Abstract
In high-altitude tectonic regions, significant geothermal activity influences groundwater arsenic levels, presenting crucial resource and environmental challenges. The present study examines the Gonghe-Guide Basin located in the northeastern region of the Qinghai-Tibet Plateau. The study employs a comprehensive approach encompassing field sampling, hydrochemical analysis, thermodynamic modeling, and statistical analysis to ascertain the composition and origins of arsenic in geothermal groundwater. The research data indicates that the geothermal groundwater in the area displays weak alkalinity and medium to high mineralization, with the principal hydrochemical types being SO4−Cl·Na and Cl·Na. The concentration of arsenic has a notable inverse relationship with Cl− and a positive correlation with water temperature and DO. According to thermodynamic calculations, the most common kind of arsenic is As5+. The hydrochemical properties of the research area are shaped by rock weathering, evaporative concentration, and ion-exchange adsorption working together. These factors contribute to the favorable circumstances for the formation and migration of arsenic throughout the environment. Notably, the ion-exchange between sodium ions and calcium and magnesium ions significantly impacts the arsenic concentration. This study marks the first discovery of a unique arsenic contamination pattern in geothermal groundwater within the Gonghe-Guide Basin in the northeastern Tibetan Plateau, revealing a positive correlation between arsenic levels, water temperature, and dissolved oxygen content. This provides a new perspective on understanding arsenic pollution in geothermal groundwater in high-altitude regions.
Highlights
-
Discovery of unique arsenic patterns in Qinghai–Tibet Plateau geothermal waters linked to environmental factors.
-
Identification of arsenic behavior influenced by water chemistry and geothermal activity.
-
First-time insights into arsenic’s origins and effects in high-altitude geothermal systems.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
Geothermal hot springs, possessing distinctive characteristics as natural reservoirs of thermal energy, provide significant therapeutic, energy, and research advantages to human society [1]. Globally, the utilization of geothermal hot springs has increasingly exhibited a trend towards scaling and industrialization [2, 3]. Based on the most recent data, the application currently extends its reach to more than fifty countries, making a substantial contribution of billions of dollars to the global economy. However, as the extent of their utilization intensifies, the corresponding environmental issues have increasingly gained prominence. Among these, the pervasive presence of arsenic emerges as an issue that cannot be overlooked: numerous studies have shown that arsenic concentrations in geothermal hot springs greatly above safety regulations, and prolonged exposure can present a grave danger to human health [4, 5]. More critically, the movement and buildup of arsenic have led to groundwater contamination in certain areas, compromising drinking water safety for local residents and permanently affecting the local environment [6,7,8,9,10,11]. The World Health Organization (WHO) recommends a safety standard of 10 μg/L for arsenic in drinking water and this standard is widely adopted by various countries, including regulatory bodies such as the United States Environmental Protection Agency (EPA) [6, 9]. Thus, delving into the geochemical behavior and environmental effects of arsenic in geothermal hot springs and groundwater not only helps address current environmental issues but is also crucial for the sustainable and safe use of geothermal resources.
The presence of arsenic in geothermal systems is influenced by a complex interaction of several factors. The mobilization of arsenic from rocks and minerals to geothermal fluids is facilitated by temperatures within the range of 150–250 °C. However, it is important to note that excessively high temperatures can lead to the release of deadly arsenic [5, 12, 13]. Furthermore, it is commonly seen that deep neutral chloride waters compared to shallow acidic sulfate waters. The forms in which arsenic exists relate to the subterranean redox conditions, encompassing various biological and abiological processes such as oxidation, reduction, and methylation [14, 15]. The migration of arsenic within geothermal systems is closely linked to its origins and geochemical circumstances. In deep geothermal reservoirs, arsenopyrite and chalcopyrite are the primary sources of arsenic [13]. In some regions, the arsenic concentration in geothermal water is associated with rock leaching, and alkaline geothermal waters typically have elevated arsenic levels. Notably, neutral hot springs, representative of deep circulating geothermal fluids, have more prominent arsenic concentrations compared to acidic springs.
The Qinghai-Tibet Plateau is renowned for its substantial geothermal resources, which are accompanied by a significant emission of elevated levels of arsenic in its geothermal fluids [16]. It is believed that these heightened arsenic concentrations might be related with magma chambers contaminated by crustal materials. Additionally, rock leaching, fluid-rock interactions, and specific chemical components of magmatic fluids also influence the release of arsenic. Renowned as the "Roof of the World," the Qinghai-Tibet Plateau also holds a globally significant position in terms of groundwater and geothermal resource scale. Current regional studies and data indicate that the objective conditions of arsenic in the geothermal and groundwater of the Qinghai-Tibet Plateau is characterized by a significant level of severity. In comparison to other comparable places or adjacent areas, the concentration of arsenic is noticeably higher [17]. This arsenic poisoning hampers further development and usage of geothermal and groundwater resources. The distribution, shape, and association of mercury in geothermal groundwater have been elucidated through the utilization of integrated field tests, hydrochemical thermodynamic simulations, and statistical methods. These investigations have shed light on the influence of the geological environment on the merger cycle, specifically in relation to rock winding, evaporation enrichment, and ion exchange absorption processes. Hence, conducting a comprehensive investigation into the geochemical properties of arsenic in the groundwater and geothermal systems of the Qinghai-Tibet Plateau is crucial. This study seeks to clarify the origins of arsenic, its mechanisms of migration, and the factors that influence its presence. By doing so, it not only facilitates the development of efficient remediation approaches but also guarantees the responsible utilization of these essential resources.
