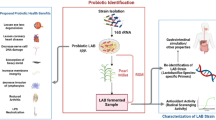
Abstract
People on vegan diets are at risk of being deficient in varied nutrients such as vitamin B12 and certain amino acids. In this study, we investigated vitamin B12-producing lactic acid bacteria (LAB) as well as the probiotic and antioxidant properties. Lactiplantibacillus plantarum HY7720 was screened from 22 strains of LAB that were isolated from different plant foods, and its growth ability and extracellular vitamin B12-producing capacity in vitamin B12-deficient medium were investigated. To determine whether HY7720 functions as a probiotic, survival rate in the simulated gastrointestinal tract and adhesion property to human intestinal epithelial cells of HY7720 were compared with positive control, Lacticaseibacillus rhamnosus GG (LGG). Moreover, the results showed that HY7720 recovered the gene expression levels of tight junction-associated proteins (TJPs), including TJP1, TJP2, occludin (OCLN), and claudin-1 (CLDN1) and inhibited the secretion levels of pro-inflammatory cytokines, including interleukin (IL)-6 and IL-8, in tumor necrosis factor (TNF)-α-stimulated Caco-2 cells. Furthermore, we verified that HY7720 exhibit the antioxidant potential, by showing its intracellular reactive oxygen species (ROS) scavenging ability in hydrogen peroxide (H2O2)-stimulated Caco-2 cells. The ability of HY7720 to ameliorate H2O2-induced cytotoxicity in Caco-2 cells was inhibited by mitogen-activated protein kinase (MAPK) inhibitors, indicating that its antioxidant responses are related to extracellular signal-regulated kinase (ERK) and c-JUN N-terminal kinase (JNK). This study also investigated the nutritional qualities of three plant-based materials (brown rice, white rice, and soy milk) fermented using HY7720. Collectively, HY7720 could be used as a promising probiotic strain for the prevention of nutritional deficiencies among people on vegetarian diets.
Article Highlights
-
Lactiplantibacillus plantarum HY7720 is a promising probiotic candidate and fermentation starter for people on vegetarian as well as omnivorous diets.
-
L. plantarum HY7720 has an extracellular vitamin B12-producing capacity.
-
L. plantarum HY7720 can help the body’s probiotic and antioxidant function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Vegetarian and vegan diets lead to a low intake of saturated fatty acids and animal protein and high intake of fiber and phytochemicals [1]. However, whether the health benefits of vegetarian diets exceed the risks of nutrients deficiencies has not been cleared. In addition, the numerous studies have emphasized that vegetarian diets are at risk of vitamin B12 (cobalamin) deficiency in growing children and adolescents [2]. Moreover, half of the vegans and 7% of vegetarians were categorized as vitamin B12 deficient in the cross-sectional analysis involving 689 men [3]. Vitamin B12, generally supplied in animal-source foods, is synthesized by specific archaea or bacteria, not by plants [2]. Vitamin B12 is necessary for synthesis of nucleic acids, erythrocytes, and nucleoprotein [4]. Recently, as increasing number of people who follow the vegetarian and vegan diets and improve their health through intaking balanced nutrition, the interests of vitamin B12 have been growing steeply in the food industry [5].
A diversity of fermented foods and beverages, including kimchi, jangajji, and makgeolli, in major source of beneficial lactic acid bacteria (LAB) in different regions of Korea are produced. The fermented products are known for various pro-healthy benefits owing to the presence of LAB with probiotic, antioxidant, and antibacterial properties. These properties are primarily demonstrated to probiotics belonging to LAB, particularly Lactobacillus [6]. In addition, the fermented products have a high content of vitamins, minerals, and dietary fibers. Accordingly, a variety of researches have attempted to evaluate the health-promoting constituents and investigate metabolic processes of the probiotics for application in agriculture, nutrition, and pharmaceutical industries [7].
Probiotics is generally expected to prevent intestinal barrier dysfunction, providing maintenance of the host’s health. Thus, it is essential to assess whether a novel LAB strain has protective effect on injury and disruption of intestinal barrier for validating its potential application as probiotic strain in food and pharmaceutical industries. Injury of intestinal epithelial cell leads to increase of intestinal permeability, which is closely related to the development of inflammatory diseases [8]. Tight junctions (TJs), intercellular adhesion protein complexes between epithelial cells, play crucial roles in regulation of epithelial barriers through paracellular pathway [9]. The various studies have reported that the dysfunction of intestinal barrier is associated with disturbance in the expression and translocation of TJ proteins (TJPs) [10]. A study by J.-D.Schulzke has reported that the number of TJ chains is reduced, accompanied by alternation of the expression levels of TJ proteins, in the intestinal tissues from patients with inflammatory bowel disease (IBD) [11]. Therefore, gene regulatory effect of a novel probiotic on intestinal TJ components, including TJP1, TJP2, occludin (OCLN), and claudin-1 (CLDN1), indicates that it plays potential role in maintenance of gut barrier. In addition, pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 are known as risk factors related to the pathogenesis of IBD and colorectal cancer. Hence, it is also important to evaluate the novel probiotic has the inhibitory effects on release of the pro-inflammatory cytokines in model of intestinal injury for using therapeutic implication or preservative solution for diverse intestinal disorders.
The certain probiotics play also antioxidant roles by alleviating oxidative stress. Oxidative stress is an imbalance condition between the generation and removal of reactive oxygen species (ROS) [12]. The long-term ROS exposure cause the development of various intestinal diseases, including IBD, ischemic intestinal injury, and colorectal cancers [13]. As antioxidants, the modes of action of probiotics are belong to chelating metal ion, producing antioxidant metabolites, up-regulating anti-oxidase activities, and regulating signaling pathways [14].
Lactiplantibacillus plantarum has a variety of beneficial properties for host, including improving immune system, balancing intestinal microecology, and increasing absorption of nutrients [15]. Owing to its functions, L. plantarum has been widely used in the dairy and food industry as the probiotic. Probiotics must survive in gastrointestinal environment if they colonize in small intestinal and regulate intestinal flora, thereby providing beneficial effects on human health [16]. Lactobacillus species are known to be resistant to acid condition intrinsically [17]. However, even strains belong to the same species exhibit a diversity of metabolic capacities and probiotic properties, showing strain-specific variability [18]. Therefore, it is important to precisely examine its certain probiotic potentials for application of industrial strain [19].
