Abstract
Background
The incidence, mortality, and recurrence rates of oral cancer are high worldwide. It is a common and aggressive type of tumor. Owing to the challenges associated with early illness diagnosis, squamous cell carcinoma, a kind that is prevalent of oral cancer, has an unacceptably high fatality rate. The management of the condition and the prevention of cancer, on the other hand, depend greatly on early detection. Therefore, alternative methods for the treatment and early diagnosis are essential for oral cancer. The detection of tongue squamous cell carcinoma is aided by coupled surface plasmon resonance, which can occur in gold nanoparticles (AuNPs). Compared to the currently utilized imaging contrast chemicals, AuNPs are more biocompatible and capable of targeting specific surface molecules. In the current study, AuNPs were synthesized in one step via citrate reduction and applied to tongue samples of a Caucasian man's Homo sapiens (Squamous cell carcinoma from ATCC cell-lines) in order to improve early detection using and laser-induced fluorescence and Raman spectroscopy.
Results
UV–visible spectroscopy, Zeta potential, TEM, and FTIR spectroscopic technique were used to characterize the synthesized nanoparticles. The synthesized AuNPs measured 13 ± 3 nm with uniform size distribution and high stability. Results demonstrate the significance of AuNPs in improving the identification of tongue squamous cell carcinoma.
Conclusion
Obtained results revealed that the use of AuNPs modifies the emitted spectra in the two employed spectroscopic techniques and provides more significant receiver operating characteristic curve parameters, hence a higher detection rate of cancer.
Article Highlights
-
AuNPs improved oral squamous cell carcinoma detection using laser-induced fluorescence and Raman spectroscopy.
-
Cancer cells with NPs showed significant protein-dominant peaks.
-
ROC curves were created before and after using AuNPs to evaluate their efficacy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Background
Particularly in underdeveloped nations, oral cancer either in the lip and the oral cavity is a very common form of cancer. Most oral cancer patients in the early stages do not exhibit any symptoms; consequently they do not try to find medical attention until they express clear symptoms like bleeding, swelling, or lumps in the mouth or neck, which happen when lymphatic dissemination is established [1]. As a result, it is typically detected at progressive stages (III and IV) with a survival rate below 40% [2]. Physical examination, vital staining techniques, imaging techniques, spectroscopic methods (including Raman, FTIR, optical coherence tomography and laser-included tissue autofluorescence), biomarker detection, and histopathological examination are some of the methods that are currently used to diagnose oral cancer [3,4,5,6,7,8,9]. Additionally, near-infrared spectroscopy (NIRS) is a non-destructive and fast technique that is increasingly being used in medical diagnostics [10, 11]. In general, optical diagnosing methods are highly preferred because it’s high safety and simplicity [12,13,14]. However, the current lack of sensitivity and specificity limits the use of such conventional noninvasive approaches. The principal optical wave interactions with biological tissues are absorption, autofluorescence and scattering. Absorption results from the essential absorbers like melanin and hemoglobin, while fluorescence is caused by proteins like elastin and collagen or by intracellular cofactors. In contrast, multiple light reflections at the boundaries between tissue components with different refractive indices cause scattering [15]. These processes have long been used as a source of supplemental metabolic and structural data on biological tissues and conditions that are diseased [16].
Nanotechnology is now a helpful tool for keeping track of several diseases, including cancer [8, 17, 18]. Cancers of the mouth, throat, nasopharynx, lungs, breast, gastric system, and cervical cancer have all been successfully diagnosed using nanotechnology [19, 20]. Oral cancer has been identified with a variety of nanomaterials, including liposomes, polymeric nanoparticles and inorganic nanoparticles [9, 21,22,23]. Colloidal gold nanoparticles (AuNPs) have been used in the current investigation to enhance the detection of oral squamous cell cancer utilizing Laser-induced fluorescence and Raman spectroscopy. Prior to and during the usage of the nanoparticles, spectroscopic signals of healthy epithelial and malignant cells can be captured. Furthermore, the receiver operating characteristic (ROC) curves were developed before and after utilizing AuNPs to assess the effectiveness of the proposed results. The nanoparticles group accumulates together and generates hot spots, which improves the observed spectrum according to the obtained results. As a consequence, the application of AuNPs can significantly increase the recognition of oral squamous cell cancer.