2 Materials and methods
2.1 Overview of the study area
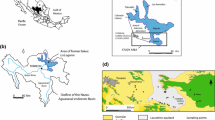
The Gonghe-Guide Basin is located on the northeastern periphery of the Qinghai-Tibet Plateau, principally shaped by the uplift of the Qinghai-Tibet Plateau and the northward collision of the Indian Plate. The geological structure exhibits a complex configuration, mostly consisting of three prominent faults: the northeast-oriented Gonghe Fault, the north–south oriented Gandu Fault, and the southeast-oriented Suli Fault, alongside other subsidiary faults and strike-slip faults. The basin has experienced the formation of various structural uplifts and depressions as a result of tectonic movements [18]. The study area is situated in the northeastern part of the Gonghe Basin, largely overlapping with the midsections of the Chabcha River and located inland. It boasts a high average elevation, with its surroundings characterized by high mountains and a relatively flat central area. The region is only accessible to the outside through a lower elevated uplift in the northwest, forming an open shape. Structurally, the area is positioned at the southwestern end of the Wali Guan large slope, with its primary structures including the nose-like anticline and saddle-shaped syncline within the Ayihei fold bundle.
The primary source of groundwater in the study area is derived from precipitation occurring in the mountains regions and rivers that traverse the basin. The presence of a geothermal gradient is evident in the mountainous areas that encompass the basin. As a result, the infiltration of mountain precipitation into the subsurface results in its heating, so giving rise to the creation of underground thermal water. Loose rock layers with high porosity and permeability, such as sand and gravel rocks, conglomerates, and sandstones, constitute substantial groundwater reservoir and migration layers, promoting the storage and flow of subsurface thermal water [19]. Furthermore, the storage and flow of subsurface thermal water are influenced by hydrogeological structures such as joints and fissures. Regarding the genesis of underground thermal water, the thermal water in the Gonghe-Guide Basin primarily arises due to the interplay between regional tectonics and the Earth's internal heat. This interaction induces thermal expansion and pressure-induced deformation in subsurface rocks, resulting in the creation of thermal water storage chambers that are relatively stable. Concurrently, the phenomenon of groundwater movement facilitates the introduction of a specific quantity of dissolved ions, so enhancing the subterranean hot water with a substantial concentration of mineral elements. The primary sources of heat in the basin are deep crustal geothermal energy, an internal geothermal energy within the Earth, and volcanic events.
The study region primarily consists of subsurface thermal waters that are primarily found inside the low-temperature geothermal reservoirs of the Lower Pleistocene and Neogene periods [20, 21]. The caprock of the Lower Pleistocene geothermal reservoir remains stable within the depths range of 100–200 m. This caprock is composed of subclay and subsandy soils, which possess ample water resources, exhibiting temperatures ranging from 18 to 42 °C. The geothermal input for these waters is sourced from the Quaternary Lower Pleistocene strata, which consists of a combination of fine silty sand, medium to coarse sand, and gravel. This combination forms a thermal reservoir with a granularity ranging from medium to coarse, and a thickness greater than 100 m. The Neogene geothermal reservoir, which has the potential to attain water temperatures ranging from 40 to 85 °C, is composed of siltstone, fine sandstone, medium sandstone, and pebbly medium-coarse sandstone from the Neogene era. These sedimentary layers are buried at depths ranging from 800 to 1,150 m. The primary heat source for this reservoir is the geothermal convection system linked to the tectono-magmatic belt of the Ela and Waligong mountains [22]. In this work, the two geothermal reservoirs are identified by employing a threshold of 40 °C. The thermal settings are classified into two categories: high-temperature hot springs (HT) and low-temperature hot springs (LT). Temperatures of the LT hot springs exhibit a range of 14–37.3 °C, whereas the HT hot springs demonstrate temperatures that span from 40 to 83 °C [23].
