltransferase, adenylyltransferase, and phosphotransferase are three metabolic enzymes responsible for inactivating these antimicrobial medicines. The amino groups of aminoglycosides use these enzymes for site remodeling of aminoglycoside resistance genes (aac6'la and aac6'lb) (Fig. 1), which encode these enzymes [3]. One of the five apramycin-resistant dairy bacterial isolates, Escherichia coli J62-1, produced the aac (1) enzyme as described previously [92].
The protein synthesizing enzyme DNA gyrase, the deconstruction and remodeling enzymes that superbugs use are both inhibited by the phytochemicals found in plants. In addition to lowering efflux pump activity, they can selectively target porins for vascular permeability. Phytochemicals are able to disrupt the superbug's target sites before those sites may be modified
2.1.2 Target modification by nosocomial bacterial pathogens
Pathogens can alter the molecular targets of antibiotics, preventing the drugs from binding effectively. Altered penicillin-binding proteins (PBPs) in methicillin-resistant Staphylococcus aureus (MRSA) was reported by Sousa et al. [90]. Mutations in DNA gyrase and topoisomerase IV that confer resistance to fluoroquinolones in Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Acinetobacter baumannii, Enterobacter species and Citrobacter freundii as reported by Sousa et al. [90].
2.1.3 Reduced drug accumulation by nosocomial bacterial pathogens
Bacteria can reduce the intracellular concentration of antibiotics through increased efflux pump activity that actively expels drugs out of the cell, as seen in Pseudomonas aeruginosa and Acinetobacter baumanni as reported by Xia et al. [88]; Walsh et al. [89]. Decreased porin expression, limiting drug entry into the bacterial cell, observed in Gram-negative bacteria comprising Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Acinetobacter baumannii and Neisseria meningitidis was also reported by Xiao et al. [87]; Xia et al. [88]; Walsh et al. [89].
2.1.4 Bypass of metabolic pathways by nosocomial bacterial pathogens
Some bacteria comprising Staphylococcus aureus and Neisseria meningitidis can bypass the metabolic pathways targeted by certain antibiotics, rendering the drugs ineffective. The overproduction of the target enzyme to overcome inhibition by antimetabolite drugs like sulfonamides which are antimetabolite drugs that target the enzyme dihydropteroate synthase (DHPS) in Campylobacter jejuni and Streptococcus pneumoniae was reported by Sousa et al. [90], as well as the development of alternative metabolic pathways that do not require the targeted enzyme in Pseudomonas species, Zymomonas mobilis, Vibrio cholerae and Escherichia coli also reported by Sousa et al. [90].
These resistance mechanisms, often combined in a single pathogen, contribute to the emergence and spread of "superbug" infections in healthcare settings, posing significant challenges to effective treatment and control.
2.1.5 Enzymatic inactivation of antibiotics by nosocomial bacterial pathogens to evade beta-lactams and glycopeptides
It comprises destructive and modifying enzymes [3]. Bacteria chemically destroy antibiotics to escape being killed by them, changing their chemical composition. Beta-lactamases attach to the beta-lactam ring of penicillins, cephalosporins, and carbapenems, degrading them [40, 93, 94]. In earlier studies, the antibacterial effects of baicalein restored the efficacy of the beta-lactam antibiotics tetracycline and ciprofloxacin against MRSA by blocking NorAEP [95]. Bayode et al. [16] reported that Penicillins, cephalosporins, monobactams, and carbapenems are among the antibiotics of the beta-lactam class that inactivate glycopeptide transpeptidases to prevent the formation of bacterial cell walls. Bitter leaves (Vernonia amydalina) also demonstrate potent antimicrobial activity via their inherent phytochemicals (polyphenol family) that inhibit the beta-lactamase enzymes responsible for the pathogenicity of Enterobacteriaceae infections, as reported by [94].
2.1.6 The potential of porin-targeting phenolics in combating antibiotic resistance in gram-negative bacteria-causing nosocomial infections
Porins govern active diffusion across lipid bilayers [96]. Gram-negative bacteria are vulnerable to porin-targeting phenolics. By altering their porins, bacteria can evade antimicrobial agents [96] (Fig. 1). Gram-negative bacteria employ this method to avoid antibiotics [96]. Additionally, polyphenol families found naturally in bitter leaves (Vernonia amygdalina) have shown strong antibacterial activity by inhibiting the beta-lactamase enzymes responsible for the pathogenicity of Enterobacteriaceae infections [94].
2.1.7 Modification of drug-binding sites as mechanism of antibiotic resistance in nosocomial bacterial pathogens
One type of drug resistance is the modification of drug-binding sites. This form of resistance develops when an antibacterial agent's specific targeting site is modified in a way that prevents the agent from reacting with the bacterium. As a consequence, the effectiveness of antibacterial compounds is greatly diminished [96]. Antibiotic-resistant bacteria may have multiple methods of resistance, one of which involves peptidyltransferase modification of the treatment site. Epigenetic modification of this enzyme at a specific amino acid residue reduces antibiotic selectivity to the action site peptidyltransferase [96] (Fig. 1). Clinical isolates of Staphylococcus aureus that have developed resistance to antimicrobial agents almost always do so by employing this resistance mechanism.
2.2 Phenolic compounds as efflux pump inhibitors to combat antibiotic resistance in nosocomial pathogens
The phenol group, which is connected to numerous regions of each molecule, gave rise to the name phenolic class of phytochemical compounds. Phenolics are a diverse group of bioactive, naturally occurring substances that are widely used in medicine. In several ways, these substances, which are bioactive molecules, contribute significantly to improving antibiotic action against bacteria that have developed resistance [97]. Some of the most important strategies for treating this disease include lowering efflux pump (EP) activity and functioning as multidrug efflux inhibitors (MEIs). The efficacy of these substances for efflux pump inhibition (EPI) against pathogenic bacteria has been encouraging. Natural phenolic compounds have antimicrobial properties beyond their capacity to obstruct efflux pumps. Numerous phenolic compounds have been discovered thus far, each with a unique mode of action [97]. Among plant families, the Fabaceae family possesses the most phenolic chemical byproducts [98]. Maximizing the combinatorial effect of phytonutrients with failed antibiotics, which reportedly restores optimal antibacterial activity, is pertinent as supported by Brown, [99]; Porras et al. [100].
When produced at sufficiently high concentrations, phenolic compounds show promising EPI activity against pathogenic germs, increasing the efficacy of antibiotics by inhibiting the development of resistance [101]. Phenolics’ antibacterial action can be attributed to two main processes namely by chemical interference with the creation or operation of key components of bacteria and by side-stepping of well-established antibacterial resistance mechanisms. Cell wall phenolics target multiple enzymes, including dihydrofolate reductase, urease, and sortase A [33]. Understanding the systems of resistant bacteria is crucial for finding solutions to slow the development of antibiotic resistance. These systems typically involve strengthening the efflux pump, eliminating antibacterial agents using neutralizing enzymes, distorting antibiotics using modifying enzymes, and evolving target structures in the bacterium that have lower resistance to antibacterial recognition [3, 35]. Recently, Lawani et al. [1] reported that employing alternative compounds such as phytochemicals alone or in combination with other antibacterial agents appears to be an effective and safe strategy for battling microbial superbug pathogens implicated in nosocomial infections.