2 Methods
2.1 Preparation and characterization of AuNPs
Following the conventional wet chemical process, AuNPs were synthesized [24, 25]. HAuCl4 solution (1 mM, 20 mL) heated to boiling and mixed quickly with trisodium citrate (38.8 mM, 2 mL). The solution color changed from yellow to deep red. After changing the color, the heater was turned off and the solution was left to stir till attainment room temperature.
2.1.1 Characterization of AuNPs
A double beam spectrophotometer (Cary 5000, USA) used to assess the spectrum absorption of the produced AuNPs using quartz cells with a wavelength range of 400–750 nm. Morphology was captured with Transmission Electron Microscope (HRTEM, Netherlands), the produced AuNPs were examined using a Dynamic Light Scattering (DLS) particle size analyzer (Zetasizer Nano ZN, Malvern Panalytical Ltd, United Kingdom). The samples underwent triplicate analysis. Zeta potential was calculated using the same apparatus. FTIR spectrometer (4100 Jasco, Japan) was used to measure FTIRs in the 400–4400 cm−1 range, then processed and lyophilized via SP Scientific (BTP-3ESE0X, England) freeze drying technology.
2.1.2 Viability assay
Cell viability was assessed by SRB assay [26, 27]. 100 μL cell suspensions (5 × 103 cells) were in 96-well plates and incubated in complete media for 24 h. Cells were treated with another 100 μL media containing AuNPs at various concentrations from 0.01 to 100 μg/ml. After 48 h. of AuNPs exposure, cells were fixed by replacing media with 150 μL of 10% TCA and incubated at 4 °C for 1 h. The TCA solution was removed, and the cells were washed 5 times with distilled water. Aliquots of 70 μL SRB solution (0.4% w/v) were added and incubated in a dark place at room temperature for 10 min. Plates were washed 3 times with 1% acetic acid and allowed to air-dry overnight. Then, 150 μL of TRIS (10 mM) was added to dissolve protein-bound SRB stain; the absorbance was measured at 540 nm using a BMG LABTECH®- FLUOstar Omega microplate reader (Ortenberg, Germany).
2.2 Oral cell-lines
Oral epithelial cell lines (OECs) were acquired from Nawah Scientific Inc. in Mokatam, Cairo, Egypt. The samples were kept at 37 °C in DMEM medium with 10% heat-inactivated fetal bovine serum, 100 units/mL penicillin, and 100 mg/mL streptomycin added. Twenty oral squamous cell carcinoma (SCC from ATCC cell-lines) samples were extracted from the tongue of a Caucasian man Homo sapiens and kept at − 80 °C till examination. After thawing at room temperature, the samples were divided into four groups: normal cells (5 samples), normal cells mixed with AuNPs (5 samples), oral squamous cell carcinoma of the tongue (5 samples), and oral SCC of the tongue mixed with AuNPs (5 samples) comprise group. Colloidal AuNPs with a diameter of 13 ± 3 nm was mixed 1:1 with the second and fourth groups.
2.3 Spectroscopic techniques
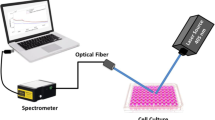
The optical setup for determining the fluorescence features of malignant and healthy cells is depicted in Fig. 1a. A CW diode pumped solid-state (DPSS) laser source (XL-R405SD, Xinl and international Co., Ltd., China) with a wavelength of 405 nm and an output power of 100 mW serves as the excitation source. The laser beam has a 2 mm diameter. The resulting fluorescence emission from the interaction with the tested samples has been transmitted through an optical fiber to a digital spectrometer (STDFSM, Touptek Photonics Co. Ltd, Zhejiang China). Through a USB cable, the spectrometer is linked to the computer. The measuring detector, a Sony ILX511 2048 Linear CCD array (Toshiba TCD1304AP Linear CCD array), has 3648 pixels with an 8200 m pixel size and a response range of 200–1100 nm wavelengths. Utilizing Matlab R2018b and the spectrometer software (Toup Spm), data processing and analysis were carried out.