Fermentation using probiotics can be an efficient nutritional and preservative strategy to improve the quality of food products. L. plantarum, widely used as a starter culture of fermented food, has been known that it improves organoleptic characteristics of products [20]. Furthermore, it has abilities to synthesis a variety of bioactive compounds, including riboflavin, folic acid, and γ-aminobutyric acid, in the fermented foods using the species [21]. In addition, its enzymatic hydrolysis can raise the protein bioavailability by increasing the content of free amino acids [22]. According to the findings, L. plantarum has a potential in development of nutritional and functional properties in fermented foods.
The aim to this study is to suggest a novel probiotic as a suitable alternative to vegetarian or vegan and present potential to its application in plant-based products. Herein, we selected the L. plantarum HY7720 that exerted high potential as vitamin B12 producing bacteria and examined its probiotic and antioxidant properties. We also verified that its fermentative characteristics, including fortification of deficient nutrition in vegan diets and antioxidant activity, in plant-derived ingredients.
2 Materials and methods
2.1 Media and treatments
Modified eagle medium (MEM), antibiotic–antimycotic solution, fetal bovine serum (FBS), and trypsin–EDTA were purchased from Gibco (Grand Island, NY, USA). Recombinant human TNF-α protein was obtained from R&D Systems (Minneapolis, MN, USA). Hydrogen peroxide (H2O2), α-amylase from human saliva, pepsin from porcine gastric mucosa, pancreatin from porcine pancreas, bile extract porcine, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) diammonium salt, potassium persulfate, ERK inhibitor (PD98059), JNK (SP600125), and p38 inhibitor (SB203580) were obtained from Sigma-Aldrich (St. Louis, MO, USA). 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) was purchased from Invitrogen (Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits for IL-6 and IL-8 were purchased from BD Biosciences (San Diego, CA, USA).
2.2 Isolation and preparation of bacterial strains
Twenty-two LAB strains were isolated from fermented plant food collected from a variety of regions in Korea and stored in a microbial strain library at hy Co., Ltd. (Yongin, Republic of Korea) and used in the present study. Each sample was aseptically mixed with PBS with the ratio of 1:9 and homogenized using a stomacher. 1 mL of the samples was serially diluted and spread onto MRS agar. After incubation for 2 days at 37 °C, representative colonies were inoculated into MRS broth and each culture were stored with sterilized 10% skim milk at − 80 °C. Lacticaseibacillus rhamnosus GG (ATCC53103) (LGG) was obtained from the American Type Culture Collection (Manassas, VA, USA). LAB strains were obtained by streaking each freezer stock onto de Man, Rogosa and Sharpe (MRS) agar plates and incubating at 37 °C for 72 h. Single colonies were inoculated into MRS medium and incubated overnight at 37 °C. The cultures were centrifuged and the pellets were washed twice with sterile phosphate-buffered saline (PBS). Subsequently, the cell pellets were resuspended in PBS saline, or plant-based materials (brown rice, whited rice, and soy milk) for respective experiments.
2.3 Selection of vitamin B12-producing lactic acid bacteria
The single-colony cultures were inoculated into 3 mL of vitamin B12-free modified chemically defined media (CDM) based on a previous study with some modification [23]. After incubation for 3 days at 37 °C, Lactiplantibacillus plantarum HY7720, which was originally isolated from fermented vegetable foods, and showing exceptional absorbance at 600 nm, was preliminarily determined as a vitamin B12 producer.
2.4 Quantitative analysis of vitamin B12
To investigate extracellular vitamin B12 derived from HY7720, the cells were incubated overnight at 37 °C in vitamin B12-free modified CDM, and the supernatant was centrifuged at 13,000 × g for 20 min. The vitamin B12 content of the supernatant was analyzed via high-performance liquid chromatography (HPLC) using a slightly modified method of a previous study [24]. The supernatant was filtered using a 0.22 μm membrane filter and stored in the dark. The operating conditions were as follows: column, Supelco Discovery C18 column (250 mm × 4.6 mm I.D., 5 μm); flow rate, 0.5 mL/min; column temperature, 30 °C; injection volume, 10 μL; wavelength of detector, UV 254 nm (Agilent 1260 G4212B); mobile phase, 0.1% formic acid in methanol (A) and 0.1% formic acid in water (B); gradient, 0–2 min 20% A, 2–3 min 20–28% A, 3–11 min 28–35%, 11–19 min 35–43% A, 19–20 min 43–100% A, 20–22 min 100–100% A, 22–26 min 100–20% A, and 26–36 min 20% A.
2.5 Survivability in the gastrointestinal tract simulation model
The probiotic potential of HY7720 was determined by measuring survival rate in the simulated gastrointestinal tract (GIT) model system. The simulated digestion fluids, including simulated salivary fluid (SSF), simulated gastric fluid (SGF), and simulated intestinal fluid (SIF), were prepared with electrolyte stock solutions, enzymes, bile extract, CaCl2, and water, as previously described [25]. The each simulated digestion fluids used as follows: SSF, 15.1 mmol/L KCl, 3.7 mmol/L KH2PO4, 13.6 mmol/L NaHCO3, 0.15 mmol/L MgCl2(H2O)6, and 0.06 mmol/L (NH4)2CO3; SGF, 6.9 mmol/L KCl, 0.9 mmol/L KH2PO4, 25 mmol/L NaHCO3, 47.2 NaCl, 0.1 mmol/L MgCl2(H2O)6, and 0.5 mmol/L (NH4)2CO3; and SIF, 6.8 mmol/L KCl, 0.8 mmol/L KH2PO4, 85 mmol/L NaHCO3, 38.4 NaCl, and 0.33 mmol/L MgCl2(H2O)6. The cell culture suspensions of HY7720 and LGG, a well-known probiotic strain, were mixed with SSF (75 U/mL α-amylase, 0.75 mM CaCl2) to adjust pH to 7.0 (50:50, v/v). After 2 min of incubation at 37 °C, the mixtures were mixed with SGF (1000 U/mL porcine pepsin, 0.075 mM CaCl2) to adjust pH to 3.0 (50:50, v/v) and incubated for 2 h at 37 °C with continuous shaking. Subsequently, the mixtures were mixed with SIF (40 U/mL porcine pancreatin, 0.3 mM CaCl2, 0.24% bile extract) to adjust pH to 7.0 (50:50, v/v). Aliquots of these mixtures obtained at the end of each step were used to estimate the survival rates.