2.3 Phenolic compounds as efflux pump inhibitors for the inhibition of DNA gyrase and antibiotic resistance nosocomial bacterial pathogens
Anthraquinones [102], chebulinic acid [103], and tannic acid found in Nigella sativa (Curmin), Piper nigrum (Black Pepper), Annona muricata (Soursop) are all phenolic compounds that have inhibitory effects on DNA gyrase [104]. Epigallocatechin Gallate (ECG) found in Nigella sativa, Piper nigrum (Black Pepper) has the propensity to inhibit β-lactamase activity [105] (Table 2), Annona muricata has the potential to block the B subunit of DNA gyrase from connecting to ATP [106]. Chebulinic acid was identified as the most effective bioactive component in a virtual screening against quinolone-resistant mutations of Mycobacterium tuberculosis DNA gyrase [107]. However, the results can be evaluated only via in silico prediction because no in vitro experimental research has been conducted. An in vitro study examined the DNA gyrase inhibitor chebulinic acid and its antituberculosis potential as Haloemodins are semisynthetic anthraquinone esters. These agents prevented the entry of vancomycin-resistant Enterococcus faecalis DNA gyrase and MRSA. Various halogenated analogs of the plant-derived chemical emodin have high activity against bacterial DNA gyrase [104]. Among bacteria, five efflux pump (EP) families are found in most Gram-positive and Gram-negative bacteria [108].
The RND family of Gram-negative bacteria is a trimeric complex spanning both membranes. Some of the most well-known EPs are members of the MFS family and include Staphylococcus aureus NorA and Streptococcus pneumoniae PmrA. It has been determined that this family of EPs is the most prevalent among these microbes. This antibiotic resistance has been detected in S. aureus, Pseudomonas aeruginosa, Acinetobacter baumannii, and Candida albicans, among others [109]. Ferreira et al. [110] studied the EPI action of this medication against Arcobacter butzleri and Arcobacter cryaerophilus. Baicalein, a phytophenol from Nigella sativa and tetracycline prevented efflux pump (EP) from killing E. coli, a prominent betalactamase-producing bacteria as affirmed by Cho et al. [111] and Fujita et al. [112] as illustrated in (Table 2). The results revealed that this drug could inhibit MRSA EPs by decreasing NorA protein production. Gholamnezhad et al. [40] also investigated antibiotic-flavonoid fusions for MRSA. The hybrid compounds had increased antibacterial stability and activity, confirming their dual mode of action [113].
2.4 Mechanisms of action, chemotherapeutic synergy, metabolic pathways and antioxidant properties of phytophenolic derivatives as potential antimicrobials
The genetic and genomic differences related to the potential ameliorative effects of phytophenolic derivatives on microbial superbugs and their synergistic selectivity for chemotherapeutics can be elucidated by elucidating the mechanisms of antibiotic resistance, interactions with cellular pathways, and antioxidant properties. The origins and evolution of antibiotic resistance have been extensively studied over the past few decades, leading to the urgent need for action. This phenomenon is particularly relevant in the context of microbial superbugs, where the mechanisms of antibiotic resistance play a crucial role in persistence and virulence [92].
Phytophenols have been shown to interact with muscle proteins, modifying their structural characteristics and textural properties in food products. This interaction may also have implications for the mechanisms of action of phytophenolic derivatives on microbial superbugs, especially in terms of disrupting microbial cell membranes or metabolic pathways [147]. Furthermore, a review of tumor-associated macrophages (TAMs) and TAM-based antitumor nanomedicines provides insights into the molecular mechanisms and nanodrug delivery systems based on TAMs. This finding is relevant because it sheds light on the potential mechanisms of action of phytophenolic derivatives on microbial superbugs, especially in the context of nanomedicine and targeted drug delivery [148].
In addition, studies of microbial infections caused by quorum quenching have elucidated the underlying mechanisms and implications of quorum quenching, which could be relevant for understanding how phytophenolic derivatives may interfere with microbial communication and virulence factors [149]. Moreover, the inhibition of hazardous compound formation in muscle foods by antioxidative phytophenols highlights the potential of phytophenolic derivatives for mitigating the production of carcinogenic compounds, which could be extrapolated to their effects on microbial superbugs [150]. Phytophenolic bioactive substances can disrupt regular cellular membrane communication, cause cellular components to coagulate, and alter different cellular pathways, all of which might eventually result in microbial death.
The study of the effectiveness of Lactobacillus and phytophenols in reducing high-fat diet-induced mTOR activation in rats provides insights into the modulation of cellular pathways by phytophenols, which could be relevant for understanding their effects on microbial cells [151]. The synthesis of a cardanol-based levelling agent as a biodegradable alternative to synthetic surfactants underscores the potential of plant-derived and biodegradable surfactants, which could have implications for the development of phytophenolic derivatives as antimicrobial agents [152, 153].
The opioid receptor activation that triggers the downregulation of cAMP improves the effectiveness of anticancer drugs, highlighting the potential for synergistic effects between phytophenolic derivatives and chemotherapeutics in combating cancer cells [154]. The radical scavenging activity of dietary phytophenols in combination with coantioxidants provides insights into the antioxidant properties of phytophenolic derivatives, which could be relevant in understanding their potential mechanisms of action against microbial superbugs [155].
3 Conclusions
Phytoactives, which are chemicals derived from plants, have been used to treat a wide variety of infectious diseases, with encouraging results. They have even shown antibacterial activity against a good number of human infections. A few of these chemicals not only exhibited antibacterial effects but also altered bacterial resistance to antimicrobial agents. Antimicrobial polyphenols have shown promise, but additional research is needed to confirm their activity pathways and stability. The move from in vitro to in vivo drug testing has hindered the development of new phytochemicals to attack microbial superbugs.
Data availability
The authors declare that the data supporting the findings of this review are available within the paper.
Abbreviations
- ABC:
-
ATP binding cassette
- ATP:
-
Adenosine triphosphate
- DNA:
-
Deoxyribose nucleic acid
- EGCG:
-
Epigallocatechin gallate
- EMIE:
-
Electrochemical impedance
- ENT:
-
Ear, nose and throat
- EP:
-
Efflux pump
- EPI:
-
Efflux pump inhibition
- HA:
-
Hemagglutinin
- HIV:
-
Human immune deficiency virus
- ITC:
-
Itraconazole
- LTR:
-
Long terminal repeat
- MATE:
-
Multidrug and toxic efflux
- MDR:
-
Multidrug resistant
- MEIs:
-
Multidrug efflux inhibitors
- MFC:
-
Minimum fungicidal concentration
- UFLC-QTOF-MS:
-
Ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry
- RND family:
-
Resistance-Nodulation-Division family of Gram-negative bacteria
- MFS family:
-
Major Facilitator Superfamily of Gram-negative bacteria
- Tat/TAR-RNA complex:
-
Trans-activator of transcription/TAR-RNA complex
- DENV-2:
-
Dengue virus serotype 2
- Camp:
-
Cyclic adenosine monophosphate
- ED50:
-
50% Effective dose
- LTR transcription:
-
Long Terminal Repeat transcription
- NALP3:
-
Nucleotide-binding domain, leucine-rich repeat, and pyrin domain-containing protein 3
- CRISPR/Cas9:
-
Clustered Regularly Interspaced Short Palindromic Repeats/CRISPR-associated protein 9
- TC50:
-
Tissue Culture 50
References
Lawani BT, Bayode MT, Sadibo ME, Awodire EF, Aro OP, Akindele AA, et al. Antibiotic resistance microbes’ (ARM) mechanisms and management: a phytomedicinal approach. Proc Natl Acad Sci India Sect B Biol Sci. 2023. https://doi.org/10.1007/s40011-023-01525-9.
