Abstract
Phenanthrene, a common three-ring polyaromatic hydrocarbon [PAH], originates from sources like grilled meals, tobacco, crude oil, coal tar, and automobile exhaust. Recognized as a hazardous PAH, it is often targeted for bioremediation due to its sustainability and potential for full mineralization. In this study, we focus on biodegrading phenanthrene using the strain Alcaligenes ammonioxydans [VITRPS2], isolated from petroleum-contaminated soil. At 5 mg/ml, degradation occurred at a rate constant of 0.0181/day, with half-life values of 2.7 and 4.49 according to first and second-order kinetics, respectively. Employing a one-factor-at-a-time [OFAT] approach, we optimized biodegradation conditions within Luria–Bertani [LB] media. Under optimal conditions—pH 8.0, 8% inoculum concentration, and 37 °C incubation over seven days—the strain achieved maximal growth with phenanthrene as the sole carbon source. It exhibited a degradation efficiency of up to 72% for phenanthrene under these conditions. Gas chromatography-mass spectrometry [GC–MS] analysis revealed principal metabolites of the breakdown pathway, including salicylic acid, catechol, and various phthalic acid derivatives. This underscores the strain's potential for remediating environments polluted by PAH metabolites, showcasing its remarkable capability for complete phenanthrene degradation.
Graphical abstract

Highlights
-
The study explores factors that might hinder the bacteria in the biodegradation process
-
Optimization and kinetics parameters were carried out to study the growth and degradation as well.
-
GC–MS analysis revealed principal metabolites: salicylic acid, catechol, and various phthalic acid derivatives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Over time, there has been a significant expansion in the comprehension of the promising adverse impacts of pollutants on both the natural environment and public health, which is occurring at a worldwide level [1]. Polycyclic aromatic hydrocarbon [PAH] is a hydrocarbon with more than two fused benzene rings. PAH with up to three rings is classified as low/light molecular weight PAH, whereas those with four or more rings are classified as high/heavy molecular weight PAH [2, 3]. PAH are poisonous to aquatic creatures and birds, and they severely degrade marine habitats [4]. Phenanthrene, a hazardous chemical belonging to the class of hydrocarbons that are PAH, is known to be deposited and assembled in the soil as a result of continuous pollution caused by crude oil with its derivatives [5]. Petroleum contamination arises from leaks in both above-ground and below-ground storage tanks, spills of petroleum products during transportation, the abandonment of petrol manufacturing sites, inadvertent discharges, and continuous industrial operations. Petroleum possesses a range of hazardous substances, namely phenanthrene and other PAH, which have the potential to adversely impact the well-being of animals, plants, and humans. Exposure to PAHs has been associated with significant toxicological risks, demonstrating mutagenic, carcinogenic, and, genotoxic properties [6]. According to the United States Environmental Protection Agency [UEPA], a total of sixteen PAHs have been classified as having toxic effects on human health [7]. Phenanthrene and its isomers have been categorized as part of the group of sixteen priority PAHs with toxic properties. The potential dangers linked to exposure to PAHs underscore the importance of implementing a thorough remediation strategy for environments contaminated with PAHs [8].
Over the last few decades, the utilization of bioremediation has experienced a surge in popularity due to its cost-effectiveness, feasibility, and ability to ensure safety while effectively addressing the issue of contamination in various locations [9]. Bioremediation involves the utilization of microorganisms that can metabolize hazardous substances, such as PAHs, and subsequently produce non-toxic compounds. Since the early 2010s, several strains of bacteria have been classified as “PAH Degraders”. These strains belong to various genera, such as Mycobacterium, Rhodococcus, Pseudomonas, Proteus, Alcaligenes, Stenotrophomonas, and Bacillus [10]. Understanding the degradation rate is critical for forecasting intermediate hydrocarbon concentrations during a proposed bioremediation procedure. This may be seen by analyzing the degradation kinetics using first-order and second-order kinetic models. It has been shown that the breakdown rate of low MW PAH is quicker than high MW PAH [11].