2.6 Adhesion assay to intestinal epithelial cells
The adhesion ability to human intestinal epithelial cells was assessed as previously reported [26]. Briefly, human colonic carcinoma Caco-2 cells were obtained from the ATCC and maintained in MEM supplemented with 10% FBS and 1% antibiotic–antimycotic at 37 °C under 5% CO2. Caco-2 cells were plated onto 24-well plates (1.0 × 105 cells/well). When the cells reached 100% confluency, the medium was replaced with MEM without FBS. HY7720 and the reference strain LGG were diluted in PBS and inoculated into Caco-2 cells at a density of 1.0 × 108 cells/mL/well. After 2 h of incubation at 37 °C, the cells were washed four times with PBS and treated with 0.05% trypsin–EDTA for 5 min to separate them from the plates. The cell viability of HY7720 and the reference strain was determined using MRS agar plates.
2.7 Reverse transcription-PCR (RT-qPCR) analysis
Caco-2 cells were plated at 1.0 × 105/well in 12-well plates and maintained in a cell culture medium (MEM supplement with 10% FBS and 1% antibiotic–antimycotic). To obtain mature differentiated monolayer, the culture medium was changed with fresh medium every 2–3 days for a total of 21 days. The differentiated cells were pretreated with or without 1.0 × 107 CFU/mL of HY7720 or LGG in a serum-free medium for 1 h, followed by TNF-α (100 ng/mL) treatment for 72 h. Phosphate-buffered saline (PBS) was used vesicle control. Total RNA was extracted using an Easy-BLUE™ Total Extraction kit (iNtRON Biotechnology Inc., Seongnam, Republic of Korea). cDNA was synthesized from 1 μg of total RNA using an Omniscript® Reverse Transcription kit (Qiagen, Hilden, Germany). The mRNA levels were quantified using QuantStudio 6 Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) and a TaqMan Universal PCR Master Mix (Applied Biosystems). The levels of TJP1 (Hs01551861_m1), TJP2 (Hs00910543_m1), OCLN (Hs00170162_m1), CLDN1 (Hs00221623_m1), and GAPDH (Hs99999905_m1) transcripts were quantified using gene-specific primers.
2.8 Cytokine measurement
The culture media were collected under the conditions described above and used to measure the levels of IL-6 and IL-8 using ELISA kits according to the manufacturer’s instructions.
2.9 ABTS+ radical scavenging assay
ABTS+ radical scavenging activities of HY7720, LGG, and the fermented samples were determined using the modified method of a previous study [27]. Briefly, to prepare ABTS radical cation stock solution, 7 mM of ABTS was mixed with 2.4 mM of potassium persulfate in equal quantities and allowed to react in the dark at room temperature for 16 h. The stock solution was diluted with distilled water appropriately to show absorbance of 1.0 ± 0.02 at 734 nm. All samples and the positive control (L-ascorbic acid) were reacted with the working solution in the dark at room temperature for 15 min, and absorbance was measured at 734 nm. Antioxidant activity was calculated as follows: antioxidant activity (%) = [1 − (Asample − Ablank)/(Avehicle sample − Avehicle blank)] × 100.
2.10 Intracellular ROS scavenging assay
Caco-2 cells (2.0 × 104/well; 96-well plate) were pretreated with HY7720 or LGG (2.0 × 105 CFU/mL) for 1 h, followed by H2O2 treatment (500 μM) for 1 h. The cells were incubated with 10 μM of H2DCFDA solution in the dark at 37 °C for 30 min and washed with PBS. DCF fluorescence was measured (excitation 485 nm, emission 528 nm) using a Synergy HTX multi-mode reader (BioTek Instruments, Inc., Winnoski, VT, USA).
2.11 Lactate dehydrogenase (LDH) leakage assay
Caco-2 cells (2.0 × 104/well; 96-well plate) were pretreated with specific MAPK inhibitors (20 μM of PD98059, 20 μM of SP600125, or 10 μM of SB203580) for 30 min, prior to HY7720 or LGG (2.0 × 105 CFU/mL) treatment. After 1 h incubation, the cells were treated with 1 mM of H2O2 for 2 h, and the culture media were collected and subjected to LDH measurement. A CytoTox 96® Non-Radioactive Cytotoxicity Assay kit (Promega, Madison, WI, USA) was used following the manufacturer’s instructions.
2.12 Fermentation of plant-based materials
To investigate the effects of HY7720 on the fermentation quality of plant-based materials, brown rice (BR) and white rice (WR) extracts were obtained from Eco trading co., Ltd. (Seongnam, Republic of Korea), and soy milk (SM) was obtained from hy Co., Ltd. All fermentation solutions of these planted-based materials were diluted with distilled water in a 1:10 (w/w) ratio, and then centrifuged. The fermentation solutions were inoculated with a pure culture of HY7720 at a final concentration of 1.0 × 109 CFU/mL and cultured at 37 °C for 24 h. After centrifugation of the samples (3 plant-based materials before fermentation and 3 plant-based materials after fermentation), the supernatants were filtered using 0.22 μm pore-sized membrane filter to examine different parameters.
2.13 Analytical determination of lactate and amino acids
The level of lactate in the samples was quantified via HPLC using the method of a previous study [28]. The operating conditions were as follows: column, Aminex HPX-87H (Bio-Rad, 300 mm × 7.8 mm); flow rate, 0.6 mL/min; column temperature, 50 °C; injection volume, 20 μL; wavelength of detector, UV 210 nm; and mobile phase, 4 mM of sulfuric acid. The level of amino acids was analyzed using an L-8500 high-speed amino acid analyzer (Hitachi Ltd., Tokyo, Japan) with #2622SC (Hitachi, 4.6 mm I.D. × 60 mm), as described previously [29]. For the pretreatment, the samples and 5% trichloroacetic acid (TCA) were mixed in equal quantities and centrifuged at 10,000 rpm for 15 min, followed by acid hydrolysis with 0.02 N of hydrochloric acid (HCl). The operating conditions were according to the manufacturer’s instructions.