WHO. Antimicrobial resistance: Global report on surveillance, vol. 2021. Geneva: WHO; 2021.
Khameneh B, Iranshahy M, Soheili V, Bazzaz BSF. Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Res Infect Dis. 2019;8:118.
Bakal SN, Bereswill S, Heimesaat MM. Finding novel antibiotic substances from medicinal plants—antimicrobial properties of Nigella sativa directed against multidrug-resistant bacteria. Eur J Microbiol and Immunol. 2017;7(1):92–8. https://doi.org/10.1556/1886.2017.00001.
Patil SM, Ramu R, Shirahatti PS, Shivamallu C, Amachawadi RG. A systematic review on ethnopharmacology, phytochemistry and pharmacological aspects of Thymus vulgaris Linn. Heliyon. 2021;7: e07054. https://doi.org/10.1016/j.heliyon.2021.e07054.
Takooree H, Muhammad ZA, Kannan RR, Rengasamy KN, Venugopala RJ, Zengin G, Mohamad FMA. Systematic review on black pepper (Piper nigrum L.): from folk uses to pharmacological applications. Crit Rev in Food Sci Nut. 2019. https://doi.org/10.1080/10408398.2019.1565489.
Quílez AM, Fernández-Arche MA, García-Giménez MD, De laPuerta R. Potential therapeutic applications of the genus Annona: local and traditional uses and pharmacology. J Ethnopharmacol. 2018;225:244–70. https://doi.org/10.1016/j.jep.2018.06.014.
Yajid AI, Rahman HS, Wong MP, Zain WZ. Potential benefits of Annona muricata in combating cancer: a review. Malays J Med Sci. 2018;25(1):15.
Campos LC, Ari SOL, Irley OM, Diniz LAC, Thiago PS, et al. Antifungal Annona muricata L. (soursop) extract targets the cell envelope of multidrug resistant Candida albicans. J Ethnopharmacol. 2023;301:115856. https://doi.org/10.1016/j.jep.2022.115856.
González-Villarreal JA, González-Lozano KJ, Aréchiga-Carvajal ET, Morlett-Chávez JA, Luévanos-Escareño MP, et al. Molecular mechanisms of multidrug resistance in clinically relevant enteropathogenic bacteria. Exp Therapeut Med. 2022;24(6):1–1. https://doi.org/10.3892/etm.2022.11689.
Daoud Z, Dropa M. Editorial: the global threat of Carbapenem-resistant gram-negative bacteria. Front Cell Infect Microbiol. 2023. https://doi.org/10.3389/fcimb.2023.1196488.
Cassini A, Högberg L, Plachouras D, Quattrocchi A, Hoxha A, et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect Dis. 2019;19(1):56–66. https://doi.org/10.1016/s1473-3099(18)30605-4.
Huguet C, Bourjot M, Bellanger J, Prévost G, Urbain A. Screening for antibacterial activity of French mushrooms against pathogenic and multidrug resistant bacteria. Appl Sci. 2022;12(10):5229. https://doi.org/10.3390/app12105229.
Alkofide H, Alhammad A, Alruwaili A, Aldemerdash A, Almangour T, et al. Multidrug-resistant and extensively drug-resistant enterobacteriaceae: prevalence, treatments, and outcomes—a retrospective cohort study. Infect Drug Resist. 2020;13:4653–62. https://doi.org/10.2147/idr.s283488.
Donkor E, Muhsen K, Johnson S, Kotey F, Dayie N, et al. Multicenter surveillance of antimicrobial resistance among gram-negative bacteria isolated from bloodstream infections in Ghana. Antibiotics. 2023;12(2):255. https://doi.org/10.3390/antibiotics12020255.
Bayode MT, Olalemi AO, Oladejo BO. Multiple antibiotic resistant index and detection of qnrS and qnrB genes in bacterial consortium of urine samples from clinical settings. Euro J Biol Res. 2021;11(1):45–56. https://doi.org/10.5281/zenodo.4304311.
Yan J, Zheng D, Gu H, Yu Y, Zeng J, Chen Q, et al. In situ sprayed biotherapeutic gel containing stable microbial communities for efficient anti-infection treatment. Adv Sci. 2022. https://doi.org/10.1002/advs.202205480.
Kantele A, Kuenzli E, Dunn S, Dance D, Newton P, et al. Dynamics of intestinal multidrug-resistant bacteria Colonisation contracted by visitors to a high-endemic setting: a prospective, daily, real-time sampling study. The Lancet Microbe. 2021;2(4):e151–8. https://doi.org/10.1016/s2666-5247(20)30224-x.
Kwansa-Bentum B, Okine BA, Dayie AD, Tetteh-Quarcoo PB, Kotey FC, Donkor ES, et al. In Vitro effects of petroleum ether, dichloromethane, methanolic and aqueous leaf extracts of Eucalyptus grandis on selected multidrug-resistant bacteria. PLoS ONE. 2023;18(3):e0283706. https://doi.org/10.1371/journal.pone.0283706.
Kryvtsova M, Caлaмoн I, Koščová J, Bucko D, Spivak M. Antimicrobial, antibiofilm and biochemichal properties of thymus vulgaris essential oil against clinical isolates of opportunistic infections. Biosyst Divers. 2019;27(3):270–5. https://doi.org/10.15421/011936.
Kováčová M, Daneu N, Tkáčiková Ľ, Búreš R, Dutková E, Stahorský M. Sustainable one-step solid-state synthesis of antibacterially active silver nanoparticles using mechanochemistry. Nanomaterials. 2020;10(11):2119. https://doi.org/10.3390/nano10112119.
Khalil P, Masood S, Rehman A, Iqbal A, Islam Z, Javaid N, et al. Proximate and sensory analysis of wheat bread supplemented with Nigella sativa oil and Nigella sativa extract. Pure Appl Biol. 2021. https://doi.org/10.19045/bspab.2021.100122.
Mekky A. Study of phytochemical analysis and antimicrobial activity of ethanolic extract of Nigella sativa and Matricaria chamomilla. Al-Azhar J Agric Res. 2022;47(2):38–51. https://doi.org/10.21608/ajar.2022.277829.
Zakaria M, Putri Y, Rahaju A, Fatmawati S, Cahyanto A. Inhibitory effect of Calcium hydroxide combined with Nigella sativa against Enterococcus faecalis. Dental J (Majalah Kedokteran Gigi). 2021;54(4):181–5. https://doi.org/10.20473/j.djmkg.v54.i4.p181-185.