2.2 Sample collection and chemical analysis
A complete hydrochemical analysis was conducted in April 2022 by meticulously collecting 48 groundwater samples from hot springs, thermal wells, and geothermal boreholes located within the Lower Pleistocene and Neogene thermal reservoirs of the Gonghe Basin (Fig. 1). The hot springs are predominantly located in the middle section of the Chabcha River, while the thermal wells are mainly distributed in the upper reaches of the Chabcha River and the Ayihei Gully. The deep geothermal drilling wells are primarily situated within Gonghe County. Sampling began once it was confirmed that the discharge from the hot spring or wellhead had reached a stable state, which required continuous monitoring of the outflow. Afterwards, samples were carefully collected immediately from the area above the submersible pumps or at the location where the hot springs first appeared. The temperature of the samples during collection varied between 14 and 83 °C, representing the thermal variation among the sampling locations. For the purpose of chemical analysis, samples were held in two 500 mL polyethylene bottles. After cooling, all water samples were immediately filtered through a 0.45 μm membrane filter according to field practices in order to remove any particulate matter. In addition, the pH of the samples was meticulously regulated below 2 through the utilization of reagent grade HNO3 in order to impede oxidation during the cation analysis. After the tests were completed, the relative measurement error was calculated based on the standards announced in the “Standard Test Methods for Drinking Water—Quality Control in Water Quality Analysis (GB/T 5750.3–2006)” and DD2014-2015 “Technical Requirements for Quality Control of Sample Analysis in Groundwater Pollution Investigation and Evaluation”.
The temperature of the water was measured on site with a digital thermometer to the accuracy of 0.3 °C. Total dissolved solids (TDS), pH, electrical conductivity (Ec), redox potential (ORP), and dissolved oxygen (DO) were determined by the Hach HQ40d at the field site (Beijing Hach Instruments Co., Ltd.) with the precision of ± 2% full scale for TDS, ± 0.01 for pH, ± 0.5% full scale for Ec, ± 0.1 mV for ORP and ± 0.1 mg/L for DO. The Ca2+, Mg2+, Na+, K+ and As were determined using an Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES, Thermo Electron Corporation, USA) within 2 weeks after sampling. The CO32− and HCO3− were determined using potentiometric titrations. The contents of SO42− and Cl− were measured on an unaltered sample using Ion Chromatography (Dionex-900). The chemical studies were conducted by Qinghai Geological and Mineral Testing Application Center. The accuracy of the experimental data was checked and verified at the conclusion of the hydrochemical run, utilizing chemical equilibrium theory principles such as charge balance and precipitation balance.
2.3 Data processing
Analyzed for spatial distribution features, the acquired data was examined using the Mapgis software. The process of gathering, arranging, and analyzing data was carried out using Excel 2007 and SPSS version 25.0. The estimation of arsenic valence states and the mineral saturation index (SI) was performed using the PHREEQC software, which utilized the wateq4f.dat database. The investigation and examination of water chemistry properties and causal connections were conducted through the utilization of Piper trilinear diagrams, Gibbs diagrams, chloro-alkaline indices, and Gaillardet plots.
3 Results and discussions
3.1 Hydrochemical characteristics
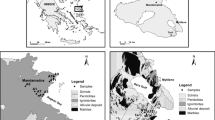
Statistical analysis indicated that the geothermal water temperatures in the area under study varied between 14 and 83 °C, with a mean value of 35.52 °C (Table 1). The pH values observed in the range of 7.22–9.01, with an average of 8.18, suggest the presence of a moderately alkaline environment. The TDS measurements exhibited a range of mineralization levels, reaching a maximum of 2239 mg/L, a minimum of 297.3 mg/L, and an average of 1256.90 mg/L. These findings indicate the significant water–rock interactions taking place within the geothermal systems. According to the ion analysis, sodium (Na+) was identified as the primary cation with an average concentration of 409.37 mg/L. The elevated levels of calcium (Ca2+) and magnesium (Mg2+) in the thermal reservoir, measuring 35.95 mg/L and 10.38 mg/L respectively, suggest an extended period of groundwater circulation and its interaction with carbonate minerals. In terms of anions, the average concentrations of chloride (Cl−) and sulfate (SO42−) were 380.38 mg/L and 257.11 mg/L, respectively. On the other hand, the average concentration of bicarbonate (HCO3−) was 235.53 mg/L. In the research region, the hydrochemical facies of the groundwater in the study area are primarily classified as sulfate–chloride-sodium (SO4−Cl·Na) and chloride-sodium (Cl·Na) types, as depicted in Fig. 2.