Effective degradation and removal of phenanthrene using microbes is significant because of its low solubility, severe toxicity, and the inability of most organisms to use Phenanthrene as the primary carbon source [12]. Hence present study centers on the growth inhibition kinetics and optimization of degradation of phenanthrene by Alcaligenes ammonioxydans [strain VITRPS2], isolated from petroleum-contaminated soil. Also, we have identified the metabolic intermediates through gas chromatography-mass spectrometry [GC–MS] analysis, to elucidate the complete metabolic breakdown pathway of phenanthrene using Alcaligenes ammonioxydans.
2 Materials and methods
2.1 Sample collection
Ennore serves as a significant oil supply port in Chennai, hosting major oil refinery brands with their facilities. Soil samples were aseptically collected from a depth of 5–10 cm. These samples were stored in airtight plastic containers at room temperature, transported to the laboratory, and subsequently kept at − 80 °C in a cold room for bacterial isolation.
2.2 Enrichment and isolation of phenanthrene-degrading bacteria
The soil sample was subjected to enriching in Luria‐Bertani [LB] medium which was kept in an incubated shaker for 100 rpm [rotation per minute] at 37 °C for 7 days. The culture from the enriched media was transferred to a freshly prepared LB and kept in an incubator for 24 h. Using the conventional surface spray-plate technique, the enriching media was taken to perform streak in agar plate and incubated at 37 °C to observe the bacterial growth. Then, a loopful of the colony from the plate was taken and inoculated in a freshly prepared petri plate with the same conditions said above and incubated at the same conditions. The above process is repeated several times until a well-defined separate colony appears. The best phenanthrene degrading colony is selected and taken for further identification and characterization studies.
2.3 Sequencing and phylogenetic analysis
The ABI PRISM® BigDyeTM Terminator Cycle Sequencing Kits with AmpliTaq® DNA polymerase [FS enzyme] [AppliedBiosystems] was used to sequence the amplification product resulting from polymerase chain reaction [PCR]. The nucleotide BLAST search in GenBank was employed for the similarity search of the 16S rRNA nucleotide sequence of the isolate [13]. The phylogenetic relationship was examined by comparing the genetic sequences of the subject organism with those of closely related species available in the GenBank database. Additionally, a phylogenetic tree was constructed. Furthermore, the nucleotide sequence of the isolated bacteria was duly submitted to GenBank, where it was assigned an accession number.
2.4 Utilization of Phenanthrene by A. ammonioxydans as a lone carbon source
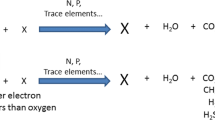
Two sets of LB medium were prepared and inoculated with A. ammonioxydans [8.0%] after the addition of phenanthrene [5 mg/ml]. The second medium served as the abiotic control. Samples were taken every 24 h from incubation at 37 °C with 100 RPM. Biomass concentration was determined by centrifuging to separate the bacterial biomass pellet, followed by drying. The cell-free supernatant was extracted from the pellet using ethyl acetate. Residual phenanthrene was dissolved in methanol [2.0 ml] after evaporating ethyl acetate. The concentration of phenanthrene was quantified using GC–MS by comparing peak areas to reference peaks [14]. The percentage degradation of phenanthrene was calculated using the equation.
Co and C are the initial & residual concentrations [mg/ml].
3 Optimization of phenanthrene-degrading bacteria
3.1 Impact of inoculum size on phenanthrene degradation
The influence of inoculum concentration on the breakdown of phenanthrene was studied by incorporating varying percentages of inoculum [ranging from 2 to 12%] into LB medium supplemented with 5 mg/ml phenanthrene, followed by incubation at 37 °C for 7 days. Subsequently, every 24 h during the incubation period, 1 mL of cell-free supernatant was extracted, and the degradation of phenanthrene was assessed at 254 nm [15].
3.2 Impact of substrate [phenanthrene] concentration on the degradation
The percentage degradation of phenanthrene was examined by elevating its concentration in the LB medium. Initially, 8% of inoculum was introduced into conical flasks containing 100 ml of LB enriched with varying concentrations of phenanthrene [ranging from 2 to 12 mg/ml]. These flasks were then placed in a shaking incubator at 37 °C with agitation set at 100 rpm. Over a period of 7 days, samples of cell-free supernatant were collected at regular intervals, specifically every 24 h, and the degradation of phenanthrene was assessed at 254 nm. [16].