Abdulrahman K, Bamosa A, Bukhari A, Siddiqui I, Arafa M, Mohsin A, et al. The effect of short treatment with Nigella sativa on symptoms, the cluster of differentiation (CD) profile, and inflammatory markers in mild COVID-19 patients: a randomized, double-blind controlled trial. Intl J Environ Res Public Health. 2022;19(18):11798. https://doi.org/10.3390/ijerph191811798.
Gavamukulya Y, Maina E, Meroka A, Madivoli E, El-Shemy H, Magoma G, et al. Liquid chromatography single quadrupole mass spectrometry (lC/SQ MS) analysis reveals presence of novel antineoplastic metabolites in ethanolic extracts of fruits and leaves of Annona muricata. Pharmacog J. 2019;11(4):660–8. https://doi.org/10.5530/pj.2019.11.104.
Neglo D, Tettey C, Essuman E, Amenu J, Mills-Robertson F, Sedohia D. Evaluation of the modulatory effect of Annona muricata extracts on the activity of some selected antibiotics against biofilm-forming MRSA. Evid-Based Complemen Altern Med. 2021;2021:1–9. https://doi.org/10.1155/2021/9342110.
Akanji O. Antibacterial activity of Annona muricata leaves’ extracts. Intl J Frontline Res Sci Technol. 2023;2(1):024–8. https://doi.org/10.56355/ijfrst.2023.2.1.0053.
Mohammed R, Ramadhan H, Shari F. Hypoglycemic, hypolipidemic, renal protective and antioxidant activity of Annona muricata in streptozotocin-induced diabetic rats. Res J Pharm Technol. 2021. https://doi.org/10.52711/0974-360x.2021.01121.
Tacconelli E, Mazzaferri F, Smet AMGA, Bragantini D, Eggimann P, Huttner B, et al. ESCMID-EUCIC clinical guidelines on decolonization of multidrug-resistant gram-negative bacteria carriers. Clin Microbiol Infect. 2019;25(7):807–17. https://doi.org/10.1016/j.cmi.2019.01.005.
Bush K, Bradford PA. β-lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med. 2016;6(8): a025247. https://doi.org/10.1101/cshperspect.a025247.
El-Tahir KE-DH, Bakeet DM. The black seed Nigella sativa Linnae21us-A mine for multi cures: a plea for urgent clinical evaluation of its volatile oil. J Taibah Univ Med Sci. 2006;1:1–19. https://doi.org/10.1016/S1658-3612(06)70014-2.
Shaaban HA, Sadek Z, Edris AE, Saad-Hussein A. Analysis and antibacterial activity of Nigella sativa essential oil formulated in microemulsion system. J Oleo Sci. 2015;64:223–32. https://doi.org/10.5650/jos.ess14177.
Kuete V. Thymus vulgaris. In: Kuete V, editor. Medicinal spices and vegetables from Africa. 1st ed. Elsevier Inc.; 2017. p. 599–609.
DebMandal SM. Thyme (Thymus vulgaris L.) oils. In: Preedy V, editor. Essential oils in food preservation, flavor and safety. London: Academic Press; 2016. p. 825–34.
Mogosanu GD, Grumezescu AM, Bejenaru C. Natural products used for food preservation. In: Preservation F, editor. Academic Press. London: UK; 2017. p. 365–411.
Gavamukulya Y, Wamunyokoli F, El-Shemy HA. Annona muricata: is the natural therapy for most disease conditions, including cancer, growing in our backyard? This is a systematic review of its research history and future prospects. Asian Pac J Trop Med. 2017;10:835–48. https://doi.org/10.1016/j.apjtm.2017.08.009.
Vouillamoz JF, Christ B. Thymus vulgaris L.: thyme. In: Novak J, Blüthner WD, editors. Medicinal aromatic and stimulant plants. Handbook of plant breeding. Cham: Springer; 2020. p. 547–57.
Dehkordi FR, Kamkhah AF. Anti-hypertensive effect of Nigella sativa seed extract in patients with mild hypertension. Fundam Clin Pharmacol. 2008;22:447–52. https://doi.org/10.1111/j.1472-8206.2008.00607.x.
Gholamnezhad Z, Keyhanmanesh R, Boskabady MH. Anti-inflammatory, antioxidant, and immunomodulatory aspects of Nigella sativa for its preventive and bronchodilatatory effects on obstructive respiratory diseases: a review of basic and clinical evidence. J Funct Food. 2015;17:910–27. https://doi.org/10.1016/j.jff.2015.06.032.
Babayemi OO, Oke EA, Bayode MT. Antibacterial activity of Jatropha tanjorensis leaf extracts against bacteria associated with wound infections from the clinical setting. Nusantara Biosci. 2021;13:239–46. https://doi.org/10.13057/nusbiosci/n130215.
Satyal P, Murray BR, McFeeters R. Essential oil characterization of Thymus vulgaris from various geographical locations. Foods. 2016;5:70–5.
Benameur Q, Gervasi T, Pellizzeri V. Antibacterial activity of Thymus vulgaris essential oil alone and in combination with cefotaxime against blaESBL producing multidrug resistant Enterobacteriaceae isolates. Nat Prod Res. 2019;33:2647–54. https://doi.org/10.1080/14786419.2018.1466124.
Tian F, Lee SY, Chun HS. Comparison of the antifungal and anti-aflatoxigenic potential of liquid and vapor phase of Thymus vulgaris essential oil against Aspergillus flavus. J Food Protect. 2019;82:2044–8. https://doi.org/10.4315/0362-028x.jfp-19-016.
Prasanth RV, Ravi VK, Varsha PV. Review on Thymus vulgaris traditional uses and pharmacological properties. Med Aromat Plants. 2014;3:1–3. https://doi.org/10.4172/2167-0412.1000164.
Badrie N, Schauss AG. Soursop (Annona muricata L.): composition, nutritional value, medicinal uses, and toxicology. In: Watson RR, Preedy, VR (eds.), Bioactive foods in promoting health. Oxford, (2009) pp. 621–643. https://doi.org/10.1186/1750-9378-6-S2-S9
Moghadamtousi SZ, Fadaeinasab M, Nikzad S, Mohan G, Ali HM, et al. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Intl J Mol Sci. 2015;16(7):15625–58.
Patel MS, Patel JK. A review on a miracle fruits of Annona muricata. J Pharmacog phytochem. 2016;5(1):137.
Zubaidi SN, Mohd Nani H, Ahmad Kamal MS, Abdul Qayyum T, Maarof S, Afzan A, Mohmad Misnan N, Hamezah HS, Baharum SN, Mediani A. Annona muricata: comprehensive review on the ethnomedicinal, phytochemistry, and pharmacological aspects focusing on antidiabetic properties. Life (Basel). 2023;13(2):353. https://doi.org/10.3390/life13020353.
Takahashi JA, Pereira CR, Pimenta LP, Boaventura MAD, Silva LGE. Antibacterial activity of eight Brazilian Annonaceae plants. Nat Prod Res. 2006;20(1):21–6. https://doi.org/10.1080/14786410412331280087.