The pH values of the high-temperature (HT) and low-temperature (LT) groups exhibited minor change in relation to temperature fluctuations. The oxidation–reduction potential (ORP) in the HT group was − 206.14 mV, while in the LT group it was − 42.34 mV. This difference may indicate variations in the redox surroundings under different heat settings. The average Total Dissolved Solids (TDS) in the HT group was 1608.86 mg/L, indicating a higher level of mineralization. In contrast, the LT group had an average TDS of 1111.97 mg/L, demonstrating a relatively lower degree of mineralization and reflecting more severe water–rock interactions in the HT group samples. The HT group demonstrated a mean sodium (Na+) concentration of 531.24 mg/L, whereas the LT group revealed a concentration of 359.19 mg/L. This disparity could perhaps be attributed to the HT group's heightened interaction with sodium-bearing minerals, such as feldspars, resulting from elevated temperatures. There was a notable disparity in the mean potassium (K+) concentration between the HT and LT groups, with recorded values of 11.55 mg/L and 2.92 mg/L respectively. This observation implies that increased temperatures could potentially facilitate the breakdown of potassium minerals. The HT group had elevated mean concentrations of chloride (Cl−) and sulfate (SO42−) at 440.74 mg/L and 340.39 mg/L respectively, in comparison to the LT group's readings of 355.53 mg/L and 222.82 mg/L. The HT group may exhibit a higher level of evaporation concentration processes or dissolution of sulfate and chloride minerals. The difference in bicarbonate (HCO3−) levels, with mean values of 294.54 mg/L in the HT group and 211.23 mg/L in the LT group, suggests that more carbonate minerals dissolve at higher temperatures due to increased CO2 pressures.
Distinct aspects of water–rock interactions within two discrete geothermal environments are shown by the observed discrepancies between the HT and LT groups in terms of water temperature, mineralization, major cation, and anion contents. The high-temperature environment of the HT group facilitates the dissolving of a larger quantity of minerals, hence enhancing mineralization and influencing the hydrochemical composition. Conversely, the cooler temperatures of the LT group lead to comparatively lesser mineralization and the emergence of different chemical fingerprints.
3.2 Causes of hydrochemical characteristics
The Gonghe Basin is situated on the fault zone of the eastern edge of the Tibetan Plateau. The presence of active fault structures in this particular area has the potential to result in the interaction between groundwater and chemicals, such as sulfides, that are released by deep geological structures. Consequently, this interaction can have an impact on the hydrochemical properties of the groundwater. The diversity of rock types and mineral compositions through which water flows is attributed to the effect of geological structures. The ion concentration in water is influenced by various factors, including the mineral composition of subterranean rocks, solubility, and ion exchange [24]. Consequently, there are variations in the hydrochemical properties of the groundwater across different regions within the research area. The occurrence of alkaline water bodies can potentially be linked to the liberation of sodium, calcium, magnesium ions, and bicarbonate anions as a result of the acidification process within silicate minerals such as feldspar and mica found in rocks. Furthermore, it is worth noting that tectonic events have the potential to expedite the circulation of groundwater, hence augmenting the contact between groundwater and rocks. Consequently, this can lead to an elevation in ion concentration and mineralization within the groundwater [25].
Regarding causal disparities, elevated temperatures may potentially facilitate the breakdown of rocks, hence elucidating the elevated quantities of salt and chloride ions seen in the HT group. In the LT group, the dissolution of specific minerals such as feldspar and mica may be delayed due to lower temperatures, resulting in calcium and sulfate being the prevailing ions. Furthermore, the geological tectonic activity in the Gonghe Basin may facilitate increased interaction between the groundwater in the HT group and deep rocks, thereby influencing the presence of compounds like sulfides produced from these deep geological structures. On the other hand, the LT group may have a greater susceptibility to the influence of rocks and soils in proximity to the surface.
3.3 Arsenic speciation and origin
3.3.1 Arsenic speciation
The geological implications of the valence state of arsenic in groundwater are significant, as it offers comprehensive understanding of the geochemical conditions and processes occurring inside aquifers [26]. Different valence states of arsenic, particularly As(III) and As(V), distinctly indicate the redox conditions of the groundwater system and the presence of associated electron acceptors and donors [27]. The valence states of arsenic play a significant role in determining its mobility, solubility, and reactivity of arsenic within the aquifer. Additionally, these states provide valuable insights on the sources and routes of arsenic. Consequently, this facilitates a more profound comprehension of the geological circumstances within the aquifer.
Based on the data, it's apparent that the levels of both arsenic species are subject to the influence of groundwater temperature. Under hyperthermic conditions, there is a noticeable decrease in the concentration of As3+, whereas As5+ experiences a considerably higher increases. The observed fluctuations in concentration could potentially be attributed to distinct chemical reactions that are facilitated by elevated temperatures. In the groundwater of the study area, As5+ is the predominant ion particularly when exposed to oxidizing circumstances. The reduction of As5+ to As3+ can be facilitated by the microbial breakdown of organic waste under reducing circumstances [28]. Nevertheless, even in the ORP-lower LT conditions, the concentration of As3+ remains low in the samples collected from the research area. This observation suggests the existence of a mechanism that restricts the conversion of As5+ to As3+, or alternatively, As3+ undergoes fast oxidation back to As5+ upon its production. In the high temperature (HT) environment, there's a marginal elevation in As5+ concentration, which may be attributed to the impact of elevated temperatures on the chemical reaction equilibrium. The elevated Total Dissolved Solids (TDS) levels seen in both environments provide additional evidence of significant interactions between groundwater and the adjacent rocks and minerals, potentially impacting the concentration and distribution of arsenic (Table 2).