Based on the interaction of light with the sample's chemical bonds, Raman spectroscopy is a method for providing a thorough chemical analysis of the subject under study where a powerful laser beam at a certain wavelength is directed at the sample, and the light reflected by its molecules is then measured (Fig. 1b). A small amount of the scattered light is scattered at different wavelengths (Raman scattering) as a result of the inelastic collision brought on by the modification of the sample's molecule's rotational and vibrational energy. A significant portion of the dispersed light (Rayleigh scattering) has the same wavelength as the incident light. The resultant Raman spectrum exhibits many peaks that correspond to the wavelength position and intensity of the scattered Raman light. These peaks are linked to the material under investigation's molecular bond vibration, either individually or in groups.
A LabRAM HR Evolution Raman spectroscope (HORIBA, France SA) was used in current study to identify the Raman spectra of the samples under investigation. The measurement wavelength of the spectrometer is from 200 to 2200 nm. A 25-mW power laser source operating at 532 nm was used. A 50 focal length lens (NA = 0.5) was used to acquire Raman spectra in the 400–3000 cm−1 range. Three integrations were carried out in an 8-s integration period. For appropriate Raman investigation, the samples have been thoroughly mixed and homogenized to prevent any concentration gradients or particle settling. Furthermore, the sample has been properly dried after deposition to remove any moisture or leftover solvent that can affect the Raman observations. Enough time has been given for the solvents to completely evaporate.
A number of variables, including nanoparticle characteristics, surface modifications, uptake pathways, cell type considerations, and SERS signal enhancement, must be taken into account while optimizing the SERS setup to guarantee AuNPs' absorption not only on the cell surface but also in the cytoplasm and other organelles. This was accomplished in the current study by employing imaging techniques (TEM) to ascertain the AuNPs' intracellular distribution in addition to including negative controls devoid of AuNPs to account for any non-specific binding or background signals, as well as conducting control tests to confirm the specificity of AuNP uptake and intracellular localization. Moreover, Raman spectroscopy is combined with confocal microscopy to provide spatially resolved Raman spectra. Raman spectroscopy combined with confocal examination allows for the exact localization of Raman signals from AuNPs within specific cellular compartments. This integration enables the viewing and characterization of AuNPs' intracellular distribution at high spatial resolution.
2.4 Statistical analyses using ROC
The ROC curve is a visual representation of the relation between sensitivity (the true positive rate) and (1-specificity), which is the true negative rate, in terms of a predetermined threshold (cut-off value). Additionally, a resultant area under the ROC curve is used to calculate accuracy [24, 25]. The performance of the collected measurements and the sensitivity and accuracy of the reported results have both been tested based on the obtained ROC parameters [28, 29]. Our proposed ROC curves were generated using an in-house function written in the MATLAB R2018b compiler (MathWorks, USA) [30, 31]. This function exhibits the 1-specificity and sensitivity of two classes of data (i.e., SCC and OCE (5 samples in each group) both with and without the addition of AuNPs, and computes the ROC curve's parameters.
3 Results
Figure 2a demonstrates how the produced AuNPs exhibit visible-range absorption caused by Surface Plasmon Resonance (SPR) at 522 nm [26]. TEM images were used to elucidate the morphology of the generated AuNPs which have a uniform size distribution and are roughly spherical in shape and about 13 ± 3 nm in size Fig. 2b. With a maximum viability loss of 13% at 100 g/ml as shown in Fig. 2c, cytotoxicity of AuNPs on oral epithelial cell lines exposed that the AuNPs had a good cell viability proportion at all of the varied doses ensuring the safety of utilizing AuNPs in diagnostic approach.
The hydrodynamic diameter and exterior charge of AuNPs were examined via DLS as shown in Fig. 3.
AuNPs was further characterized via FTIRs as presented in Fig. 4. AuNPs has two peaks at 3390 and 1628 cm−1 in addition peak at 1265 cm−1. (O–H stretching vibrations) resulting from intermolecular hydrogen bonding coincides with the large peak observed at 3400 cm−1. Whereas, peaks at 1629 and 1263 cm−1 assigned to –C=O stretching and C-O stretching.
The OCE and SCC in presence of AuNPs showed a fluorescence peak at 489.2 nm following the analysis of the samples excited by 405 nm laser irradiation as shown in Fig. 5. However, compared to those without adding the NPs, this peak is comparatively higher in the SCC combined with AuNPs.
Figure 6, displays the Raman spectra of cancer and normal cell lines that were obtained both before and after the addition of AuNPs. Higher peaks at 594, 665, 1096, 1679, and 1881.1 nm, which correspond to lipid-dominant biomarkers linked to (CH) bending, can be seen in normal cells containing AuNPs. Conversely, cancer cells containing AuNPs have high protein-dominant peaks with peaks at 541, 965, 1105, 1213, 2117, 2314, and 2786.08 cm−1.