Florence NT, Benoit MZ, Jonas K, Alexandra T, Désiré DD, et al. Antidiabetic and antioxidant effects of Annona muricata (Annonaceae), aqueous extract on streptozotocin-induced diabetic rats. J Ethnopharmacol. 2014;151(2):784–90. https://doi.org/10.1016/j.jep.2013.09.021.
Nícolas CC, Lara MC, Anna CS, Ari SO, Thiago PS, Rossana CN, Caroline CL, Marcos JS, Ana CM, Elita S, Rodrigo LF. Antimicrobial Annona muricata L. (soursop) extract targets the cell membranes of gram-positive and gram-negative bacteria. Ind Crops Prod. 2017;107:332–40. https://doi.org/10.1016/j.indcrop.2017.05.054.
Tiji S, Rokni Y, Benayad O, Laaraj N, Asehraou A, Mimouni M. Chemical composition related to antimicrobial activity of Moroccan Nigella sativa extracts and isolated fractions. Evid-Based Complemen Altern Med. 2021;2021:1–14. https://doi.org/10.1155/2021/8308050.
Denizkara A, Atik İ, Atik A, Akarca G (2021) Records of Agric and Food Chem. 1(1–2): 19–26. https://doi.org/10.25135/rfac.4.2112.2294
Sanad S, Mekky A, Ahmed A. Pyrazolo[5,1-b]quinazolines and their bis-analogues linked to different spacers: Regio-selective synthesis, antibacterial screening and Swiss ADME prediction study. Chem Select. 2023. https://doi.org/10.1002/slct.202300171.
Sirimongkolvorakul S, Jasancheun A. Screening of in vitro antimicrobial effects of Helicteres isora extract against Staphylococcus aureus. Vet World. 2021. https://doi.org/10.14202/vetworld.2021.2313-2316.
Gedikoğlu A, Sökmen M, Çivit A. Evaluation of thymus vulgaris and Thymbra spicata essential oils and plant extracts for chemical composition, antioxidant, and antimicrobial properties. Food Sci Nut. 2019;7(5):1704–14. https://doi.org/10.1002/fsn3.1007.
Mihaylova Y, Ermenlieva N, Stamova S, Mihaylova S, Georgieva E, Tsvetkova A, et al. In vitro study of antimicrobial activity of commercial essential oils of the Lamiaceae family against Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922 and Candida albicans ATCC 10231. Proceed of CBU Nat Sci ICT. 2022;3:8–11. https://doi.org/10.12955/pns.v3.318.
Mohsenipour Z, Hassanshahian M. The inhibitory effect of Thymus vulgaris extracts on the planktonic form and biofilm structures of six human pathogenic bacteria. Avicenna J Phytomed. 2015;5(4):309.
Jalal M, Ansari M, Alzohairy M, Ali S, Khan H, Almatroudi A, et al. Anticandidal activity of biosynthesized silver nanoparticles: effect on growth, cell morphology, and key virulence attributes of Candida species. Intl J Nanomed. 2019;14:4667–79. https://doi.org/10.2147/ijn.s210449.
Murad K. Antimicrobial effect of tetraspanin CD9 peptides on Pseudomonas aeruginosa. J Pure Appl Microbiol. 2023;17(3):1764–75. https://doi.org/10.22207/jpam.17.3.41.
Bitar R, Fahmi R, Borjac J. Annona muricata extract reduces inflammation via inactivation of NALP 3 inflammasome. J Nat Rem. 2019;19(1):12–23. https://doi.org/10.18311/jnr/2018/22709.
Vignera S, Basile L, Aversa A, Calogero A, Grillo A, Cannarella R, et al. The use of ellagic acid and Annona muricata improves semen quality in men with high-risk papillomavirus infection. J Clin Med. 2022;11(16):4691. https://doi.org/10.3390/jcm11164691.
Oliviero M, Romilde I, Beatrice MM. Evaluations of thyme extract effects in human normal bronchial and tracheal epithelial cell lines and in human lung cancer cell line. Chem-Biol Int. 2016;256:125–33. https://doi.org/10.1016/j.cbi.2016.06.024.
Sarwar A, Latif Z. GC-MS characterization and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Nat Prod Res. 2015;29:447–51. https://doi.org/10.1080/14786419.2014.947493.
Emeka LB, Emeka PM, Khan TM. Antimicrobial activity of Nigella sativa L. seed oil against multidrug resistant Staphylococcus aureus isolated from diabetic wounds. Pak J Pharm Sci. 2015;28:1985–90.
Solomon-Wisdom GO, Ugoh SC, Mohammed B. Phytochemical screening and antimicrobial activities of Annona muricata (L.) leaf extract. Am J Biol Chem Pharm Sci. 2022;2(2014):1–7.
Mutakin M, Fauziati R, Fadhilah FN, Zuhrotun A, Amalia R, et al. Pharmacological activities of soursop (Annona muricata Linn). Mol. 2022;27(4):1201.
Liaqat F, Sheikh AA, Nazir J, Hussain T, Rabbani M, Shaheen AY, et al. Report-isolation identification and control of vancomycin resistant Staphylococcus aureus. Pak J Pharm Sci. 2015;28:997–1004.
Dianez F, Santos M, Parra C. Screening of antifungal activity of 12 essential oils against eight pathogenic fungi of vegetables and mushroom. Lett Appl Microbiol. 2018;67:400–10. https://doi.org/10.1111/lam.13053.
Gucwa S, Milewski T, Dymerski G. Investigation of the antifungal activity and mode of action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Mol. 2018;23:1116. https://doi.org/10.3390/molecules23051116.
Scalas D, Mandras N, Roana J. Use of Pinus sylvestris L. (Pinaceae), Origanumvulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Compl Alt Med. 2018;18:1–13. https://doi.org/10.1186/s12906-018-2219-4.
Pinto L, Bonifacio MA, De Giglio E, Cometa S, Logrieco AF, Baruzzi F. Unravelling the antifungal effect of red thyme oil (Thymus vulgaris L.) compounds in vapor phase. Molecules. 2020;25(20):4761.
Pai BM, Rajesh G, Shenoy R, Rao A. Antimicrobial efficacy of soursop leaf extract (Annona muricata) on oral pathogens: an in vitro study. J Clin Diag Res. 2016;10(11):ZC01.
Cesar KKFA, Batista AKR, Paula LR, da Silva RT, da Silva, FL (2021) Ação antifúngica de extratos e frações de Annona muricata L. sobre Candida spp. Res Soc Developmt. 10(5): e28010514938-e28010514938.
Vimalanathan S, Hudson J. Anti-influenza virus activity of essential oils and vapors. Amer J Essent Nat Prod. 2014;2:47–53.
Feriotto G, Marchetti N, Costa V. Chemical composition of essential oils from Thymus vulgaris, Cymbopogon citratus, and Rosmarinus officinalis, and their effects on the HIV-1 Tat protein function. Chem Biodivers. 2018. https://doi.org/10.1002/cbdv.201700436.
Wahab NZA, Ibrahim N, Kamarudin MKA, Lananan F, Ghazali A, et al. Cytotoxicity and antiviral activity of Annona muricata aqueous leaves extract against dengue virus type. J Fund Appl Sci. 2018;10:580–9. https://doi.org/10.4314/jfas.v10i1s.41.