3.3 Impact of temperature on phenanthrene degradation
The impact of temperature on the degradation of phenanthrene by A. ammonioxydans was examined in an LB medium supplemented with 5 mg/ml phenanthrene. Conical flasks were inoculated with an 8% inoculum and then incubated across a temperature range of 25 to 50 °C [17]. Samples of cell-free supernatant were collected periodically, specifically every 24 h, and the degradation of phenanthrene was assessed at 254 nm.
3.4 Impact of initial pH on phenanthrene degradation
The impact of pH concerning the degradation of phenanthrene by A. ammonioxydans was investigated by altering the pH of LB carrying 5 mg/ml phenanthrene to a range of 5.5–8.0 [18]. The culture medium was then injected with 8% inoculum of bacterial suspension & incubated at 37 °C with shaking [100 rpm]. The control group was kept in a comparable environment. The cell-free supernatant was collected every 24 h for up to 7 days, and the degradation of phenanthrene was evaluated at 254 nm.
3.5 Growth, degradation, and inhibition kinetics
The growth of A. ammonioxydans and the degradation of phenanthrene were investigated by increasing the concentration of phenanthrene [19]. The organism was introduced into an LB medium supplemented with varying concentrations of phenanthrene [ranging from 2 to 12 mg/ml] as the sole carbon source. Over a period of 7 days, both the cell suspension and the cell-free supernatant were collected periodically [every 24 h] and measured at 600 nm and 254 nm, respectively, to estimate growth and phenanthrene degradation [20]. The biomass, residual phenanthrene, and degradation percentage were calculated using methods previously described.
The following degradation models were used to study the kinetics of phenanthrene breakdown.
Co and Ct = initial and residual Phenanthrene concentrations (mg/l).
k = degradation rate constant [/day for first order and \(\frac{{\text{ml}}}{{\text{mg}}/{\text{day}}}\) for second-order kinetics respectively].
With the help of Eqs. (4) and (5), the half-lives for Phenanthrene degradation under first and second-order kinetics were calculated respectively.
Using Eq. 6 [The Andrew-Haldane model] was used to examine the effect of Phenanthrene rise on Alcaligenes growth. The plot of ln X/Xo and time was used to calculate Alcaligenes at various Phenanthrene concentrations [5 mg/ml].
where μ = precise growth rate (/day)Ks = substrate saturation constant (mg/L)Ki = substrate inhibition constant (mg/L)μmax = maximum specific growth rate (/day)S = substrate concentration (mg/L).
3.6 Phenanthrene degradation pathway
Individual A. ammonioxydans was inoculated at 8.0% into the medium with 5 mg/ml Phenanthrene. For 7 days, the inoculated medium was kept spinning at 100 RPM at room temperature. As an abiotic control, the uninoculated media was retained. On the seventh day of incubation, degraded metabolites were collected from the sample using ethyl acetate and methanol, as previously reported. GCMS was then used to examine the methanol fraction for degraded metabolites [21].
4 Results and discussion
4.1 Microbial identification
To facilitate the identification of the isolate, a targeted amplification of a specific region within the 16S rRNA gene was conducted utilizing the polymerase chain reaction [PCR] technique. Upon subjecting the amplified product derived from the isolate to 1.5% of agarose gel electrophoresis, an observed fragment length of approximately 1160 base pairs was obtained. The sequence was submitted to the GenBank and assigned an accession number i.e., OR366524. Based on the biochemical characterization and phylogenetic analysis (Fig. 1) the organism was identified as A. ammonioxydans VITRPS2.
4.2 A. ammonioxydans utilize Phenanthrene as a singular carbon supply
Bacteria use the simpler molecules as primary substrates for development, whereas the complex Phenanthrene is degraded as a secondary substrate. Various studies showed that the isolation of bacteria using Phenanthrene as the only carbon source is extremely difficult because of their severe toxicity property [22]. In the present study, A. ammonioxydans effectively used Phenanthrene as a sole carbon source, as evidenced by an increase in biomass of up to 5 mg/ml. Within 7 days, the strain destroyed more than 72% of the given phenanthrene. Most petroleum hydrocarbon-polluted locations have high levels of A. ammonioxydans, indicating their capacity to metabolize complex compounds [23, 24].