To elucidate the speciation of arsenic in geothermal waters and its interaction with hydrochemical properties, this study employs an analysis of arsenic valence states and the calculation of mineral saturation indices (SI). This approach aims to illuminate the mechanisms of arsenic migration, deposition, and valence transformation in groundwater. By examining the relationship between arsenic valence states (As(III) and As(V)) and the saturation indices of calcite and gypsum under varying temperature conditions, we assess how geochemical environments affect the transformation of arsenic forms and their distribution within geothermal systems. Figure 3 indicates that there is a moderate positive correlation between the saturation index (SI) of calcite and the concentration of As(III) (R2 = 0.3156), suggesting that the precipitation and dissolution processes of calcite may influence the concentration of As(III). Figure 4 reveals that the correlation between calcite's SI and As(V) concentration is relatively weak (R2 = 0.1677), while a stronger positive correlation exists between gypsum's SI and As(V) concentration (R2 = 0.6291), indicating that the precipitative-dissolutive processes of gypsum are a significant factor affecting As(V) concentrations.
3.3.2 Source of arsenic
There exists a correlation between the content of arsenic in groundwater and several ions and properties. Specifically, the ion Cl− demonstrates a notable inverse relationship with arsenic content, but water temperature and DO display a positive correlation with arsenic levels (Table 3). This observation is consistent with previous studies, indicating a potential correlation between the presence of arsenic and the occurrence of sodium, calcium, magnesium, sulfate, and chloride ions in groundwater. The potential correlation between these ions and arsenic could be attributed to the process of dissolution from stratified rocks containing arsenic upon contact. Furthermore, despite earlier research suggesting a higher pH level in groundwater with high arsenic groundwater, our data does not show a significant correlation between pH and arsenic concentration. This observation suggests that the relationship between the presence of arsenic and pH value is not necessarily linear, and may be influenced by additional geological and chemical factors.
3.4 Arsenic formation environment
3.4.1 Evaporative concentration mechanism
In order to understand how arsenic is formed, moves, and spreads in groundwater, we utilized the Gibbs diagram to analyze the hydrochemical composition and its sources in groundwater [29, 30]. The Gibbs approach is capable of classifying the various elements that influence the chemistry of natural water into three distinct categories: “Atmospheric Precipitation Control Type,” “Rock Weathering Type,” and “Evaporation-Concentration Type.” The vertical axis of this method depicts the logarithmic scale of total dissolved solids(TDS) in groundwater, while the horizontal axis illustrates the ratio of Na+/(Na+ + Ca2+) or Cl−/(Cl− + HCO3−) (Fig. 5).
Based on our data analysis, it can be shown that the samples predominantly cluster in regions associated with evaporative concentration and rock weathering [31]. This observation provides additional evidence that the generation and movement of arsenic in the groundwater of the Guide Basin are primarily driven by rock weathering processes. Given the annual precipitation in the Guide Basin amounts to merely 200 mm, its impact on the hydrochemical composition of groundwater within the basin is relatively limited. The generation, movement, and enrichment of arsenic are likely influenced by various factors, such as the paths through which groundwater runoff occurs and the speed of circulation. This is supported by the observation of a low ratio of HCO3−/Cl− in the samples.
Expanding on prior studies, elevated levels of arsenic in groundwater can be influenced by the replenishment of precipitation in particular regions, such as the mountainous areas in the southern part of the study area. The extended durations of runoff paths and water circulation in these regions may potentially result in the migration and enrichment of arsenic. Furthermore, the concentration of arsenic in sediment increases as the depth increases.. The principal mechanisms responsible for the enrichment of arsenic in groundwater are iron-manganese oxides and organic debris. These findings provide essential references for future research and the management of groundwater resource.
3.4.2 Ion exchange adsorption
Ion exchange adsorption is a major mechanism in the groundwater chemistry process, since it governs the ion concentrations and equilibrium within the aqueous system [32]. According to Gibbs' theory, ion exchange can be described as the reciprocal replacement of cations and anions on solid-phase surfaces, or vice versa. This procedure assumes a crucial function in ascertaining the levels of particular ions, such as arsenic, within groundwater.