Balderrama-Carmona AP, Silva-Beltrán NP, Gálvez-Ruiz JC, Ruíz-Cruz S, Chaidez-Quiroz C, et al. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts Plants (Basel). 2020;9(12):1650. https://doi.org/10.3390/plants9121650.
Padmaa MP, Chansouria JPN, Khosa RL. Wound healing activity of Annona muricata extract. J Pharm Res. 2009;2:404–6.
Elmi A, Sayem SAJ, Ahmed M, Mohamed F. Natural compounds from Djiboutian medicinal plants as inhibitors of COVID-19 by in-silico investigations. ChemRxiv. 2020. https://doi.org/10.26434/chemrxiv.12325844.v1.
Ahmed M, Zhong L, Cong S, Yang Y, Doi Y. Colistin and its role in the era of antibiotic resistance: an extended review (2000–2019). Emerg Microbes Infect. 2020;9(1):868–85. https://doi.org/10.1080/22221751.2020.1754133.
Nguyen M, Joshi S. Carbapenem resistance in Acinetobacter baumannii, and their importance in hospital-acquired infections: a scientific review. J Appl Microbiol. 2021;131(6):2715–38. https://doi.org/10.1111/jam.15130.
Getahun Y, Ali D, Taye B, Alemayehu Y. Multidrug-resistant microbial therapy using antimicrobial peptides and the CRISPR/CAS9 system. Vet Med Res Reports. 2022;13:173–90. https://doi.org/10.2147/vmrr.s366533.
Bhatta D, Koirala S, Baral A, Amatya N, Parajuli S, Shrestha R. Methicillin-resistant Staphylococcus aureus contamination of frequently touched objects in intensive care units: potential threat of nosocomial infections. Canad J Infect Dis Med Microbiol. 2022;2022:1–6. https://doi.org/10.1155/2022/1023241.
Roy S, Roy J, Guo B. Nanomaterials as multimodal photothermal agents (PTAS) against ‘superbugs.’ J Mat Chem B. 2023;11(11):2287–306. https://doi.org/10.1039/d2tb02396b.
Xiao Y. Nosocomial infections and bacterial resistance. In: Li L, editor. Infectious microecology advanced topics in science and technology in China. Berlin: Springer; 2014. https://doi.org/10.1007/978-3-662-43883-1_5.
Xia J, Gao J, Tang W. Nosocomial infection and its molecular mechanisms of antibiotic resistance. Biosci Trends. 2016;10(1):14–21. https://doi.org/10.5582/bst.2016.01020.
Walsh TR, Gales AC, Laxminarayan R, Dodd PC. Antimicrobial resistance: addressing a global threat to humanity. PLoS Med. 2023;20(7): e1004264. https://doi.org/10.1371/journal.pmed.1004264.
Sousa SA, Feliciano JR, Pita T, Soeiro CF, Mendes BL, Alves LG. Bacterial nosocomial infections: multidrug resistance as a trigger for the development of novel antimicrobials. Antibiotics (Basel). 2021;10(8):942. https://doi.org/10.3390/antibiotics10080942.
Rana R, Sharma R. Repurposing of existing statin drugs for treatment of microbial infections: how much promising? Infect Disord Drug Targets. 2018. https://doi.org/10.2174/1871526518666180806123230.
Davies J, Davies D. Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev. 2010;74(3):417–33. https://doi.org/10.1128/mmbr.00016-10.
Chan BCL, Ip M, Lau CBS, Lui SL, Jolivalt C, Ganem-Elbaz C, et al. Synergistic effects of baicalein with ciprofloxacin against NorA over-expressed methicillin-resistant Staphylococcus aureus (MRSA) and inhibition of MRSA pyruvate kinase. J Ethnopharmacol. 2011;137(1):767–73. https://doi.org/10.1016/j.jep.2011.06.039.
Olusola-Makinde OO, Bayode MT. Comparative antimicrobial study of Vernonia amygdalina Del. and Lawsonia inermis L. against microorganisms from aqueous milieu. Eur J Biol Res. 2021;11(3):283–93.
Ramirez MS, Tolmasky ME. Aminoglycoside modifying enzymes. Drug Resist Updates. 2010;13(6):151–71.
Prajapati JD, Kleinekathöfer U, Winterhalter M. How to enter a bacterium: bacterial porins and the permeation of antibiotics. Chem Rev. 2021;121(9):5158–92. https://doi.org/10.1021/acs.chemrev.0c01213.
Farhadi F, Khameneh B, Iranshahi M, Iranshahy M. Antibacterial activity of flavonoids and their structure–activity relationship: an update review. Phytother Res. 2019;3(1):13–40. https://doi.org/10.1002/ptr.6208.
Górniak I, Bartoszewski R, Króliczewski J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem Rev. 2019;18(1):241–72. https://doi.org/10.1007/s11101-018-9591-z.
Brown D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void? Nat Rev Drug Discov. 2015;14(12):821–32. https://doi.org/10.1038/nrd4675.
Porras G, Chassagne F, Lyles JT, Marquez L, Dettweiler M, et al. Ethnobotany and the role of plant natural products in antibiotic drug discovery. Chem Rev. 2020;121:3495–560. https://doi.org/10.1021/acs.chemrev.0c00922.
Gradisar H, Pristovsek P, Plaper A, Jerala R. Green tea catechins inhibit bacterial DNA gyrase by interaction with its ATP binding site. J Med Chem. 2007;50(2):264–71. https://doi.org/10.1021/jm060817o.
Patel K, Tyagi C, Goyal S, Jamal S, Wahi D, Jain R, et al. Identification of chebulinic acid as potent natural inhibitor of M. tuberculosis DNA gyrase and molecular insights into its binding mode of action. Comput Biol Chem. 2015;59:37–47. https://doi.org/10.1016/j.compbiolchem.2015.09.006.
Duan F, Li X, Cai S, Xin G, Wang Y, Du D, et al. Haloemodin as novel antibacterial agent inhibiting DNA gyrase and bacterial topoisomerase I. J Med Chem. 2014;57(9):3707–14. https://doi.org/10.1021/jm401685f.
Lomovskaya O, Warren MS, Lee A, Galazzo J, Fronko R, Lee M, et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob Agents Chemother. 2001;45(1):105–16.
Mahamoud A, Chevalier J, Alibert-Franco S, Kern WV, Pages JM. Antibiotic efflux pumps in gram-negative bacteria: the inhibitor response strategy. J Antimicrob Chemother. 2007;59(6):1223–9. https://doi.org/10.1093/jac/dkl493.
Elafify M, Shi C. Response of foodborne pathogens to phytochemicals. In: Ding T, Liao X, Feng J, editors. Stress responses of foodborne pathogens. Cham: Springer; 2022. https://doi.org/10.1007/978-3-030-90578-1_13.
Cabarkapa I, Colovic R, Đuragi O. Anti-biofilm activities of essential oils rich in carvacrol and thymol against Salmonella Enteritidis. Biofouling. 2019;35:361–75. https://doi.org/10.1080/08927014.2019.1610169.
Khameneh B, Diab R, Ghazvini K, Fazly Bazzaz BS. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog. 2016;95:32–42. https://doi.org/10.1016/j.micpath.2016.02.009.