In the OCE spectrum, the peak at 2934 cm-1 can be associated with CH2 antisymmetric stretch. Moreover, the peak at 2848.8 cm−1 in the SCC spectrum can be attributed to phospholipids, lipid components, and CH2 stretching. On the other hand, the peak at 2870 cm−1 corresponds to CH3 vibrational modes caused by the asymmetric stretch of lipids and proteins, which are particularly strong in the cytoplasm. Finally, the peak at 2940 cm−1 corresponds to nucleic acid CH vibrational modes, which are typically prominent in the nucleus and nucleolus [32, 33]. Protein characterization is aided by significant spectral features such as the amide I band’s C=O stretching mode, which is often observed around 1650–1700 cm−1 and phenylalanine's ring breathing mode which is typically observed around 1000–1030 cm−1. Regarding the structure of the peptide backbone and aromatic amino acids, these bands offer important information [34, 35].
To assess the efficacy of the proposed results, the ROC curves were produced before and after utilizing the AuNPs using the two spectroscopic approaches (i.e. LIF and Raman) as shown in Fig. 6a, b respectively. Additionally, Table 1 summarizes the whole parameters of the ROC curves. The main criterion for evaluation is the gained area under the ROC curve, or “AUC”.
As illustrated in Fig. 7 and Table 1, higher AUC (0.65 compared to 0.59 in LIF and 0.7 compared to 0.62 in Raman spectroscopy) was obtained after using AuNPs. In general, the overall ROC curve parameters obtained using the samples that are mixed AuNPs showed higher values than those without adding the AuNPs.
4 Discussion
The American academy of oral medicine documented that the patients with oral cancer distinguished in the early stages need less aggressive treatment and experienced fewer complications than the ones with advanced stage, with improved survival rate. Therefore, the development and use of nanomaterials were becoming increasingly common in the early detection of oral cancer. The in vitro detection technology based on AuNPs is excellent in the early screening and diagnosis of diseases [36]. Several reducing agents, including chitosan [27, 37], trisodium citrate and ascorbic acid [38], are responsible for the creation of AuNPs. Here, AuNPs were created using trisodium citrate. An indication that AuNPs were forming the creation of a dispersion with a ruby color [39]. The produced AuNPs exhibit absorption in the visible range as a result of Surface Plasmon Resonance (SPR) at 520 nm, as seen in Fig. 2a, which demonstrates the AuNPs’ stable condition. The suspension of AuNPs had a deep purple color with narrow band reflects AuNPs’ monodisperse character, devoid of agglomeration and aggregation [20]. AuNPs were 106 nm and − 14.5 mV via particle size and zeta potential as revealed in Fig. 3.
The zeta potential indicates the constancy of the nanoparticles. The negative value is caused by the presence of 3 deprotonated citrate ion carboxyl groups and are used to avoid accumulation in colloidal AuNPs [40, 41]. FTIRs analysis Fig. 4, approves that AuNPs was coated with citrate groups. Citrate groups has an vital role in the stability of produced nanoparticles [42]. With the purpose of estimating AuNPs potential for biomedical application, cytotoxicity of AuNPs was assessed against oral epithelial cell line (OEC) at different concentrations after 48 h using the SRP assay. AuNPs induced a concentration-dependent cell viability reduction with maximum 13% at100 μg/ml (Fig. 2c) which indicates the safety of AuNPs on normal oral epithelial cell line. The main cause of AuNPs cytotoxicity was the induction of apoptosis as reported in previous work [43, 44].
On the other hand, the present study considered wider spectrum in Raman spectroscopy to obtain a more thorough molecular study of the cells by. Although biomolecular information is primarily studied in the bio fingerprint region and high wavenumber region, extending the spectral range enables us to investigate other molecular vibrations and interactions that could offer important insights into the structure of cells and biochemical processes. Additionally, there may be overlapping spectrum contributions from several cellular constituents or chemical vibrations in the bio fingerprint and high wavenumber regions.
