Abstract
Many recent studies have indicated that some modified silicate glass–ceramics are considered and recommended to be part of the process of dental applications. Examples from the recommended glass–ceramics include modified lithium silicate with various dopants (ZrO2, CeO2), mica-based glass–ceramics, and leucite-based glass–ceramics. The present study employs a new direction of research in the preparation of modified lithium silicate glasses containing varying dopant percents of ZrO2 to produce the parent glasses using nano-silica as an essential chemical component in comparison with silica. The ZrO2-doped glass–ceramic reveals the crystalline phase of lithium aluminosilicate (Li5AlSi2O8) due to the effect of ZrO2. The modified lithium silicate glasses doped with ZrO2 prepared from nano silica indicate their high mechanical properties and can be primarily recommended for dental applications.
Article Highlights
-
Utilizing the melt and quench technique, lithium silicate glasses incorporating normal and nano-silica were synthesized.
-
The quantitative crystal phase composition of the resulting glass ceramics is influenced by the chemical composition.
-
ZrO2 effectively adjusts the hardness of the glasses, aligning them with the specifications for dental applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
It is accepted that the introduction of nano-material in a glass considered an essential component increases the physicochemical properties of the resultant vitreous candidate. The nanomaterials introduce primarily large surface areas leading to multiple contacts and generally higher reactivity. Actually, a large specific surface area is a very important factor for the surface chemistry of glass containing nano-component constituents. Nano silica powders have been recommended to be valuable and essential components in many diverse applications. Significant improvements are made in the physicochemical qualities, bioactivity, biodegradability, and biological properties of the bioactive candidates as a result of processing bioactive glass including nanoparticles with particle size below the typical micrometer scale [1,2,3,4]. Details of the importance of bioactive glasses with nano-structures are extensively outlined in the review chapter by El-Fiqi [5].
The interest in using silica nanoparticles comes from the ease of preparation, low-cost and large-scale preparation, larger specific surface area, volume, and controlled particle size [6]. When it comes to DNA synthesis and transport, silica nanoparticles are highly reactive [7]. Because of their smaller size, chemical formulation, and increased surface area, silica nanoparticles have been utilized to increase the viability of the seeds of the maize plant [8]. A further application of nano silica is recommended for the improvement of building cement material [9]. Höland and Beall in their third edition of Glass–ceramic technology [10] have differentiated between medical and dental glass–ceramics. They are different in their application environments and preferred properties. The materials used in dental applications must adhere to the requirements for the use of biomaterials, including compatibility with the oral environment. However, there must be no bioactivity on the surface of the dental repair. The prepared glass–ceramic's surface characteristics, such as color, translucency, toughness, and wear, match or must be comparable to those of real teeth. Also, chemical durability is of prime importance to avoid the formation of cavities. The reference glass–ceramics for dental restoration, include many silicate-based candidates including mica-based glass–ceramic, leucite-based glass–ceramics, and lithium disilicate glass–ceramic as examples [10, 11] previous publication of Höland et al. [10, 11] focused and compared on two types of biomaterials: glass–ceramic and sintered ceramics and referred to the importance of ZrO2 as an essential component to reach acceptable chemical and mechanical stability. The main aim of this present study is to prepare a host base glass from lithium silicate binary composition together with the same composition containing varying contents of ZrO2. The prepared glasses were characterized by their optical, thermal, and mechanical properties and their building structure was investigated through FTIR analysis. Samples of the prepared glasses were heat-treated to produce their glass–ceramic derivatives. Combined investigations of both the parent glasses and their glass–ceramic derivatives were carried out through structural FTIR spectral measurements together with S.E.M and X-ray analysis. It is expected to throw some insight into the effect of nano silica on the prepared glassy or crystalline materials. Evaluations of the physical, chemical, and mechanical properties of the prepared glassy samples or their glass–ceramic derivatives were carried out.