Tegos GP, Haynes M, Strouse JJ, Khan MM, Bologa CG, Oprea TI, et al. Microbial efflux pump inhibition: tactics and strategies. Curr Pharm Des. 2011;17(13):1291–302. https://doi.org/10.2174/138161211795703726.
Ferreira S, Silva F, Queiroz JA, Oleastro M, Domingues FC. Resveratrol against Arcobacter butzleri and Arcobacter cryaerophilus: activity and effect on cellular functions. Int J Food Microbiol. 2014;180:62–8. https://doi.org/10.1016/j.ijfoodmicro.2014.04.004.
Cho H, Uehara T, Bernhardt TG. Beta-lactam antibiotics induce a lethal malfunctioning of the bacterial cell wall synthesis machinery. Cell. 2014;159(6):1300–11. https://doi.org/10.1016/j.cell.2014.11.017.
Fujita M, Shiota S, Kuroda T, Hatano T, Yoshida T, Mizushima T, et al. Remarkable synergies between baicalein and tetracycline, and baicalein and beta-lactams against methicillin-resistant Staphylococcus aureus. Microbiol Immunol. 2005;49(4):391–6. https://doi.org/10.1111/j.1348-0421.2005.tb03732.x.
Zhou D, Xie K, Wang H, Chen Y, Xie M. Inhibitory effects of biochanin a on the efflux pump of methicillin-resistant Staphylococcus aureus (MRSA). Wei Sheng Wu Xue Bao. 2014;54(10):1204–11.
Yun BY, Zhou L, Xie KP, Wang YJ, Xie MJ. Antibacterial activity and mechanism of baicalein. Yao Xue Xue Bao Acta Pharm. 2012;7:1587–92.
Cannalire R, Machado D, Felicetti T, Santos Costa S, Massari S, Manfroni G, et al. Natural isoflavonebiochanin a as a template for the design of new and potent 3-phenylquinolone efflux inhibitors against Mycobacterium avium. Eur J Med Chem. 2017;140:321–130. https://doi.org/10.1016/j.ejmech.2017.09.014.
Stermitz FR, Cashman KK, Halligan KM, Morel C, Tegos GP, Lewis K. Polyacylated neohesperidosides From Geranium caespitosum: Bacterial multidrug resistance pump inhibitors. Bioorganic Med Chem Lett. 2003;13:1915–8. https://doi.org/10.1016/s0960-894x(03)00316-0.
Holler JG, Christensen SB, Slotved HC, Rasmussen HB, Gúzman A, et al. Novel inhibitory activity of the Staphylococcus aureus NorA efflux pump by a Kaempferol rhamnoside isolated from Persea lingue Nees. J Antimicrob Chemother. 2012;67:1138–44. https://doi.org/10.1093/jac/dks005.
Siriwong S, Thumanu K, Hengpratom T, Eumkeb G. Synergy and mode of action of ceftazidime plus quercetin or luteolin on Streptococcus pyogenes. Evid Based Complement Altern Med. 2015;2015:759459. https://doi.org/10.1155/2015/759459.
Yin J, Peng X, Lin J, Zhang Y, Zhang J, Gao H, Tian X, Zhang R, Zhao G. Quercetin ameliorates Aspergillus fumigatus keratitis by inhibiting fungal growth, toll-like receptors and inflammatory cytokines. Int Immunopharmacol. 2021;93: 107435. https://doi.org/10.1016/j.intimp.2021.107435.
Sriramkumar SR, Sivakumar N, Vijayaraghavan P. Enzymatic inhibition of phytochemical from Garcinia imberti on homology-modeled beta-lactamase protein in Staphylococcus sciuri. J Young Pharm. 2020;12:37–41. https://doi.org/10.5530/jyp.2020.12.8.
Calmes B, N’Guyen G, Dumur J, Brisach CA, Campion C, Iacomi B, et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front Plant Sci. 2015;6:414. https://doi.org/10.3389/fpls.2015.00414.
Zhao WH, Hu ZQ, Hara Y, Shimamura T. Inhibition of penicillinase by epigallocatechin gallate resulting in restoration of antibacterial activity of penicillin against penicillinase-producing Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:2266–8.
Zhao WH, Asano N, Hu ZQ, Shimamura T. Restoration of antibacterial activity of beta-lactams by epigallocatechingallate against beta-lactamase-producing species depending on location of beta-lactamase. J Pharm Pharmacol. 2003;55:735–40. https://doi.org/10.1211/002235703765951320.
Bazzaz BSF, Sarabandi S, Khameneh B, Hosseinzadeh H. Effect of catechins, green tea extract and methylxanthines in combination with gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa-Combination therapy against resistant bacteria. J Pharmacopunct. 2016;19:312–8.
Wu SC, Chu XL, Su JQ, Cui ZQ, Zhang LY, et al. Baicalin protects mice against Salmonella typhimurium infection via the modulation of both bacterial virulence and host response. Phytomed. 2018;48:21–31. https://doi.org/10.1016/j.phymed.2018.04.063.
Li K, Guan G, Zhu J, Wu H, Sun Q. Antibacterial activity and mechanism of a laccase-catalyzed chitosan–gallic acid derivative against Escherichia coli and Staphylococcus aureus. Food Control. 2018;96:234–43. https://doi.org/10.1016/j.foodcont.2018.09.021.
Moura FCS, Cechinel-Filho V, Greco FA, Venzon L, Meurer MC, et al. Taxifolin and gastro-adhesive microparticles containing taxifolin promotes gastric healing in vivo, inhibits Helicobacter pylori in vitro and proton pump reversibly in-silico. Chem Interact. 2021;339: 109445. https://doi.org/10.1016/j.cbi.2021.109445.
Tyagi P, Singh M, Kumari H, Kumari A, Mukhopadhyay K. Bactericidal Activity of Curcumin I Is Associated with Damaging of Bacterial Membrane. PLoS ONE. 2015;10: e0121313. https://doi.org/10.1371/journal.pone.0121313.
Kareem S, Mahmood SS, Hindi N. Effects of curcumin and silymarin on the Shigella dysenteriae and Campylobacter jejuni In vitro. J Gastrointest Cancer. 2020;51:824–8. https://doi.org/10.1007/s12029-019-00301-1.
Mun SH, Joung DK, Kim SB, Park SJ, Seo YS, et al. The mechanism of antimicrobial activity of sophoraflavanone B against methicillin-resistant Staphylococcus aureus. Foodborne Pathog Dis. 2014;11:234–9. https://doi.org/10.1089/fpd.2013.1627.
Cha JD, Moon SE, Kim JY, Jung EK, Lee YS. Antibacterial activity of sophoraflavanone G isolated from the roots of Sophoraflavescens against methicillin-resistant Staphylococcus aureus. Phytother Res. 2009;23:1326–31. https://doi.org/10.1002/ptr.2540.
Szatmári Á, Móricz ÁM, Schwarczinger I, Nagy JK, Alberti Á, et al. A pattern-triggered immunity-related phenolic, acetosyringone, boosts rapid inhibition of a diverse set of plant pathogenic bacteria. BMC Plant Biol. 2021;21:1–20. https://doi.org/10.1186/s12870-021-02928-4.