Based on the data in this study, ICAI-1 predominantly represents the ion exchange adsorption between chloride ions and sodium and potassium ions [33]. The result demonstrates a statistically significant correlation between the ICAI-1 values and As concentration. As the strength of ion exchange adsorption between chloride and sodium/potassium intensifies, the concentration of As also increases, as depicted in Fig. 6. This might be attributed to the conditions of specific ion activity wherein certain ions are adsorbed from the aqueous phase onto the solid phase, leading to the release of As from the solid phase into the water phase. Meanwhile, the design of ICAI-2 is more extensive, taking into account a wider range of ions including bicarbonates, sulfates, carbonates, and nitrates, when adsorbing chloride ions by ion exchange. The analysis of the data reveals a nuanced relationship between ICAI-2 and As concentration, indicating that the impact of ion exchange adsorption of different ions on As concentration may be complicated. It is postulated that the concentrations of As are strongly influenced by ion exchange adsorption under certain conditions of ion activity. Nevertheless, the concentration of As is not exclusively determined by a single ion, but rather by the combined impact of many ion exchange adsorptions, as a result of the ion exchange process involving multiple ions.
3.4.3 Water–rock interaction
In order to enhance our comprehension of ion exchange adsorption, we conducted a comparative analysis γ(Na+) − γ(Cl−) and γ(Ca2+) + γ(Mg2+) − [γ(HCO3−) + γ(SO42−)] to provide a more comprehensive representation of the interplay between cations and anions. The majority of data points exhibited a tendency to aggregate around a reference line characterized by a slope of -1 (Fig. 7). The observed phenomenon clearly demonstrates the occurrence of cation exchange adsorption between sodium ions and calcium and magnesium ions. The aforementioned discovery aligns with the theoretical hypothesis on the occurrence of ion exchange between sodium and calcium and magnesium ions. Nevertheless, certain sample sites exhibit substantial deviations from the established reference line, potentially due to the influence of additional geochemical phenomena such as ion precipitation and redox reactions. Moreover, the non-uniformity observed in the data distribution may suggest the presence of diverse groundwater flow routes, indicating the existence of interaction complexities and other potential elements that may exert influence. For example, elevated levels of ions in specific regions may result from the process of mineral weathering or the influence of additional sources.
In order to gain a deeper understanding of the origins of the chemical composition of the water, we utilized the Gaillardet diagram as our principal analytical instrument. Gaillardet et al. (1999) propose that this figure provides a useful method for identifying the main chemical components of water by comparing the molar ratios of Ca2+/Na+ and HCO3−/Na+ [34]. The data shown in Fig. 8 clearly indicates that the majority of sample points exhibit a clustering pattern around the end-member of evaporite dissolution. This implies that the chemical properties of groundwater in the designated research region are primarily impacted by the process of evaporite dissolution. This finding is consistent with the geological chronology and characteristics of the subterranean basin. The presence of a substantial quantity organic matter within the confined aquifers, along with extended exposure to the surrounding rock, facilitates a lengthy period for groundwater to undergo chemical reactions with the surrounding rock. Consequently, a considerable quantity of soluble salts is dissolved into the groundwater. In addition, redox reactions occurring in the aquifers might also affect the chemical composition of the water, particularly the reduction reactions involving iron oxides, which could result in higher amounts of HCO3− [35]. The occurrence of HCO3− is associated with the structure and movement of arsenic. In alkaline environments, the formation of complexes between HCO3− and As(III) can have an impact on the mobility and solubility of As(III). The forms and quantities of arsenic are influenced by the combined impacts of evaporite dissolution processes and redox reactions. These findings are consistent with our previous results about the chemical characteristics and forms of arsenic in groundwater.
4 Conclusion
This study conducted a comprehensive analysis of geothermal waters in the Gonghe-Qiabuqia Basin in the northeastern part of the Tibetan Plateau, revealing their mildly alkaline nature and a medium to high degree of mineralization as indicated by average electrical conductivity. The hydrochemical types are predominantly SO4-Cl·Na and Cl·Na, closely related to the basin's geographical location, geological structure, and the types of rocks the water flows through. Notably, arsenic in the geothermal waters is predominantly in the form of As(V), correlating with the oxidative-reductive conditions of the groundwater. The concentration of arsenic shows significant correlations with Cl−, water temperature, and dissolved oxygen, suggesting its presence is likely associated with dissolution from contact with strata rocks. Key factors influencing arsenic's formation and migration include rock weathering, evaporative concentration, and ion exchange adsorption, with the ion exchange adsorption between sodium and calcium/magnesium ions notably affecting arsenic concentrations.
Availability of data and materials
The data generated in this study are part of an ongoing research initiative and are subject to intellectual property considerations. In accordance with the terms and conditions of our funding agreements with the State Grid Qinghai Electric Power Company Research Institute, the dissemination of this data is governed by confidentiality clauses. Consequently, the public availability of these data is restricted until the completion of the project and the resolution of any related patent processes. We commit to facilitating access to the data as far as is permissible under the prevailing intellectual property rights and funding agreement stipulations in the future.