2 Experimental details
2.1 Preparation of the parent glasses
The glass of binary composition 66.33SiO2–33.66 Li2O together with ZrO2-doped samples (0.5, 1.5, and 3% of ZrO2) were prepared from both nano-silica with chemical composition in Table 1 and laboratory chemicals of lithium carbonate together with zirconium dioxide. The weighted raw ingredients were heated into platinum crucibles for two hours at 1400 °C while being rotated periodically to ensure complete melting and uniformity. The final melts were put into warmed stainless steel molds, and the ready glassy samples were then immediately moved to an annealing muffle set at 400 °C. This temperature was chosen based on previous related studies for this system. After one hour, the muffle was turned off, and the samples inside were allowed to cool to room temperature.
2.2 Differential thermal analysis and thermal expansion measurements
DTA measurement of the base parent lithium silicate glass was carried out on powdered glass which was examined up to 1000 °C using powdered alumina as a reference material. A DTA Perkin Elmer (DTA-7) apparatus was used.
Also, the thermal expansion data of the glassy samples were measured using a computerized dilatometer type (NETSCH-402 PC, Germany) with a heating rate of 5 °C/min. The thermal expansion measurements were carried out from room temperature up to the dilatometric softening temperature of each glass.
2.3 Structural FT infrared and optical spectral measurements
FTIR spectral data were collected out for powdered samples from the glasses and their glass–ceramics derivatives using a spectrometer type (FTIR Brucker Vertex 8V, Germany) within the range from 400 to 4000 cm−1 with a resolution of 4 cm−1. Optical absorption of the parent glasses was measured by a recording spectrophotometer (type Jasco 570, Japan) within the range 200–1100 nm for polished samples with equal thickness (2 mm ± 0.1 mm).
2.4 Heat treatment regime (conversion of parent glasses to their glass–ceramic derivatives)
The parent glasses were thermally heat-treated within a two-step regime. The samples were primarily heated slowly (5 °C/min) to the first selected temperature (400 °C) at which it is expected to produce nuclei sites and kept at this temperature for 6 h. Then the furnace was raised to the second temperature (587 °C) and this temperature was fixed for another 6 h. After that, the furnace with the samples inside was shut down and left to cool to room temperature.
2.5 X-ray diffraction analysis
To determine which crystalline phases were separated during the heat treatment, X-ray diffraction analysis was performed on the heat-treated samples. A diffractometer (type Philips PW 1390) using a Ni-filter and Cu-target was used to analyze powdered samples.
2.6 Microstructure analysis using scanning electron microscope
After coating the surfaces with a thin layer of gold, the exterior textures of the produced glass–ceramics were studied using a scanning electron microscope (type JEOL-JXA-840 A, Japan).
3 Results and discussion
3.1 DTA measurements
Figure 1 reveals the DTA result of the base lithium disilicate glass. The thermal curve shows a very distinct exothermic peak at 587 °C. The collected results from DTA and thermal expansion measurements are taken into consideration in the conducted heat treatment.
3.2 Thermal expansion data
The thermal expansion curves of the examined glasses are shown in Fig. 2. The thermal curves show that the dilatometric softening temperature of the parent glasses progressively increases with the increase of ZrO2 dopant up to 1.5% percent then declines with 3% ZrO2Content. Glasses are generally considered to expand like regular solids when heated. The glass type, chemical composition, bond strength, and cation field strength are the only factors that influence the glass’s dilatometric softening temperature. The understanding and interpretation of the thermal data are summarized as follows [12,13,14,15,16,17,18,19]:
-
(a)
Glasses like solids generally expand on heating with few exceptions. The valves of the thermal expansion coefficient in glasses depend on several factors including the type and all chemical constituents of the glass, the bond strength, field strength of the cations. In the silicate system, some alkali silicate glasses show silicon oxide anomaly which reveals a minimum of around 20 mol% alkali oxide and then increases again to the glass formation limit. The presence of nonbridging oxygens is very effective in the thermal expansion data.
-
(b)
Normal expansion is described as being the response to the increased amplitude of the atomic vibrations of the constituents upon heating.