2.14 Microbial growth analyses
To compare the growth ability of HY7720 in media containing nitrogen source derived from a plant or animal source, all tested media were prepared based on MRS medium with modifications. The specific recipe was as follows: glucose, 20 g/L; yeast extract, 15 g/L; complex nitrogen source, 10 g/L; dipotassium hydrogen phosphate, 2 g/L; sodium acetate, 5 g/L; diammonium hydrogen citrate, 2 g/L; magnesium sulfate, 0.01 g/L; manganese sulfate, 0.04 g/L; and polysorbate 80, 1 g/L. The following nitrogen sources were tested: soy peptone such as Hy-Soy™ Kosher IPS (Kerry Bio-Science, Norwich, NY, USA) and HSP-349 (The Tatua Co-Operative Dairy Co., Ltd., Morrinsville, New Zealand), Broadbean peptone (Solabia Group, Pantin, France), casein peptone such as Bacto™ Tryptone from casein (Beckton Dickinson, CA, USA) and HCP-321 (The Tatua Co-Operative Dairy Co., Ltd.), and lactoalbumin peptone (Tatua-2016, The Tatua Co-Operative Dairy Co., Ltd.). The growth curves were recorded at 37 °C by measuring absorbance at 600 nm (OD600), and the maximum specific growth rate and the slope of the exponential growth phase (μmax = Δln(OD600)/Δt) were determined.
2.15 Molecular characterization of bacterial isolates
Whole genome sequencing (WSG) were conducted at Sanigen Inc. (Seoul, Republic of Korea). The genomic DNA of L. plantarum HY7720 was sequenced with Illumina MiSeq and Oxford Nanopore MinION platforms. For Illumina sequencing, the extracted genomic DNA was fragmented using Covaris M220 (Covaris, MA, USA). The sheared DNA were used to prepare a WGS library with an average insert size of 550 bp using a TruSeq Nano DNA Sample Prep kit (Illumina, CA, USA). The library was sequenced on an Illumina MiSeq platform using 300 bp paired-end sequencing mode. For nanopore sequencing, a MinION sequencing library was prepared using the Nanopore Ligation Sequencing Kit (SQK-LSK110; Oxford Nanopore Technologies, Oxford, UK). The library was sequenced with an R9.4.1 MinION flow cell (Flongle) for a 24 h run using MinKNOW with the default settings (MinKNOW core 5.0.0, Guppy 6.0.6). The qualified sequencing data were de novo assembled with Unicycler v0.4.8. The genome was annotated using Prokka v 1.14.6 and their coding sequence were identified. Based on the virulence factor database (VFDB), we surveyed the HY7720 isolates for the presence of four virulence factors such as hemolysin/cytolysin (cylA), aggregation substance (asa1), hyaluronidase (hyl), and gelatinase (gelE).
2.16 Antibiotic susceptibility of baterial isolates
HY7720 was evaluated for its susceptibilities to seven antibiotics according to the guidelines followed by the European Food Safety Authority (EFSA). The minimal inhibitory concentrations (MICs) of ampicillin, gentamicin, kanamycin, erythromycin, clindamycin, tetracycline, and chloramphenicol were then determined using MIC Test Strip (Liofilchem, Teramo, Italy) according to the manufacturer’s instructions. The MIC value was the point where the zone of inhibition that intersects each strip.
2.17 Statistical analysis
All data are expressed as mean ± standard deviation (SD). Significant differences were identified using one-way analysis of variance (ANOVA), followed by Newman–Keuls test (multiple groups) or Student’s t-test using GraphPad Prism v5 (San Diego, CA, USA).
3 Results and discussion
3.1 HY7720 is a novel extracellular vitamin B12-producing Lactiplantibacillus strain
Vegetarian or vegan diets may be selected for ethical, health, or religious reasons. In recent years, the demands for vegetarian or vegan diets have risen sharply owing to their health benefits in terms of prevention of several diseases, such as cardiovascular disease, type 2 diabetes, and cancer [30]. However, since vegetarians are more likely to have a higher socio-economic status and to follow a health-conscious lifestyle with regular exercise and no smoking, it is not clear whether the benefits of vegetarian diets overcome the risks of nutritional deficiencies, including deficiencies in vitamin B12, certain amino acids, iron, and omega-3 [2]. In particular, several studies have highlighted the health risks of vitamin B12 deficiency due to veganism and vegetarianism [2, 3, 31]. As previously stated, major strains that have high natural production level of vitamin B12 are Pseudomonas freudenreichii and P. denitrificans [32]. Additionally, several studies reported that certain Lactobacillus species show ability to produce vitamin B12, including Limosilactobacillus reuteri, Lactobacillus coryniformis, Lactiplantibacillus plantarum, and Lactobacillus rossiae [33]. Since intracellular environment is a main barrier to in situ fortification of these strains in foods, it is important to verify their ability for extracellular production [24]. In this study, we tested the growth of 22 LAB strains from plant-based sources in CDM medium without vitamin B12, but all strains did not grow. After that, the growth of these strains was evaluated using the vitamin B12-free modified CDM medium (contained cobalt, an essential component of vitamin B12) (Supplementary Table 1). After discovering HY7720 had the highest growth in that medium, we verified that HY7720 produced 38 μg/L of vitamin B12 (CN-Cbl) in the vitamin B12-free modified CDM broth extracellularly (Supplementary Fig. 1). Although CN-Cbl is a physiologically inactive form of cobalamin in contrary to adenosylcobalamin (Ado-Cbl) and methylcobalamin (Met-Cbl), CN-Cbl has higher stability toward heat and light than the active forms; therefore, it is considered most utilized commercial form of vitamin B12 [34]. However, since vitamin B12 production of HY7720 is less than industrial vitamin B12-producing strains, further examination is necessary to enhance its production of extracellular vitamin B12. The genome of HY7720 consists of 6 contigs with total size of 3,508,619 bp and a GC% of 44.15%. The average nucleotide identity (ANI) gave a value of 98.95% between HY7720 and L. plantarum CIP 103151 (NR_104573.1), identifying the strain as L. plantarum.