5 Optimization of phenanthrene degrading
The efficacy of bioremediation is frequently constrained by various chemical, physical, and, environmental factors. Several key factors have been identified as potentially inhibiting bacterial growth and the efficient digestion of PAHs]. These factors encompass the pH levels, temperature, bacteria inoculum size, and substrate concentration. The management of these factors holds significant importance in facilitating the process of biodegradation by microbial strain.
5.1 Impact of inoculum size on phenanthrene degradation
The dosage of biomass was found to have a significant impact on the degradation of PAHs. The presence of a higher population of bacteria in LB influenced the acclimatization of the cells and the synthesis of enzymes that facilitate cellular metabolism. Determining the amount of the inoculum is crucial to optimize the degradation of PAHs. The investigation focused on examining the impact of the primary biomass population within a range of 2–12% (Fig. 2). The degradation of phenanthrene exhibited optimal performance when a biomass amount of 8% was applied. However, after this dosage, a decline in performance was observed.
5.2 Effect of substrate [phenanthrene] concentration on the degradation
It is widely recognized that certain microbial species possess the ability to endure elevated concentrations of PAHs. The concentration of the substrate is a critical factor in both bacterial growth and the degradation of PAHs. For, a bacterial strain to be considered viable for biodegradation, it must possess the capacity to both tolerate and effectively degrade high concentrations of PAHs]. The study investigated the influence of varying initial concentrations of phenanthrene, ranging from 2 to 7 mg/ml on its degradation capabilities. The strain demonstrated maximum degradation efficiency when exposed to a phenanthrene concentration of 5 mg/ml (Fig. 3) after 3 days of incubation.
5.3 The impact of temperature on phenanthrene degradation
The influence of temperature on the development of phenanthrene-degrading A. ammonioxydans on LB is shown in Fig. 4. This bacterium's growth was shown to be linear from 20 to 50 °C, reaching an optimum at 37 °C after 7 days of incubation, and decreasing considerably above the optimum temperature. The considerable reduction of bacterial growth seems to be attributed to denature the proteins which may be important for the microbe's development and survival. In general, microorganisms have optimal temperatures for fast growth and metabolic activities involving enzymes and proteins.
5.4 The impact of initial pH on phenanthrene degradation
The pH level of the surrounding atmosphere is a crucial parameter in the microbial degradation of Phenanthrene as the sole carbon and energy source. The majority of microorganisms exhibit optimal growth and survival conditions in environments with pH levels that are near neutrality. The study investigated the impact of different initial pH levels ranging from pH 5.5 to 8.5, on the degradation of various PAHs. The results revealed that an optimal pH of 8.0 (Fig. 5) was identified for the degradation of phenanthrene.
In the present study, the investigation revealed that PAH degradation exhibited the highest levels at a pH of 8.0. However, their effectiveness significantly decreased as the pH increased to more alkaline values. According to previous research, it has been determined that the optimal pH range for the degradation of hydrocarbons by bacterial individual strains or consortiums falls within the interval of 6.5 to 8.0.
6 Growth, degradation and inhibition kinetics
6.1 Growth of Alcaligenes on exposure to increasing Phenanthrene concentration
The impact of the rise in Phenanthrene concentrations on A. ammonioxydans growth was investigated by exposing the bacterial strain to a Phenanthrene concentration for 7 days. A constant decline in growth was seen with the Phenanthrene surge, whereas suppression of growth was accounted for at 5 to 10 mg/ml for strain. The degradation of phenanthrene was shown to be most efficient in an A. ammonioxydans isolated from an oil-contaminated location [25, 26].
6.2 Phenanthrene Degradation by A. ammonioxydans
The metabolism of phenanthrene by the A. ammonioxydans was studied for 7 days at 5 mg/ml. On the 7th day, the strain decomposed more than 72% of 5 mg/ml phenanthrene (Fig. 6). We observed that the degradation activity of A. ammonioxydans is dependent on the concentration of hydrocarbons provided [27].