-
(c)
The high melting temperature of ZrO2 and the strong bonding of Zr4+ ions with oxygens are the causes of the rise in dilatometric softening with an increase in ZrO2.According to the building structure and bond valence-bond length considerations, Zr4+ ions are sixfold coordinated (CN = 6) in alkali-rich silicate and borosilicate glasses (i.e., glasses with a high proportion of non-bridging oxygen atoms, or NBOs) and (ZrO6)2− octahedra share corners with SiO4 tetrahedra. These glasses’ Zr–O–Si bonds serve to stabilize the glass network. In glasses with a high ZrO2 content, ZrO2 can be thought of as a reticulating oxide due to the strong bonding between Zr and the silicate network and the rising abundance of alkali ions near the oxygen atoms joining Zr and Si. Hence, the presence of Zr–O–Si bonds in the glass structure would improve the glass's strength.
3.3 FTIR spectra of the parent glasses and that for their corresponding glass–ceramics
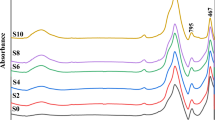
Figure 3 illustrates the FTIR spectra of the prepared parent glasses including the base Li2O–SiO2 prepared by either silica or nano silica and samples containing additional dopants of ZrO2. Figure 4 shows the FTIR of the corresponding glass ceramics. The IR spectral curves show separate vibrational bands with a range of 400 to 1650 cm−1 and a near IR broadband with a center of about 3450 cm−1. The curves show far IR bands at about 480, 559, 735 cm−1 and followed by several bands at about 908, 1018, 1136, 1250, 1433, and 1639 cm−1.
The detailed IR spectral curves of the two parent glasses reveal far IR small peaks extending from 400 to about 1000 cm−1 and showing two peaks at 455 and 466 cm−1 followed by smaller peaks at 541, 636, 798, and 933 cm−1. The previous spectrum is succeeded by a highly intense broad band extending from about 1000 to 1300 cm−1 with a distinct peak at 1093 cm−1 and another attached peak at about 1150 cm−1. A further identified two medium bands are shown with peaks at 1384 and 1635 cm−1. Small peaks can be seen in the remaining spectra at 2032, 2260, 2360, 2854, 2925, and 2958 cm−1. A last broad near-IR band with a peak at 3428 cm−1 and a range of 3000 to 3800 cm−1.
The identified IR bands observed in Fig. 4 are nearly the same as those for their parent glasses but show obvious intensities or are more defined.
The interpretation of the resultant IR spectra from the glasses or corresponding glass–ceramics can be summarized as illustrated in Table 2 and their details as follows [19,20,21,22,23,24,25]:
-
(1)
It has been agreed that the fundamental building block or unit for silicate glasses is the SiO4 tetrahedron in which the bridging oxygen atoms are shared between two tetrahedra, forming a fully polymerized network. The introduction of alkali cations (Li+, Na+) in the added alkali oxide acts as modifiers, breaking building oxygen bonds to form nonbridging oxygens (NBO)and the alkali cations located close to the negatively charged NBOs at places interstitial to the tetrahedral network.
-
(2)
Extensive studies on alkali silicate glasses have reached the following attribution to the frequently identified IR absorption bands:
-
(a)
The far IR bands at 400–500 cm−1 and extended to 600 cm−1 are generally correlated to Si–O–Si and O–Si–O bending modes.
-
(b)
The Si–O–Si symmetric stretching of bridging oxygens between tetrahedra is responsible for the IR bands between 750 and 850 cm−1.
-
(c)
The bands at 950–1100 cm−1 are related to Si–O–Si antisymmetric stretching.
-
(d)
The bands within the range 1650 cm−1 to 3500 cm−1 are attributed to vibrations of OH, water, and silanol (SiOH) groups.
-
(a)
-
(3)
The IR absorption bands of glass–ceramics are attributed to the same assignments as their parent glasses.
According to the previous discussions, Significant Zr–O–Si bonds are formed when ZrO2 is gradually added, and more alkali ions are moved from a modifier position (near NBOs) to a charge compensator position (near SiO4 units). A progressive increase in dilatometric softening was probably due to the presence of ZrO2 and the increase in the proportion of Zr–O–Si units in the studied glasses [25].