We aimed to improve spectral deconvolution and precise quantification of each molecular species by cutting these overlapping signals apart and resolving them by expanding the spectral spectrum. Even though the Raman signal in the silent region for biological samples might not be as strong as in other spectral regions, our study’s consideration of this region enables careful noise assessment, precise calibration of the instrument, investigation of subtle spectral signatures, and the possibility of new research directions. We think that by including the silent zone into our analysis, we improve the study's overall robustness and scientific rigor and further our understanding of biological sample Raman spectroscopy.
5 Conclusions
The present work demonstrates a fast, eco-friendly, low cost synthesis of AuNPs using sodium citrate in addition has almost no cytotoxic effect on Oral epithelial cell lines (OEC). A fluorescence peak was noticed at 489.2 nm in the OCE and SCC incubated with AuNPs which is comparatively advanced in the SCC combined with AuNPs. As well, Raman spectra of cancer and normal cell lines before and after the addition of AuNPs. Higher peaks correspond to lipid-dominant biomarkers can be seen in normal cells containing NPs. Conversely, cancer cells containing NPs have high protein-dominant peaks demonstrating the significance of AuNPs in enhancing detection of tongue squamous cell carcinoma utilizing laser-induced fluorescence and Raman spectroscopy. Because of the good cell vitality that has been observed, AuNPs can potentially be used in clinical and point-of-care diagnostic applications with confidence to analyze oral epithelial cells and improve disease monitoring and detection. Accordingly, our future research aims to evaluate treatment progress by employing spectroscopic method to track the utilization of AuNPs as drug carriers for oral cancer treatment.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AuNPs:
-
Gold nanoparticles
- OEC:
-
Oral epithelial cell lines
- DPSS:
-
Diode pumped solid-state laser
- HRTEM:
-
High-Resolution Transmission electron Microscope
- SPR:
-
Surface Plasmon Resonance
- FTIRs:
-
Fourier transform infrared spectroscopy
- LIFs:
-
Laser induced fluorescence spectroscopy
References
Abati S, Bramati C, Bondi S, et al. Oral cancer and precancer: a narrative review on the relevance of early diagnosis. Int J Environ Res Public Health. 2020;17:1–14. https://doi.org/10.3390/ijerph17249160.
Shibahara T. Oral cancer—diagnosis and therapy. Clin Calcium. 2017;27:1427–33.
Bick U, Trimboli RM, Athanasiou A, et al. Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European society of breast imaging. Insights Imaging. 2020;11:1–8. https://doi.org/10.1186/s13244-019-0803-x.
Nitya K, Vikram SA, Bhuvaneshwari GN. Vital staining- pivotal role in the field of pathology. Ann Cytol Pathol. 2020;5:058–63. https://doi.org/10.17352/acp.000017.
Iranmakani S, Mortezazadeh T, Sajadian F, et al. A review of various modalities in breast imaging: technical aspects and clinical outcomes. Egypt J Radiol Nucl Med. 2020;51:1–22. https://doi.org/10.1186/s43055-020-00175-5.
Xue L, Yan B, Li Y, et al. Surface-enhanced Raman spectroscopy of blood serum based on gold nanoparticles for tumor stages detection and histologic grades classification of oral squamous cell carcinoma. Int J Nanomed. 2018;13:4977–86.
Abdel-Salam Z, Harith MA. Laser spectrochemical characterization of semen. Talanta. 2012;99:140–5. https://doi.org/10.1016/j.talanta.2012.05.031.
Zou D, Cui D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol Med. 2018. https://doi.org/10.20892/j.issn.2095-3941.2018.0256.
Nour M, Hamdy O, Faid AH, et al. Utilization of gold nanoparticles for the detection of squamous cell carcinoma of the tongue based on laser—induced fluorescence and diffuse reflectance characteristics : an in vitro study. Lasers Med Sci. 2022;37:3551–60. https://doi.org/10.1007/s10103-022-03634-9.
Blondel W, Delconte A, Khairallah G, et al. Diffuse reflectance spectroscopy : spectrolive medical device for skin In Vivo optical biopsy. Electronics. 2021;10:1–18.
Hamdy O, Hassan MF, El-azab J, Solouma NH. Diffuse Reflectance Based Tissue Characterization Using Spatial Frequency Domain Imaging. In: Imaging and Applied Optics 2019 (COSI, IS, MATH, pcAOP), OSA Technical Digest (Optical Society of America, 2019), paper ITh4B. 2019; 5. pp 5–6
Hamdy O, Abdel-Salam Z, Abdel-Harith M. Optical characterization of biological tissues based on fluorescence, absorption, and scattering properties. Diagnostics. 2022;12:1–14.