3.2 HY7720 shows resistance in simulated gastrointestinal tract and adherence ability to human intestinal cells
Probiotics provide health benefits for the host when they are taken in adequate amounts; as living microorganisms, they help maintain the balances of intestinal microflora. Although various Lactobacillus species are known to have probiotic properties, only certain strains with ability to survive in the gastrointestinal tract can be considered probiotic strains [35]. Indeed, survivability and ability to adhere to the intestinal epithelium are the main criteria used to select probiotic strains [36]. The survival rates of HY7720 and LGG were not affected under the conditions of the oral phase. HY7720 had a similar gastric survival rate to LGG. Moreover, HY7720 showed non-significantly higher resistance to intestinal conditions than LGG (Fig. 1A). We also estimated the ability of the two strains to adhere to human intestinal epithelial Coco-2 cells. As shown in Fig. 1B, HY7720 exhibited a significantly higher ability to adhere to the Caco-2 monolayer than the reference strain LGG. In addition, seven antibiotics were tested according to the EFSA guidance, and HY7720 was found to be susceptible to all antibiotics (Supplementary Table 2). The WGS analysis also showed that the virulence genes, including cylA, asa1, hyl, and gelE were not detected in HY7720 isolates.
Survivability of HY7720 and LGG in the gastrointestinal tract simulation model and adhesion ability to Caco-2 cells. A The survival rates of each step were estimated compared to the initial number of each strain. B Adhesion ability was determined as the percentage of adhesion to Caco-2 cells. Data are represented as mean ± SD of three independent experiments. **p < 0.01 and ***p < 0.001 when compared to the LGG group
3.3 HY7720 inhibits TNF-α-induced down-regulation of tight junction-associated genes and pro-inflammatory cytokines production in Caco-2 cells
The pro-inflammatory cytokine TNF-α triggers an increase in epithelial barrier permeability by interrupting TJs [37]. Additionally, previous studies have reported that TNF-α leads to the activation of the nuclear transcription factor kappa B (NF-κB), followed by the expression of diverse pro-inflammatory genes [38]. Thus, TNF-α is widely used to induce an increase in intestinal epithelial TJ permeability and intestinal inflammation. In this study, we observed that HY7720 significantly increased the mRNA expression levels of TJ components, including scaffolding molecules, zonula occludens (ZOs) (also known as TJPs), and transmembrane proteins (OCLN and CLDN1), were reduced by TNF- α in Caco-2 cells (Fig. 2A–D). LGG treatment also tended to attenuate TNF-α-induced down-regulation of tight junction-associated genes. However, there was no statistical significance in TJP1 and TJP2 expression levels. The recovery effects of ZOs, OCLN, and CLDN1 expression levels by HY7720 were in line with a previous research showing the maintenance effects of their protein expression in a murine colitis model by probiotic mixtures [39]. HY7720 and LGG also significantly reduced the secretion levels of the pro-inflammatory cytokines, IL-6 and IL-8, which were increased by TNF-α in Caco-2 cells (Fig. 2E, F). Our data suggest that HY7720 can be used to reinstate the intestinal epithelial barrier, providing a novel treatment option for gut barrier dysfunction.
Effects of HY7720 on mRNA expression of tight junction-associated proteins and production of pro-inflammatory cytokines in TNF-treated Caco-2 cells. Relative mRNA levels of A TJP1, B TJP2, C OCLN, and D CLDN1 were normalized against that of GAPDH. E IL-6 and F IL-8 secretion in the culture media was determined using ELISA kits. Data are represented as mean ± SD of three independent experiments. ##p < 0.01 and ###p < 0.001 when compared with the vehicle group. *p < 0.05 and **p < 0.01 when compared to the TNF-α group
3.4 HY7720 is protective against oxidative stress-induced cytotoxicity through regulation of ERK and JNK
The roles of probiotic formulations in promoting antioxidation have also been highlighted in a number of studies [14, 40]. LGG has been proven in previous studies to have good antioxidant capacity [40]. In our work, to unveil if HY7720 had the potential to be an antioxidant, the antioxidant activities of HY7720 and LGG were evaluated by measuring their ABTS+ radical scavenging activity. At low and high concentrations of HY7720, ABTS+ radical scavenging capacity was 68.79 ± 0.11% and 93.71 ± 8.66%, respectively, showing that HY7720 expressed higher antioxidant activity than LGG (Fig. 3A). In addition, we examined its intracellular ROS scavenging activity and anti-cytotoxicity in H2O2-treated Caco-2 cells. HY7720 and LGG significantly restored the intracellular ROS level and LDH leakages, which are markers of apoptosis, increased by H2O2 in Caco-2 cells (Fig. 3C). Moreover, we hypothesized that HY7720 regulated MAPKs among the antioxidant signaling pathways known to be regulated by probiotics. Interestingly, we found that inhibiting ERK and JNK activation blocked the cytoprotective effect of HY7720 against apoptotic cell death induced by H2O2, but inhibiting the p38 inhibitor did not reveal this effect (Fig. 3D). A previous study reported that surface layer proteins from Lactobacillus strains can elevate ERK phosphorylation, thereby decreasing apoptotic cell death [41]. Also, in line with our findings, a previous study reported that a certain Lactiplantibacillus plantarum strain activates only two MAPKs (ERK and JNK) in porcine intestinal epithelial cells, promoting the expression of human defense protein (HDP) [42]. Therefore, our data suggest that the protective effects of HY7720 against oxidative injury are regulated by different signaling pathways.