3.4 Optical absorption spectra
The optical absorption spectra are an important tool for studying the translucency of glass applied in dental applications. To limit the effects of contaminants and structural defects, surface texture optimization is used to maximize the degree of translucency.
Figure 5 shows the UV–visible spectra of three selected glasses including the base lithium disilicate glass without ZrO2 and two glasses containing 1.5 and 3% ZrO2 as a dopant. The optical spectra reveal a distinct UV absorption band with a peak at about 239 nm and become more intense with the addition of ZrO2 also the curves show two small peaks at about 354 and 400 nm but with no further absorption to the rest of the measurement at 1100 nm.
It has been recognized by many authors [26, 27] that some undoped silicate, borate or phosphate glasses reveal distinct UV absorption, and the origin of this UV absorption is assumed to be connected with the presence of unavoidable trace ferric ion impurities frequently contaminated with many raw materials or laboratory chemicals. Duffy [28] classified the UV absorption as usually identified in many glasses.
He assumed the origin of most UV absorption spectra of glasses is due to charge electron transfer that occurs as a result of the existence of traces of transition metal ions (such as Fe3+, Cr6+) even when the traces are at the ppm level. Also, UV absorption may be correlated to the heavy metal ions (Pb2+, Bi3+) when they present in noticeable content in the composition. An extended study by Ehrt and her co-authors [27, 29] agreed with this postulation and recommended the need for ultrapure materials to prepare special optical glasses for lenses and leasers. The same assumption has been agreed by many scientists [30, 31].
3.5 X-ray diffraction of the glass–ceramic derivatives
Figure 6 reveals the x-ray diffraction patterns of the two crystallized derivatives of the undoped binary Li2O–SiO2 glasses prepared from silica or nano-silica. The identified crystalline phases are somewhat different. The glass–ceramic derivatives from silica show two crystalline phases from Li2Si2O5 and Li2SiO3that correspond to PDF cards No. 40-0376 and 15-0519, respectively while the second glass–ceramic from nano-silica reveals an additional crystalline from sodium silicate Na2Si2O5(PDF card No. 022-1397). This behavior is due to the presence of Na2O in the composition of nano-silica.
As a whole, the precipitation of two different silica-based crystal phases from the present glass systemis due to the influence of the chemical composition of the glasses that impacted the quantitative crystal phase composition of the final glass ceramics. Hence, in normal silica, the layered silicate Li2Si2O5 is nucleated by an internal nucleation mechanism while the addition of nano-silica enhances the nucleation to crystalize another phase (Na2Si2O5) beside the main Li2Si2O5. Such interference between these two phases is due to the presence of a percentage of sodium when using nano-silica in the chemical composition.
Figure 7 illustrates the x-ray diffraction patterns of the glass–ceramic sample containing the highest ZrO2 (3%). The identified crystalline phase is the Li5AlSi2O8 (lithium aluminosilicate phase) corresponding to PDF card No. 46-0607. It is obvious that the presence of ZrO2 in the composition of the parent glass didn’t lead to any crystalline phase containing ZrO2 but only initiated the crystallization process as a catalyst and the presence of Al2O3 in the crystalline phase due to the presence of Al2O3 in the composition of the used nano-silica.
3.6 Scanning electron microscope and microhardness investigations of the glasses and glass–ceramic derivatives
Figure 8 illustrates the SEM photographs of the prepared glass ceramics. The figures show amorphous textures for all samples and for the higher ZrO2 doped glasses some marginal stations can be identified but with no crystallinity evidence. This confirms the amorphous nature of the parent glasses. Figure 9 illustrates the SEM graphs of the crystallized glass–ceramics. The undoped glass -ceramic reveals an almost homogenous microcrystalline texture revealing that the crystalline phases are similar to ordinary lithium silicate crystalline phases. The ZrO2–doped glass–ceramics reveal some variations indicating the formation of different crystalline phases formed through the action of ZrO2 as a nucleating agent or share in the formed crystalline phases.