Hamdy O, Ismail T (2019) Study of optical power variations in multi-layer human skin model for monitoring the light dose. In: novel intelligent and leading emerging sciences conference (NILES). pp 21–24
Hamdy O, El-Azab J, Solouma NH, et al. The use of optical fluence rate distribution for the differentiation of biological tissues. In: 8th Cairo international biomedical engineering conference (CIBEC). 2016.
Shanshool AS, Lazareva EN, Hamdy O, Tuchin VV. Optical properties and fluence distribution in rabbit head tissues at selected laser wavelengths. Materials. 2022;15:5696.
Abdelazeem RM, Ahmed MM, Hamdy O. Monitoring the optical diffuse transmittance of skin during thermo-hydrotherapy via spatial frequency domain imaging : a pilot study. in: biophotonics congress: optics in the life sciences. 2021.
Faid AH, Shouman SA, Thabet NA, et al. Laser enhanced combinatorial chemo-photothermal therapy of green synthesis gold nanoparticles loaded with 6mercaptopurine on breast cancer model. J Pharm Innov. 2023;18:144–8. https://doi.org/10.1007/s12247-022-09626-0.
Hussein A, Badr YA, Shouman SA, Sliem MA. Improvement of 6-mercaptopurine efficiency by encapsulated in chitosan nanoparticles. Arab J Nucl Sci Appl. 2018;51:181–6. https://doi.org/10.21608/ajnsa.2018.2630.1040.
Zheng W, Zhou Q, Yuan C. Nanoparticles for oral cancer diagnosis and therapy. Bioinorg Chem Appl. 2021. https://doi.org/10.1155/2021/9977131.
Faid AH, Shouman SA, Badr YA, Sharaky M. Enhanced photothermal heating and combination therapy of gold nanoparticles on a breast cell model. BMC Chemistry. 2022;16:1–9. https://doi.org/10.1186/s13065-022-00859-1.
Ramadan MA, El-Tayeb TA. Photostability, cytotoxicity, and photothermal impact of AgNPs, CoAgNC, and IOAgNC on HEp-2 laryngeal carcinoma cells. SN Appl Sci. 2023;5:1–12. https://doi.org/10.1007/s42452-023-05472-y.
Baldea I, Florea A, Olteanu D, et al. Effects of silver and gold nanoparticles phytosynthesized with Cornus mas extract on oral dysplastic human cells. Nanomedicine. 2019;15:55–75. https://doi.org/10.2217/nnm-2019-0290.
Hussein A, Badr Y, Shouman S, Sliem M. Green synthesis of spherical gold nanoparticles by chitosan for 6 mercaptopurine delivery. Arab J Nucl Sci Appl. 2018;51:175–80.
Turkev J, Stevenson PC, Hillie J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.
Faid AH, Shouman SA, Badr YA, Sharaky M. Enhanced cytotoxic effect of doxorubicin conjugated gold nanoparticles on breast cancer model. BMC Chem. 2022;16:1–8. https://doi.org/10.1186/s13065-022-00889-9.
Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12.
Ramadan MA, Sharaky M, Faid AH (2022) Ionic gelation synthesis, characterization and cytotoxic evaluation of chitosan nanoparticles on different types of human cancer cell models. Egyptian Journal of Chemistry 65:153–159. https://doi.org/10.21608/EJCHEM.2021.82733.4070
Goncalves L, Subtil A, Oliveira MR, Bermudez P de Z (2014) ROC Curve Estimation : An Overview. REVSTAT – Statistical Journal 12:1–20
Nam B-H, D’Agostino RB. Discrimination index, the area under the ROC curve. In: Statistics for industry and technology. Boston, MA: Birkhäuser; 2002. p. 267–79.
Ahmed R, Hamdy O, Awad RM. Diagnostic efficacy of systemic immune-inflammation biomarkers in benign prostatic hyperplasia using receiver operating characteristic and artificial neural network. Sci Rep. 2023;13:1–10. https://doi.org/10.1038/s41598-023-41781-3.