References
Liu L, Yang L, Shen L, Xiao H. Research progress and prospect of geothermal resources. E3S Web of Conf. 2022. https://doi.org/10.1051/e3sconf/202339301001.
Aghahosseini A, Breyer C. From hot rock to useful energy: a global estimate of enhanced geothermal systems potential. Appl Energy. 2020;279: 115769. https://doi.org/10.1016/J.APENERGY.2020.115769.
Soltani M, Moradi Kashkooli F, Dehghani-Sanij AR, et al. A comprehensive review of geothermal energy evolution and development. Int J Green Energy. 2019;16(13):971–1009. https://doi.org/10.1080/15435075.2019.1650047.
Hu Y, Cheng H, Tao S. Environmental and human health impacts of geothermal exploitation in China and mitigation strategies. Crit Rev Environ Sci Technol. 2023;53(11):1173–96. https://doi.org/10.1080/10643389.2022.2128236.
Taufiq T, Atmojo JP, Raharjo SB, et al. Correlation of Arsenic Gas with Subsurface Temperature to Determine a Heat Source on “U” Geothermal System. In: 82nd EAGE annual conference & exhibition. European association of geoscientists & engineers, 2021, 2021(1):1–5. doi https://doi.org/10.3997/2214-4609.202113265
Dilpazeer F, Munir M, Baloch MYJ, et al. A comprehensive review of the latest advancements in controlling arsenic contaminants in groundwater. Water. 2023;15(3):478. https://doi.org/10.3390/w15030478.
Taneja S, Yadav S, Pipil H, et al. Soil-water interactions and arsenic enrichment in groundwater. Hydrogeochem Aquatic Ecosyst. 2023;2023:97–120. https://doi.org/10.1002/9781119870562.ch5.
Nath B, Das A, Majumder S, et al. Geospatial machine learning prediction of arsenic distribution in the groundwater of Murshidabad District, West Bengal, India: analyzing spatiotemporal patterns to understand human health risk. ACS ES&T Water. 2022;2(12):2409–21. https://doi.org/10.1101/2022.05.21.22275403.
Raju NJ. Arsenic in the geo-environment: a review of sources, geochemical processes, toxicity and removal technologies. Environ Res. 2022;203: 111782. https://doi.org/10.1016/j.envres.2021.111782.
Das S, Mukherjee S. Implementation of biotechnological techniques in treatment of groundwater contaminated with arsenic. Int J Res Appl Sci Eng Technol. 2022;10:993–1000. https://doi.org/10.22214/ijraset.2022.40431.
Adeloju SB, Khan S, Patti AF. Arsenic contamination of groundwater and its implications for drinking water quality and human health in under-developed countries and remote communities—a review. Appl Sci. 2021;11(4):1926. https://doi.org/10.3390/APP11041926.
Wang Y, Li P, Guo Q, et al. Environmental biogeochemistry of high arsenic geothermal fluids. Appl Geochem. 2018;97:81–92. https://doi.org/10.1016/J.APGEOCHEM.2018.07.015.
Bundschuh J, Maity JP. Geothermal arsenic: occurrence, mobility and environmental implications. Renew Sustain Energy Rev. 2015;42:1214–22. https://doi.org/10.1016/J.RSER.2014.10.092.
Khanikar L, Ahmaruzzaman M. Bioremediation of arsenic A sustainable approach in managing arsenic contamination. Bioremediation of Toxic Metal (loid)s. Cambridge: CRC Press; 2022. p. 185–203. https://doi.org/10.1201/9781003229940-12.
Satyapal GK, Rani S, Kumar M, et al. Potential role of arsenic resistant bacteria in bioremediation: current status and future prospects. J Microb Biochem Technol. 2016;8(3):256–8. https://doi.org/10.4172/1948-5948.1000294.
Guo Q, Planer-Friedrich B, Liu M, et al. Magmatic fluid input explaining the geochemical anomaly of very high arsenic in some southern Tibetan geothermal waters. Chem Geol. 2019;513:32–43. https://doi.org/10.1016/J.CHEMGEO.2019.03.008.
Gu H, Ni H, Wang Y, et al. hydrogeochemical characteristics and impact of arsenic released from a gold deposit in Tibet. Mine Water Environ. 2020;39:746–57. https://doi.org/10.1007/S10230-020-00732-4.
Wang BZ, Li JY, Wang ZH, et al. Geologic evolution characteristics of the northeastern Qinghai-Tibet Plateau in the Gonghe-Guide Basin. Geol Bull. 2015;34(1):15–22 (in Chinese with English abstract).
Liu CY, Yu KN, Zhang Y, Jing JH, Liu JT. Characteristics and Driving Mechanisms of Shallow Groundwater Chemistry in Xining City. Huan Jing Ke Xue. 2023;44(6):3228–36. https://doi.org/10.13227/j.hjkx.202203297.