Vickers microhardness data were collected by measuring polished samples through indentations by a Microhardness apparatus (Type Shimadzu, Japan) with a load of 100 gms for 15 s in 5 different places. The microhardness of undoped and zirconium-doped glasses recorded values at about 430, 440, 450, and 460 kg/mm2 for 0, 0.5, 1.5, and 3% ZrO2 respectively, with no characteristic changes in glass–ceramic. The gradual increase in the microhardness value is related to the increase in the content of ZrO2. According to the microhardness data taken as a whole, ZrO2 produces compactness in the network structure of all samples, which clearly raises the microhardness values. Additionally, the glass–ceramic derivatives have defined crystalline textures upon preparation, as predicted, and this practically encourages higher microhardness levels. This could be a result of the glass samples’ crystalline phase development, which prevents crack growth.
4 Conclusions
This work includes the preparation of some glasses from the basic Li2O⋅2SiO2 composition using nano-silica with variable percents of ZrO2 as dopants. Further controlled thermal heat treatment of the parent glasses to convert them to their corresponding glass–ceramic derivatives. The parent glasses were characterized by measuring their optical, FTIR, thermal expansion, and Vickers microhardness data. The glass–ceramics were characterized by measuring their structural FTIR, X-ray diffraction for the type of crystalline phases, and SEM for revealing their crystalline textures. Structural FTIR data indicate that both the glasses and glass–ceramics are composed of the traditional SiO4 units with Li2O acting as modifier oxide to form some non-bridging oxygens with Li+ ions in interstitial positions. The FTIR spectra of the glass–ceramicare more defined or sharper due to their presence within crystalline phases with definite structural units. The collective data from the thermal and mechanical analysis indicated that undoped and zirconium-doped lithium silicate glasses are suitable for dental applications. More investigation is expected to find natural dental uses. Further research studies are in progress to measure the chemical durability of the prepared glasses and glass–ceramics towards the action of different acidic, alkaline, and neutral solutions to justify their suitability for various dental applications in comparison with other organic and inorganic materials used in dentistry operations as shown by various publications [32, 33].
Data availability
The authors stated and declare that all data is exist and is available.
References
Mackovic M, Hoppe A, Detsch R, Mohn D, Stark WJ, Spiecker E, Boccaccin AR. Bioactive glass (type 45S5) nanoparticles: in vitro reactivity on nanoscale and biocompatibility. J Nanopart Res. 2012;14:966.
Vichery C, Nedeloc JM. Bioactive glass nanoparticles: from synthesis to materials design for biomedical applications. Materials. 2016;9:288.
Zheng K, Boccaccini AR. Sol-gel processing of bioactive glass nanoparticles: a review. Adv Coll Interface. 2017;249:363–73.
Erol Taygun M, Boccaccini AR. Bioactive glasses. Sawston: Woodhead Publishing; 2018. p. 235–83.
El-Fiqi A. Bioactive glasses and glass-ceramics: fundamentals, applications and advances. 1st ed. Hanover: The America Ceramic Society, Inc, John Wiley & Sons Inc.; 2022.
Wang Y, Zhao Q, Han N, Bai L, Li J, Liu J, Che E, Hu L, Zhang Q, Jiang T, Wang S. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed Nanotechnol Med. 2015;11(2):313–27.
Gangwar RK, Tomar GB, Dhumale VA, Zinjarde S, Sharma RB, Datar S. Curcumin conjugated silica nanoparticles for improving bioavailability and its anticancer applications. J Agric Food Chem. 2013;61(40):9632–7.
Jeelani PGh, Mulay P, Venkat R, Ramalingam C. Multifaceted application of silica nanoparticles: a review. Silicon. 2020;12:1337–54.
Senff L, Hotza D, Lucas S, Ferreira VM, Labrincha JA. Effect of NanoSiO2 and Nano-TiO2 addition on the rheological behavior and the hardened properties of cement mortars. Mater Sci Eng A. 2012;532:354–6.