Sheet AH, Hamdy O, Abdel-Harith M. Scattering and absorption properties modification of optically cleared skeletal muscles : an ex vivo study. J Opt Soc Am A. 2023;40:1042–50.
Movasaghi Z, Rehman S, Rehman IU. Raman spectroscopy of biological tissues. Appl Spectrosc Rev. 2007;42:493–541. https://doi.org/10.1080/05704920701551530.
de das Carvalho LFC e. S, Sato ÉT, Almeida JD, da Silva Martinho H. Diagnosis of inflammatory lesions by high-wavenumber FT-Raman spectroscopy. Theoretical Chemistry Accounts, 2011; 130:1221–1229. https://doi.org/10.1007/s00214-011-0972-2
Zikic B, Bremner A, Talaga D, et al. Tip-enhanced Raman spectroscopy of A β (1–42) fibrils. Chem Phys Lett. 2021;768:1–21.
Kurouski D, Postiglione T, Deckert-Gaudig T, et al. Amide I vibrational mode suppression in surface (SERS) and tip (TERS) enhanced Raman spectra of protein specimens. Analyst. 2013;138:1665–73. https://doi.org/10.1039/c2an36478f.Amide.
Zhang Q, Hou D, Wen X, et al. Gold nanomaterials for oral cancer diagnosis and therapy: advances, challenges, and prospects. Materials Today Bio. 2022;15:1–18. https://doi.org/10.1016/j.mtbio.2022.100333.
Alexeree SMI, Abou-Seri HM, EL-Din HES, et al. Green synthesis of silver and iron oxide nanoparticles mediated photothermal effects on Blastocystis hominis. Lasers Med Sci. 2024;39:1–13. https://doi.org/10.1007/s10103-024-03984-6.
Malathi S, Balakumaran MD, Kalaichelvan PT, Balasubramanian S. Green synthesis of gold nanoparticles for controlled delivery. Adv Mater Lett. 2013;4:933–40. https://doi.org/10.5185/amlett.2013.5477.
Burda C, Chen X, Narayanan R, El-Sayed MA. Chemistry and properties of nanocrystals of different shapes. Chem Rev. 2005;105:1025–102. https://doi.org/10.1002/chin.200527215.
Zhang H, Liu G, Zeng X, et al. Fabrication of genistein-loaded biodegradable TPGS-b-PCL nanoparticles for improved therapeutic effects in cervical cancer cells. Int J Nanomed. 2015;10:2461–73. https://doi.org/10.2147/IJN.S78988.
Jelen Z, Majerič P, Zadravec M, et al. Study of gold nanoparticles’ preparation through ultrasonic spray pyrolysis and lyophilisation for possible use as markers in LFIA tests. Nanotechnol Rev. 2021;10:1978–92. https://doi.org/10.1515/ntrev-2021-0120.
Faid AH, Hussein FEZ, Mostafa EM, et al. Hybrid chitosan gold nanoparticles for photothermal therapy and enhanced cytotoxic action of 6-mercaptopurine on breast cancer cell line. Beni-Suef Univ J Basic Appl Sci. 2023;12:1–10. https://doi.org/10.1186/s43088-023-00419-z.
Ibrahim B, Akere TH, Chakraborty S, et al. Gold nanoparticles induced size dependent cytotoxicity on human alveolar adenocarcinoma cells by inhibiting the ubiquitin proteasome system. Pharmaceutics. 2023;15:1–20. https://doi.org/10.3390/pharmaceutics15020432.
Teraoka S, Kakei Y, Akashi M, et al. Gold nanoparticles enhance X-ray irradiation-induced apoptosis in head and neck squamous cell carcinoma in vitro. Biomed Rep. 2018;9:415–20. https://doi.org/10.3892/br.2018.1142.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
A.Z. and O.H., and A.H.F. Software, A.H.F. wrote the main manuscript text, M.N and S.S.K. prepared figures Data curation, M.N and S.S.K. Writing original draft preparation, M.N and S.S.K. Writing—review and editing, O.H and A.H.F. Visualization, O.H and A.H.F. Supervision, A.Z. and E.E. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamdy, O., Nour, M., Kamel, S.S. et al. Enhanced laser-induced fluorescence and Raman spectroscopy with gold nanoparticles for the diagnosis of oral squamous cell carcinoma. Discov Appl Sci 6, 157 (2024). https://doi.org/10.1007/s42452-024-05817-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05817-1