Antioxidant effects of HY7720 on H2O2-induced oxidative stress in Caco-2 cells. A Radical scavenging activity of HY7720 and LGG as determined by ABTS assay B Cell viability was evaluated via MTT assay. C The ROS levels are expressed as percentages of DCF fluorescence intensity to that of control cells with vehicle. D Cytotoxicity was determined by measuring the activity of LDH released from damaged cells. Data are represented as mean ± SD of three independent experiments. ###p < 0.001 when compared to the vehicle group, **p < 0.01 and ***p < 0.001 when compared to the H2O2 group, †p < 0.05 and †††p < 0.001 when compared to the HY7720 + H2O2 group. PD98059: ERK inhibitor; SP600125: JNK inhibitor; SB203580: p38 inhibitor
3.5 Fermentation with HY7720 improves the nutritional qualities and antioxidant activities of plant-based materials
We also examined whether fermentation with HY7720 enhances the nutritional status of plant-based foods as a compensatory strategy for vegetarianism or veganism (Table 1). Here, production of lactate, as a main organic acid, increased during the fermentation of plant-based materials with HY7720, as expected. After the fermentation of each solution of brown rice extract, white rice extract, and soy milk (BR, WR and SM, respectively), the levels of lactate increased markedly. Furthermore, lysine and methionine are considered to be the major limiting essential amino acids among the nutrients known as being deficient in plant-based diets in general. In addition, it is reported that lysine and methionine intakes account for the largest differences between vegetarians and omnivores, which are about 48% and 43% less in vegetarians, respectively [43]. In this study, the free-lysine contents were increased in the plant-based samples (BR, WR, and SM) after fermentation, unlike methionine. The levels of free methionine were not detected either before or after fermentation. The elevations of free lysine could be explained as being a consequence of bacterial enzymatic hydrolysis. In addition, it could not be completely excluded that the results were obtained due to the lysine biosynthetic capability of HY7720. However, it is necessary to perform further investigation to determine lysine biosynthesis by HY7720. Previous study has demonstrated that L. plantarum JSA 22, isolated fermented soybean foods, has ability to synthesize lysine using aspartate as a precursor in rice germ milk [44]. Accordingly, to elucidate that HY7720 is a suitable starter for fortified-lysine product, further studies are need to identify its lysine biosynthetic pathway. Our data, at least, demonstrate that fermentation using HY7720 enhances the level of lysine, which, as a highly bioavailable form, can benefit the nutritional status of vegetarians or vegans.
Moreover, the levels of vitamin B12 were compared before and after fermentation using HY7720 in all plant-based materials. Interestingly, among the three solutions, only SM was the solution with increased vitamin B12 content after fermentation. The level of vitamin B12 reduced in fermented BR than in uncultured BR. In the case of WR, the level of vitamin B12 was not detected either before or after fermentation. Furthermore, we hypothesized that the antioxidant activities would increase in the plant-based materials after fermentation by HY7720. According to previous studies, antioxidant activities could be enhanced in foods fermented by Lactiplantibacillus plantarum strain, highlighting its functional effects [45]. We verified that fermentation using HY7720 markedly increased antioxidant activities in BR and WR, but its effect seemed less important in SM. Collectively, these results indicate that L. plantarum HY7720 is not only a probiotic formulation but also has potential in the development of fermented foods and beverages with health benefits. More specifically, our findings support for the proposition that L. plantarum HY7720 would be used as a proper probiotic starter into plant-based juice for prevention of nutritional deficiencies that may occur in vegan or vegetarian. Further study is needed to evaluate the optimal fermentation conditions for each plant-based sample or combination and its sensory characteristics and shelf life to validate acceptable quality.
3.6 Nitrogen sources derived from plant ingredients support the growth of HY7720 better than that animal ingredients
Food products that are suitable for vegans do not contain animal-derived sources. According to the vegan definition agreed upon by the consumerism ministers of the German federal states, vegan foods also do not have ingredients and processing aids from animal origin added at all steps of their production [46]. Thus, to investigate the possibility of industrialization for vegan probiotic products, we compared the growth parameters (μmax and ODmax) during culture of HY7720 in plant-derived and animal-derived nitrogen sources media. As shown in Fig. 4, the nitrogen-based media derived from the hydrolysis of soy or broadbean performed better than the media based on animal-derived nitrogen sources (casein or lactoalbumin peptone) in terms of growth yield. Growth on the plant-derived nitrogen sources, HSP-349, Hy-Soy™ Kosher IPS, and Broadbean peptone, yielded a maximum population density of 0.871, 0.825, and 0.879, respectively, and a maximum specific growth rate of 0.780, 0.814, and 0.771, respectively (Table 2). Although the media based on Hy-Soy™ Kosher IPS showed a higher tendency for maximum specific growth and maximum population density than the media based on casein or lactoalbumin peptone, there was no statistically significant relation. A variety of types of LAB have adapted to live and grow on plant microbiomes even though plants are generally thought to be poor settings for numerous microbes. In this study, it has not been determined the relationship between certain factors, such as nutrients and metabolites, affecting the growth parameters of HY7720 and its origin. However, our findings imply that the HY7720 is adapted for growth on specific plants, especially legumes. Further development of media for growing HY7720 on the basis of the above data may lead to its utilization in probiotic manufacturing process that follows the vegan ethos.
Growth curves of HY7720 in plant-derived, or animal-derived, nitrogen-based media at 37 °C, as recorded with an optical density (OD) at 600 nm. The growth media are named as each complex nitrogen source. The plant-derived, nitrogen-based media include soy peptone (HSP-349 and Hy-Soy™ Kosher IPS) and broad bean peptone. The animal-derived, nitrogen-based media include casein peptone (Bacto™ Tryptone and HCP-321) and lactoalbumin peptone (Tatua-2016)
4 Conclusion
This study showed that Lactiplantibacillus plantarum HY7720 has the ability to produce extracellular vitamin B12 and shows potential probiotic and antioxidant properties. Fermentation with HY7720 also enhances the nutritional qualities, such as lysine and vitamin B12, and antioxidant properties of plant-based materials. In addition, we verified that plant-based media provide a good alternative to animal-based media for growing HY7720. Therefore, HY7720 could be suggested as a potential probiotic supplement, as well as a starter culture candidate, in applications of plant-based products for vegetarian or vegan diets.
Data availability
All data generated or analyzed in the current study are available on request to the corresponding author on reasonable request.
References
Craig WJ. Nutrition concerns and health effects of vegetarian diets. Nut Clin Pract. 2010;25:613–20. https://doi.org/10.1177/0884533610385707.
Rudloff S, Bührer C, Jochum F, Kauth T, Kersting M, Körner A, Koletzko B, Mihatsch W, Prell C, Reinehr T. Vegetarian diets in childhood and adolescence. Mole Cell Pediatr. 2019;6:1–7. https://doi.org/10.1177/0884533610385707.
Gilsing AM, Crowe FL, Lloyd-Wright Z, Sanders TA, Appleby PN, Allen NE, Key TJ. Serum concentrations of vitamin B12 and folate in British male omnivores, vegetarians and vegans: results from a cross-sectional analysis of the EPIC-Oxford cohort study. Eur J Clin Nutr. 2010;64:933–9. https://doi.org/10.1038/ejcn.2010.142.