Saravanakumar T, Park HS, Mo AY, Choi MS, Kim DH, et al. Detoxification of furanic and phenolic lignocelluloses-derived inhibitors of yeast using laccase immobilized on bacterial cellulosic nanofibers. J Mol Catal Enzym. 2016;134:196–205.
Neetu N, Katiki M, Dev A, Gaur S, Tomar S, Kumar P. Structural and biochemical analyses reveal that chlorogenic acid inhibits the shikimate pathway. J Bacteriol. 2020;202:e00248-e320. https://doi.org/10.1128/jb.00248-20.
Eumkeb G, Sakdarat S, Siriwong S. Reversing β-lactam antibiotic resistance of Staphylococcus aureus with galangin from Alpinia officinarum Hance and synergism with ceftazidime. Phytomed. 2010;18:40–5. https://doi.org/10.1016/j.phymed.2010.09.003.
Ouyang J, Sun F, Feng W, Xie Y, Ren L, Chen Y. Antimicrobial activity of galangin and its effects on murein hydrolases of vancomycin-intermediate Staphylococcus aureus (VISA) Strain Mu50. Chemother. 2017;63:20–8. https://doi.org/10.1159/000481658.
Morel C, Stermitz FR, Tegos AG, Lewis K. Isoflavones as potentiators of antibacterial activity. J Agric Food Chem. 2003;51:5677–9. https://doi.org/10.1021/jf0302714.
Wu T, He M, Zang X, Zhou Y, Qiu T, et al. A structure-activity relationship study of flavonoids as inhibitors of E. coli by membrane interaction effect. Biochim Biophys Acta. 2013;1828:2751–6. https://doi.org/10.1016/j.bbamem.2013.07.029.
Lee BW, Park IH, Yim D, Choi SS. Comprehensive evaluation of the anti- Helicobacter pylori Activity of Scutellariae Radix. Nat Prod Sci. 2017;23:46. https://doi.org/10.20307/nps.2017.23.1.46.
Lechner D, Gibbons S, Bucar F. Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis. J Antimicrob Chemother. 2008;62(2):345–8. https://doi.org/10.1093/jac/dkn178.
Qian W, Liu M, Fu Y, Zhang J, Liu W, et al. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its anti-biofilm properties. Microb Pathog. 2020;142: 104056. https://doi.org/10.1016/j.micpath.2020.104056.
Diniz-Silva HT, Magnani M, de Siqueira S, de Souza EL, de Siqueira-Júnior JP. Fruit flavonoids as modulators of norfloxacin resistance in Staphylococcus aureus that overexpresses norA. LWT-Food Sci Technol. 2017;85:324–6. https://doi.org/10.1016/j.lwt.2016.04.003.
Kubo I, Muroi H, Himejima M. Antibacterial Activity of Totarol and Its Potentiation. J Nat Prod. 1992;55:1436–40. https://doi.org/10.1021/np50088a008.
Chung KT, Lin WF, Wei CI. Growth inhibition of selected food-borne bacteria by tannic acid, propyl gallate and related compounds. Lett Appl Microbiol. 1993;17:29–32. https://doi.org/10.1111/j.1472-765X.1993.tb01428.x.
Hisano M, Yamaguchi K, Inoue Y, Ikeda Y, Iijima M. Inhibitory effect of catechin against the superantigen staphylococcal enterotoxin B (SEB). Arch Dermatol Res. 2003;295:183–9. https://doi.org/10.1007/s00403-003-0411-x.
Sinsinwar S, Vadivel V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl Microbiol Biotechnol. 2020;104:1–19. https://doi.org/10.1007/s00253-020-10853-z.
Olsen I. New promising beta-lactamase inhibitors for clinical use. Eur J Clin Microbiol Infect Dis. 2015;34(7):1303–8. https://doi.org/10.1007/s10096-015-2375-0.
Guo A, Xiong Y. Myoprotein–phytophenol interaction: implications for muscle food structure-forming properties. Comp Rev Food Sci Food Saf. 2021;20(3):2801–24. https://doi.org/10.1111/1541-4337.12733.
Cheng Y, Song S, Wu P, Lyu B, Qin M, Sun Y, Zhang Q. Tumor-associated macrophages and TAMs-based anti-tumor nanomedicines. Adv Healthcare Mat. 2021. https://doi.org/10.1002/adhm.202100590.
Dong Y, Wang L, Zhang L. Quorum-quenching microbial infections: mechanisms and implications. Phil Trans R Soc B. 2007;362(1483):1201–11. https://doi.org/10.1098/rstb.2007.2045.
Xiong Y. Inhibition of hazardous compound formation in muscle foods by antioxidative phytophenols. Ann New York Acad Sci. 2017;1398(1):37–46. https://doi.org/10.1111/nyas.13368.
Kling D, DeBose-Scarlett E, Teixeira L, Gezan S, Lorca G, Gonzalez C. Sex modulates Lactobacillus johnsonii n6.2 and phytophenol effectiveness in reducing high fat diet induced mTor activation in Sprague-dawley rats. Front Microbiol. 2018. https://doi.org/10.3389/fmicb.2018.02649.
Singh BR, Sinha DK, Agrawal RK, Singh V. Alternative Approaches to Mitigate Antimicrobial Drug Resistance. Bareilly: Division of Epidemiology, ICAR-Indian Veterinary Research Institute; 2021.
Chen K, He J, Tawiah B, Zhou X, Zhou Y. Facile synthesis of a cardanol-based levelling agent as a biodegradable alternative to tristyrylphenol ethoxylates for the dyeing of polyester fabric. Color Technol. 2021;138(3):266–77. https://doi.org/10.1111/cote.12588.
Friesen C, Hormann I, Roscher M, Fichtner I, Alt A, Hilger R, Miltner E. Opioid receptor activation triggering downregulation of cAMP improves effectiveness of anticancer drugs in treatment of glioblastoma. Cell Cycle. 2014;13(10):1560–70. https://doi.org/10.4161/cc.28493.
Acknowledgements
The authors appreciate the literature reviews of scholars cited in the review article.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the review study literature draft. Conceptualization, investigation, drafting of the review outline, proofreading and reviewing of the final draft were performed by Michael Tosin Bayode. Writing and drafting of the literature review for major aspects of the review outline were performed by Elizabeth Foluke Awodire, Emmanuel Femi Ojo, Muyideen Enitan Sadibo, and Philemon Olayemi Aro. Proofreading and reviewing of the edited and revised draft was performed by Michael Tosin Bayode, Blessing Temitope Lawani, Bodun Oluwaseun Lawrence, Patience Iye Abbah, Gladys Oluwafisayo Adenikinju, Shina Samuel Oguntuase and Adeola Eyitayo Adeyolanu. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Authors do not have any competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Bayode, M.T., Awodire, E.F., Ojo, E.F. et al. Phytophenolic derivatives as potential ameliorative agents for microbial superbugs: mechanisms of action, cellular pathways and synergistic selectivity with chemotherapeutics. Discov Appl Sci 6, 484 (2024). https://doi.org/10.1007/s42452-024-06107-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-06107-6