6.3 The Kinetics of Phenanthrene degradation is concentration-dependent
The degradation kinetics were found to be dependent on the concentration of Phenanthrene, implying that first-order and second-order kinetics [28] were more appropriate. The values of k for A. ammonioxydans degradation of 5 mg/ml Phenanthrene was 0.0181/day, with corresponding half-lives of first-order 2.7 days, and the values of k for A. ammonioxydans with corresponding half-lives of second-order 4.49 days respectively (Fig. 7A&B) (Table 1).
6.4 Andrew-Haldane model for the analysis of growth and inhibition kinetics
The Andrew-Haldane model was used to investigate the bacterial growth and inhibition kinetics of Phenanthrene [5 mg/ml]. Andrew-Haldane model is considered to be a more suitable method to investigate the growth kinetics under substrate inhibitory circumstances; however, the Monod model flopped to capture the same in a similar situation [29]. The specific growth rates for A. ammonioxydans were estimated by monitoring microbial biomass concentrations period at 5 mg/ml Phenanthrene concentration, at 0.1832/day. The experimental results were fitted to a non-linear Andrew-Haldane kinetic equation, and then, µmax, Ks, and Ki values were determined. The µmax, Ks, and Ki values for A. ammonioxydans were 0.165/day, 2.17 mg/ml, and 21 mg/ml, respectively. It was observed that the low Ks values [2.17 mg/ml] (Fig. 8), showed the potential of the strain, to grow at low concentration, while the values of Ki indicate the concentration beyond which metabolism and specific growth rate. Many studies suggested that the majority of the growth and inhibition kinetic experiments are on low MW PAH as most of the microorganisms are not able to survive on high MW PAH even at low amounts, contributing to their significant toxicity (Fig. 9).
6.5 Phenanthrene metabolism by A. ammonioxydans
6.5.1 A. ammonioxydans degrades Phenanthrene by salicylate pathway
The breakdown mechanism of Phenanthrene by Alcaligenes ammonioxydans was examined in this study by culturing them in LB medium [Phenanthrene = 5 mg/ml] for 7 days (Fig. 10). On the seventh day, a methanol extract of cell-free supernatant was analyzed and the metabolites were determined by comparing the mass spectra and retention time [Rt] obtained from the NIST library to those obtained from genuine standards. Based on the detected metabolites, 1-hydroxy-2-naphthoic acid, catechol, salicylic acid, gentisic acid,o-phthalic, and protocatechuic acid, and the routes described from PAH-degrading isolates, the pathways depicted in Fig. 9. Despite the absence of dihydrodiols or diols, the first step in phenanthrene biodegradation was observed to be dioxygenation at the 3,4-C locations due to the finding of 1-hydroxy-2-naphthoic acid, which was typically detected in phenanthrene biodegradation by A. ammonioxydans [30, 31]. The metabolic pathway was initially isolated from the generated 1-hydroxy-2-naphthoic acid by ring breakage or decarboxylation to yield 1,2-dihydroxynaphthalene. The earlier might then react with protocatechuic acid and o-phthalic acid to generate the [TCA] cycle while the final might be converted to salicylic acid before being branched again by creating catechol or gentisic acid to the TCA cycle (Fig. 11) [Table 2].
7 Conclusion
The current investigation centered on assessing the degradation potential of bacteria extracted from petroleum-contaminated soils for treating phenanthrene. Results unveiled that a bacterial strain identified as Alcaligenes ammonioxydans displayed notable efficiency in degradation. This isolate utilizes and eliminates phenanthrene as its sole source of carbon and energy. Optimal conditions for phenanthrene breakdown were determined to be a substrate concentration of 5 mg/ml, temperature of 37 °C, pH of 8.0, 8% inoculum size, and a 7-day incubation period. Under these optimal conditions, the strain demonstrated the ability to degrade approximately 72% of phenanthrene within 7 days of incubation. The Andrew-Haldane model accurately depicted the growth and substrate inhibition kinetics. Analysis using gas chromatography–mass spectrometry of phenanthrene degradation products identified significant metabolites, such as catechol, phthalic acid, and salicylic acid derivatives, indicating Alcaligenes as the primary PAHs degrader. This strain exhibits proficiency in breaking down the principal component of petroleum-contaminated soils, rendering it potentially valuable for remediation efforts. The study concluded that utilizing an enrichment approach for PAH degradation in liquid media is feasible and may be further enhanced with this strain.