Butola LK, Kute PK, Anjankar A, Dhok A, Gusain N, Vagga A. Vitamin B12-do you know everything. J Evol Med Dent Sci. 2020;9:3139–47. https://doi.org/10.14260/jemds/2020/688.
Calvillo Á, Pellicer T, Carnicer M, Planas A. Bioprocess strategies for vitamin B12 production by microbial fermentation and its market applications. Bioengineering. 2022;9:365. https://doi.org/10.3390/bioengineering9080365.
Haghshenas B, Kiani A, Mansoori S, Mohammadi-Noori E, Nami Y. Probiotic properties and antimicrobial evaluation of silymarin-enriched Lactobacillus bacteria isolated from traditional curd. Sci Rep. 2023;13:10916. https://doi.org/10.1038/s41598-023-37350-3.
Kiani A, Nami Y, Hedayati S, EliehAliKomi D, Goudarzi F, Haghshenas B. Application of Tarkhineh fermented product to produce potato chips with strong probiotic properties, high shelf-life, and desirable sensory characteristics. Front Microbiol. 2021;12:657579. https://doi.org/10.3389/fmicb.2021.657579.
Wells JM, Brummer RJ, Derrien M, MacDonald TT, Troost F, Cani PD, Theodorou V, Dekker J, Méheust A, De Vos WM. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–93. https://doi.org/10.1152/ajpgi.00048.2015.
Benson K, Cramer S, Galla H-J. Impedance-based cell monitoring: barrier properties and beyond. Fluids Barriers CNS. 2013;10:1–11. https://doi.org/10.1186/2045-8118-10-5.
Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol. 2006;68:403–29. https://doi.org/10.1146/annurev.physiol.68.040104.131404.
Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035–44. https://doi.org/10.1113/jphysiol.2011.224568.
Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. 2016. https://doi.org/10.1155/2016/7432797.
Wang Y, Chen Y, Zhang X, Lu Y, Chen H. New insights in intestinal oxidative stress damage and the health intervention effects of nutrients: a review. J Funct Foods. 2020;75: 104248. https://doi.org/10.1016/j.jff.2020.104248.
Wang Y, Wu Y, Wang Y, Xu H, Mei X, Yu D, Wang Y, Li W. Antioxidant properties of probiotic bacteria. Nutrients. 2017;9:521. https://doi.org/10.3390/nu9050521.
Seddik HA, Bendali F, Gancel F, Fliss I, Spano G, Drider D. Lactobacillus plantarum and its probiotic and food potentialities. Probiotics Antimicrob Proteins. 2017;9:111–22. https://doi.org/10.1007/s12602-017-9264-z.
Stavrou G, Kotzampassi K. Gut microbiome, surgical complications and probiotics. Ann Gastroenterol. 2017;30:45. https://doi.org/10.20524/aog.2016.0086.
Tannock GW. A special fondness for lactobacilli. Appl Environ Microbiol. 2004;70:3189–94. https://doi.org/10.1128/AEM.70.6.3189-3194.2004.
Strahinic I, Busarcevic M, Pavlica D, Milasin J, Golic N, Topisirovic L. Molecular and biochemical characterizations of human oral lactobacilli as putative probiotic candidates. Oral Microbiol Immunol. 2007;22:111–7. https://doi.org/10.1111/j.1399-302X.2007.00331.x.
Bosch M, Rodriguez M, Garcia F, Fernández E, Fuentes M, Cune J. Probiotic properties of Lactobacillus plantarum CECT 7315 and CECT 7316 isolated from faeces of healthy children. Lett Appl Microbiol. 2012;54:240–6. https://doi.org/10.1111/j.1472-765X.2011.03199.x.
Cui Y, Wang M, Zheng Y, Miao K, Qu X. The carbohydrate metabolism of Lactiplantibacillus plantarum. Int J Mol Sci. 2021;22:13452. https://doi.org/10.3390/ijms222413452.
Zhou Y, Cui Y, Qu X. Exopolysaccharides of lactic acid bacteria: structure, bioactivity and associations: a review. Carbohydr polym. 2019;207:317–32. https://doi.org/10.1016/j.carbpol.2018.11.093.
Arora S, Jood S, Khetarpaul N. Effect of germination and probiotic fermentation on nutrient composition of barley based food mixtures. Food Chem. 2010;119:779–84. https://doi.org/10.1016/j.foodchem.2009.07.035.
Kim J-Y, Choi E-J, Lee J-H, Yoo M-S, Heo K, Shim J-J, Lee J-L. Probiotic potential of a novel vitamin B2-overproducing Lactobacillus plantarum strain, HY7715, isolated from Kimchi. Appl Sci. 2021;11:5765. https://doi.org/10.3390/app11135765.
Li P, Gu Q, Yang L, Yu Y, Wang Y. Characterization of extracellular vitamin B12 producing Lactobacillus plantarum strains and assessment of the probiotic potentials. Food Chem. 2017;234:494–501. https://doi.org/10.1016/j.foodchem.2017.05.037.
Minekus M, Alminger M, Alvito P, Ballance S, Bohn T, Bourlieu C, Carrière F, Boutrou R, Corredig M, Dupont D. A standardised static in vitro digestion method suitable for food–an international consensus. Food Funct. 2014;5:1113–24. https://doi.org/10.1039/C3FO60702J.
Kim JY, Bang S-J, Kim J-Y, Choi EJ, Heo K, Shim J-J, Lee J-L. The probiotic strain bifidobacterium animalis ssp lactis HY8002 potentially improves the mucosal integrity of an altered intestinal microbial environment. Front Microbiol. 2022;13:817591. https://doi.org/10.3389/fmicb.2022.817591.
Shah P, Modi H. Comparative study of DPPH, ABTS and FRAP assays for determination of antioxidant activity. Int J Res Appl Sci Eng Technol. 2015;3:636–41.
Jeon H, Kim Y-T, Jang WY, Kim J-Y, Heo K, Shim J-J, Lee J-L, Yang D-C, Kang SC. Effects of Lactobacillus curvatus HY7602-fermented antlers in dexamethasone-induced muscle atrophy. Fermentation. 2022;8:454. https://doi.org/10.3390/fermentation8090454.
Shiau C-Y, Pong Y-J, Chiou T-K, Chai T-J. Free amino acids and nucleotide-related compounds in milkfish (Chanos chanos) muscles and viscera. J Agri Food Chem. 1996;44:2650–3. https://doi.org/10.1021/jf9601574.