Ma YH, Tang BC, Su SY, et al. Geochemical characteristics of geothermal fluids and water-rock interaction in geothermal reservoirs in and around the Gonghe Basin, Qinghai Province. Earth Sci Front. 2020;27(1):123–33. https://doi.org/10.13745/j.esf.2020.1.14.
Zhang SQ, Wu HD, Zhang Y, et al. Characteristics of regional and geothermal geology of the Reshuiquan HDR in Guide County, Qinghai Province. Acta Geol Sin. 2020;94:1591–605. https://doi.org/10.19762/j.cnki.dizhixuebao.2020159.
Tang XC, Wang GL, Ma Y, et al. Geological model of heat source and accumulation for geothermal anomalies in the Gonghe Basin, northeastern Tibetan plateau. Acta Geol Sin. 2020;94:2052–65. https://doi.org/10.19762/j.cnki.dizhixuebao.2020221.
Zhang HS, Feng QD, Zhang DY, Zhu GL, Yang L. Bacterial community structure in geothermal springs on the northern edge of Qinghai-Tibet plateau. Front Microbiol. 2023;31(13): 994179. https://doi.org/10.3389/fmicb.2022.994179.PMID:37180363;PMCID:PMC10172933.
Gong WJ, Tan KX, Li XM, et al. Geochemical characteristics of fluid and mechanism for ore formation in the Baiyang copper–silver deposit, Yunnan. Geotecton Metallog. 2000;24(2):175–81.
Biswas A, Majumder S, Neidhardt H, et al. Groundwater chemistry and redox processes: depth dependent arsenic release mechanism. Appl Geochem. 2011;26(4):516–25. https://doi.org/10.1016/J.APGEOCHEM.2011.01.010.
Williams AJ, Crossey LJ, Karlstrom KE, et al. Hydrogeochemistry of the Middle Rio Grande aquifer system—Fluid mixing and salinization of the Rio Grande due to fault inputs. Chem Geol. 2013;351:281–98. https://doi.org/10.1016/J.CHEMGEO.2013.05.029.
Wen B, Zhou A, Zhou J, et al. Coupled S and Sr isotope evidences for elevated arsenic concentrations in groundwater from the world’s largest antimony mine, Central China. J Hydrol. 2018;557:211–21. https://doi.org/10.1016/J.JHYDROL.2017.12.013.
Cai X, ThomasArrigo LK, Fang X, Bouchet S, Cui Y, Kretzschmar R. Impact of organic matter on microbially-mediated reduction and mobilization of arsenic and iron in arsenic(V)-bearing ferrihydrite. Environ Sci Technol. 2021;55(2):1319–28. https://doi.org/10.1021/acs.est.0c05329.
Silkin SV, Kulikov EE, Popov IA, et al. Mineral composition modelling of natural surface water. Int J Environ Anal Chem. 2021. https://doi.org/10.1080/03067319.2021.1943372.
Faust SD, Aly OM. Chemistry of water treatment. Cambridge: CRC Press; 2018.
Nghiem AA, Stahl MO, Mailloux BJ, et al. Quantifying riverine recharge impacts on redox conditions and arsenic release in groundwater aquifers along the Red River, Vietnam. Water Resour Res. 2019;55(8):6712–28. https://doi.org/10.1029/2019WR024816.
Ausikaitis JP, Myers AL, Sweed NH, et al. Adsorption and ion exchange: recent developments. 1985.
Hao QY, Xu XT, Zhang XB, et al. Chemical characteristics and formation of shallow high-fluorine groundwater in the northwest of Shandong, Yanggu area. J Earth Sci Environ. 2020;42(5):10. https://doi.org/10.19814/j.jese.2020.04033 (in Chinese with English abstract).
Li W, Chen Xie L, et al. Natural and human-induced factors controlling the phreatic groundwater geochemistry of the Longgang River basin, South China. Open Geosci. 2020;12(1):203–19. https://doi.org/10.1515/geo-2020-0039.
West J, McKinley I G, Bateman K. The microbiology of redox processes: development of a redox model. 2008.
Acknowledgements
This work is funded by Scientific research project of State Grid Qinghai Electric Power Company (B7280722E072)
Author information
Authors and Affiliations
Contributions
Conceptualization, SS, QF and HZ; Methodology HZ; Software SS; Validation GZ and ZN; Formal analysis GZ; Investigation ZN; Resources JZ; Data curation HZ; Writing—original draft preparation, HZ and SS; Visualization TZ; Supervision WZ; Project administration WZ; Funding acquisition SS, QF, SZ and HZ. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shi, S., Feng, Q., Zhang, H. et al. Arsenic in the Tibetan Plateau’s geothermal systems: a detailed analysis of forms, sources, and geochemical behaviors. Discov Appl Sci 6, 207 (2024). https://doi.org/10.1007/s42452-024-05798-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05798-1