Data availability
Yes, I have research data to declare.
References
Ghosal D, Ghosh S, Dutta TK, Ahn Y. Current state of knowledge in microbial degradation of PAHs: a review. Front Microbiol. 2016;7(1369):1–27.
Hemalatha D, Sanil S, Kumar BC, Kumaraswami M, Rao VR, Ramu K, Ramanamurthy MV. Spatial distribution of total petroleum hydrocarbons in sediments of Pulicat Lake, Southeast coast of India. Environ Chem Ecotoxicol. 2020;2:175–81.
Nageswar Rao M, Ram A, Rokade MA, Raja P, Rakesh PS, Chemburkar P, Gajbhiye SNA. preliminary estimate of total petroleum hydrocarbons in water and some commercially important fish species in the Amba estuary, West Coast of India. Bull Environ Contam Toxicol. 2016;97:56–62.
Nzila A, Sankaran S, Al-Momani M, Musa MM. Isolation and characterisation of bacteria degrading polycyclic aromatic hydrocarbons: phenanthrene and anthracene. Arch Environ Protect. 2018;44(2):43–54.
Gupte A, Tripathi A, Patel H, Rudakiya D, Gupte S. Bioremediation of polycyclic aromatic hydrocarbon [PAHS]: a perspective. Open Biotechnol J. 2016;10(1):363–78.
Fritt-rasmussen J, Wegeberg S, Gustavson K. Review on burn residues from in situ burning of oil spills in relation to arctic waters. Water Air Soil Pollut. 2015;226:329.
Zelinkova Z, Wenzl T. The occurrence of 16 EPA PAHs in food – a review. Polycyclic Aromat Compd. 2015;35:248–84.
Sumathi K, Manian R. Bioremediation of polycyclic aromatic hydrocarbons contaminated soils: recent progress, perspectives and challenges. Environ Monit Assess. 2023;195(12):1–17.
Rabiu I, Gimba YM (2021) The use of microorganisms in the bioremediation of xenobiotics in the environment” Book Title:“Biotechnology for Sustainable Development” by Immortal Publications, India. In collections with the Dept. of Biosciences and Biotechnology Krishna University India. 2021; ISBN: 978-93-5437-925-3
Singh P, Tiwary BN. Isolation and characterization of glycolipid biosurfactant produced by a Pseudomonas otitidis strain isolated from Chirimiri coal mines. India Bioresour Bioprocess. 2016;3:42.
Khatoon K, Malik A. Screening of polycyclic aromatic hydrocarbon degrading bacterial isolates from oil refinery wastewater and detection of conjugative plasmids in polycyclic aromatic hydrocarbon tolerant and multi-metal resistant bacteria. Heliyon. 2019;5: e02742.
Subashchandrabose SR, Venkateswarlu K, Naidu R, Megharaj M. Biodegradation of high-molecular weight PAHs by Rhodococcus wratislaviensis strain 9: overexpression of amidohydrolase induced by pyrene and BaP. Sci Total Environ. 2019;651:813–21.
Tamura K, Stecher G, Kumar S. MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol. 2021;38(7):3022–7.
Leng Q, Mu J, Yang G. Efficient anaerobic bioremediation of highconcentration benzo[a]pyrene in marine environments. Environ Pollut. 2021;284: 117210.
Koutsoumanis KP, Sofos JN. Effect of inoculum size on the combined temperature, pH and aw limits for growth of Listeria monocytogenes. Int J Food Microbiol. 2005;104(1):83–91.
Bibi N, Hamayun M, Khan SA, Iqbal A, Islam B, Shah F, Khan MA, Lee I. Anthracene biodegradation capacity of newly isolated rhizospheric bacteria Bacillus cereus S13. PLoS One. 2018;13(8):e0201620.
Abatenh E, Gizaw B, Tsegaye Z, Wassie M. Review Article Application of microorganisms in bioremediation-Review. J Environ Microbiol. 2017;1(1):2–9.
Fareez A, Roslee A, Gomez-fuentes C, Zakaria NN, Shaharuddin NA, Zulkharnain A, Khalil KA, et al. Growth optimisation and kinetic profiling of diesel isolated from trinity peninsula. Antarctica Biol. 2021;10:493.