Sebastiani G, Herranz Barbero A, Borrás-Novell C, Alsina Casanova M, Aldecoa-Bilbao V, Andreu-Fernández V, Pascual Tutusaus M, Ferrero Martínez S, Gómez Roig MD, García-Algar O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients. 2019;11:557. https://doi.org/10.3390/nu11030557.
Antony AC. Vegetarianism and vitamin B-12 (cobalamin) deficiency. Am J Clin Nutr. 2003;78:3–6. https://doi.org/10.1093/ajcn/78.1.3.
Xia W, Chen W, Peng W-F, Li K-T. Industrial vitamin B 12 production by Pseudomonas denitrificans using maltose syrup and corn steep liquor as the cost-effective fermentation substrates. Bioprocess Biosyst Eng. 2015;38:1065–73. https://doi.org/10.1007/s00449-014-1348-5.
Gu Q, Zhang C, Song D, Li P, Zhu X. Enhancing vitamin B12 content in soy-yogurt by Lactobacillus reuteri. Int J Food Microbiol. 2015;206:56–9. https://doi.org/10.1016/j.ijfoodmicro.2015.04.033.
Temova Rakuša Ž, Roškar R, Hickey N, Geremia S. Vitamin B12 in foods, food supplements, and medicines—a review of its role and properties with a focus on its stability. Molecules. 2022;28:240. https://doi.org/10.3390/molecules28010240.
Kerry RG, Patra JK, Gouda S, Park Y, Shin H-S, Das G. Benefaction of probiotics for human health: a review. J Food Drug Anal. 2018;26:927–39. https://doi.org/10.1016/j.jfda.2018.01.002.
Conway P, Gorbach S, Goldin B. Survival of lactic acid bacteria in the human stomach and adhesion to intestinal cells. J Dairy Sci. 1987;70:1–12. https://doi.org/10.3168/jds.S0022-0302(87)79974-3.
Cui W, Li L, Sun C, Wen Y, Zhou Y, Dong Y, Liu P. Tumor necrosis factor alpha increases epithelial barrier permeability by disrupting tight junctions in Caco-2 cells. Braz J Med Biol Res. 2010. https://doi.org/10.1590/S0100-879X2010007500020.
Speciale A, Muscarà C, Molonia MS, Toscano G, Cimino F, Saija A. In vitro protective effects of a standardized extract from Cynara cardunculus L. leaves against TNF-α-induced intestinal inflammation. Front Pharmacol. 2022;13:809938. https://doi.org/10.3389/fphar.2022.809938.
Mennigen R, Nolte K, Rijcken E, Utech M, Loeffler B, Senninger N, Bruewer M. Probiotic mixture VSL# 3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am J Physiol Gastrointest liver Physiol. 2009;296:G1140–9. https://doi.org/10.1152/ajpgi.00093.2023.
Nie P, Wang M, Zhao Y, Liu S, Chen L, Xu H. Protective effect of Lactobacillus rhamnosus GG on TiO2 nanoparticles-induced oxidative stress damage in the liver of young rats. Nanomaterials. 2021;11:803. https://doi.org/10.3390/nano11030803.
Li P-N, Herrmann J, Tolar BB, Poitevin F, Ramdasi R, Bargar JR, Stahl DA, Jensen GJ, Francis CA, Wakatsuki S. Nutrient transport suggests an evolutionary basis for charged archaeal surface layer proteins. ISME J. 2018;12:2389–402. https://doi.org/10.1038/s41396-018-0191-0.
Wang J, Zhang W, Wang S, Liu H, Zhang D, Wang Y, Ji H. Swine-derived probiotic Lactobacillus plantarum modulates porcine intestinal endogenous host defense peptide synthesis through TLR2/MAPK/AP-1 signaling pathway. Front Immunol. 2019;10:2691. https://doi.org/10.3389/fimmu.2019.02691.
Ambroszkiewicz J, Gajewska J, Mazur J, Kuśmierska K, Klemarczyk W, Rowicka G, Strucińska M, Chełchowska M. Dietary intake and circulating amino acid concentrations in relation with bone metabolism markers in children following vegetarian and omnivorous diets. Nutrients. 2023;15:1376. https://doi.org/10.3390/nu15061376.
Choi HS, Seong H, Kim S-A, Song Y, Sim EY, Kang H, Han NS. Lysine-fortified rice germ yogurt fermented with Lactiplantibacillus plantarum JSA 22 and its beneficial health effects. J Funct Foods. 2023;109: 105787. https://doi.org/10.1016/j.jff.2023.105787.
Li C, Fan Y, Li S, Zhou X, Park K-Y, Zhao X, Liu H. Antioxidant effect of soymilk fermented by Lactobacillus plantarum HFY01 on D-galactose-induced premature aging mouse model. Front Nutr. 2021;8:667643. https://doi.org/10.3389/fnut.2021.667643.
Gerke M, Janssen M. Vegan foods: labelling practice. Ernahrungs Umschau. 2017;64:54–7. https://doi.org/10.4455/eu.2017.011.
Funding
No external funding was received to carry out the present work.
Author information
Authors and Affiliations
Contributions
Ju-Yeon Kim: conceptualization, methodology, software, validation, formal analysis, investigation, data curation, writing—original draft preparation, visualization. Eun Jung Choi: conceptualization, investigation. Woo Young Jang: conceptualization, investigation. Soo A Kim: investigation. Kyeong Heo: investigation. Heerim Kang: investigation. Jeanne Kang: investigation. Yong-Tae Kim: conceptualization, formal analysis, resources, writing—review and editing, supervision. Jae-Jung Shim: conceptualization, writing—review and editing, supervision. Jung-Lyoul Lee: conceptualization, writing—review and editing, supervision. Jae-Hwan Lee: conceptualization, writing—review and editing, supervision.
Corresponding authors
Ethics declarations
Consent for publication
The authors agree to publish this article.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, JY., Choi, E.J., Jang, W.Y. et al. Evaluation of antioxidant activity and fermentation properties of potential probiotic strain Lactiplantibacillus plantarum HY7720 in plant-based materials. Discov Appl Sci 6, 233 (2024). https://doi.org/10.1007/s42452-024-05915-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05915-0