Preethi PS, Vickram S, Das R, Hariharan NM, Rameshpathy M, Subbaiya R, Govarthanan M. Bioprospecting of novel peroxidase from Streptomyces coelicolor strain SPR7 for carcinogenic azo dyes decolorization. Chemosphere. 2023;310: 136836.
Goveas LC, Nayak S, Selvaraj R. Concise review on bacterial degradation of petroleum hydrocarbons: Emphasis on Indian marine environment. Biores Technol Reports. 2022;1(19): 101136.
Sharma S, Verma R, Pandey LM. Crude oil degradation and biosurfactant production abilities of isolated Agrobacterium fabrum SLAJ731. Biocatal Agric Biotechnol. 2019;21: 101322.
Qin W, Fan FQ, Zhu Y, Wang Y, Liu X, Ding A, Dou J. Comparative proteomic analysis and characterization of benzo[a]pyrene removal by Microbacterium sp. strain M.CSW3 under denitrifying conditions. Bioproc Biosyst Eng. 2017;40:1825–38.
Roy A, Sar P, Sarkar J, Dutta A, Sarkar P, Gupta A, Mohapatra B, Pal S, Kazy SK. Petroleum hydrocarbon rich oil refinery sludge of North-East India harbours anaerobic, fermentative, sulfate-reducing, syntrophic and methanogenic microbial populations. BMC Microbiol. 2018;18:1–22.
Wang R, Wu B, Zheng J, Chen H, Rao P, Yan L, Chai F. Biodegradation of total petroleum hydrocarbons in soil: isolation and characterization of bacterial strains from oil contaminated soil. Appl Sci. 2020;10:4173–83.
Meng L, Li W, Bao M, Sun P. Effect of surfactants on the solubilization, sorption and biodegradation of benzo [a] pyrene by Pseudomonas aeruginosa BT-1. J Taiwan Inst Chem Eng. 2019;96:121–30.
Yessica GP, Alejandro A, Ronald FC, Jose AJ, Esperanza MR, Samuel CS, Remedios ML, Ormeno-Orrillo E. Tolerance, growth and degradation of phenanthrene and benzo[a]pyrene by Rhizobium tropici CIAT 899 in liquid culture medium. Appl Soil Ecol. 2013;63:105–11.
Chettri B, Singh AK. Kinetics of hydrocarbon degradation by a newly isolated heavy metal tolerant bacterium Novosphingobium panipatense P5:ABC. Bioresour Technol. 2019;294: 122190.
Baboshin MA, Golovleva LA. Aerobic bacterial degradation of PAHs and its kinetic aspects. Microbiol. 2012;816(81):639–50.
Swain G, Sonwani RK, Singh RS, Jaiswal RP, Rai BN. A comparative study of 4-chlorophenol biodegradation in a packed bed and moving bed bioreactor: performance evaluation and toxicity analysis. Environ Technol Innovat. 2021;24: 101820.
Cho HY, Woo SH, Park JM. Effects of intermediate metabolites on phenanthrene biodegradation. J Microbiol Biotechnol. 2006;16(6):969–73.
Feng TC, Cui CZ, Dong F, Feng YY, Liu YD, Yang XM. Phenanthrene biodegradation by halophilic Martelella sp. AD-3. J Appl Microbiol. 2012;113(4):779–89.
Acknowledgements
The authors gratefully acknowledge the Management of Vellore Institute of Technology, Vellore, India, Vice-Chancellor, Management, and Dean of SBST. We are also thankful to all our laboratory colleagues and research staff members for their constructive advice and help.
Funding
No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
K. Sumathi: Conceptualization, Writing—original draft, Validation, Visualization. M. Rameshpathy: Investigation, Writing—original draft, Validation, Visualization, Supervision, Project administration.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All authors have read understood, and have complied as applicable with the statement on "Ethical responsibilities of Authors" as found in the Instructions for Authors.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sumathi, K., Manian, R. Degradation and inhibition kinetics of phenanthrene by Alcaligenes ammonioxydans, [VITRPS2] strain isolated from petroleum-contaminated soil. Discov Appl Sci 6, 255 (2024). https://doi.org/10.1007/s42452-024-05932-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05932-z