Holand W, Beall GH. Glass-ceramic technology. 3rd ed. New Jersey: The American Ceramic Society-Wiley; 2020. p. 309–39.
Holand W, Rheinberger V, Apel E, Ritzberger C, Rothbrust F, Kappert H, Krumeich F, Nesper R. Future perspectives of biomaterials for dental restoration. J Eur Ceram Soc. 2009;29:1291–7.
Newton RG. The durability of glass. Glass Technol. 1985;26:21–38.
ElBadry KM, Mostafa FA, Azooz MA, ElBatal FH. Corrosion behaviour of some selected bioglasses by different aqueous solutions. Glass Technol. 2002;43:162–76.
ISO-Standard 6872, dental ceramics, 1995(E). p. 6.
Holloway DG. The physical properties of glass. London: Wykeham; 1973.
Rawson H. Properties and applications of glasses, glass science & technology, vol. 3. Amsterdam: Elsevier; 1980.
Ezz ElDin FM, ElBatal HA. Radiation effects on thermal expansion of some ternary alkali-silicate glasses. J Non-cryst Solids. 1993;152:195–200.
Azooz MA, ElBatal HA. Preparation and characterization of invert ZnO–B2O3 glasses and its shielding behavior towards gamma irradiation. Materi Chem Phys. 2020;240:122129.
Quintas A, Caurant D, Majérus O, Loiseau P, Charpentier T, Dussossoy J. ZrO2 addition in soda-lime aluminoborosilicate glasses containing rare earths: impact on the network structure. J Alloy Compd. 2017;714:47–62.
Wong J, Angell CA. Glass structure by spectroscopy. New York: Marcel Dekkar; 1976.
Efimov AM. Vibrational spectra, related properties, and structure of inorganic glasses. J Non-Cryst Solids. 1999;253:95–118.
Merzbacher CI, White WB. The structure of alkaline earth aluminosilicate glasses as determined by vibrational spectroscopy. J Non-Cryst Solids. 1991;130:18–34.
Husung RD, Doremus RH. The infrared transmission spectra of four silicate glasses before and after exposure to water. J Mater Res. 1990;5(10):2209–17.
ElBadry KhM, Moustafa FA, Azooz MA, ElBatal FH. Infrared absorption spectroscopy of some bio-glasses before and after immersion in various solutions. Indian J Pure Appl Phys. 2000;38:741–61.
Khalil EMA, ElBatal FH, Hamdy YM, Zidan HM, Aziz MS, Abdelghany AM. Infrared absorption spectra of transition metals-doped soda lime silica glasses. Phys B. 2010;405:1294–300.
Sigel GH Jr, Ginther RJ. The effect of iron on the ultraviolet absorption of high purity soda-silica glass. Glass Technol. 1969;9:66–73.
Möncke D, Ehrt D. Irradiation induced defects in glasses resulting in the photoionization of polyvalent dopants. Opt Mater. 2004;25:425–37.
Duffy JA. Charge transfer spectra of metal ions in glass. Phys Chem Glasses. 1997;38:289–92.
Ehrt D, Ebeling P, Natura U. UV Transmission and radiation-induced defects in phosphate and fluoride–phosphate glasses. J Non-Cryst Solids. 2000;263–264:240–50.
Marzouk MA, Elbatal HA, Abdelghany AM. Gamma irradiation effect on structural and spectral properties of CeO2, Nd2O3, Gd2O3 or Dy2O3—doped strontium borate glass. SILICON. 2018;10:29–37.
Marzouk MA, Ghoneim NA. Gamma irradiation and crystallization effects on the photoluminescence properties of soda lime fluorophosphates host glass activated with Ce4+, Dy3+ or Pr3+ ions. Rad Phys Chem. 2020;174:108893.
Hassan SM, Gad NA. Two-body wear and surface roughness of three different ceramic systems and their enamel antagonist: an in vitro study. Al Azhar Dent J Girls. 2017;4(4):347–57.
Jafari N, Habashi MS, Hashemi A, Shirazi R, Tanideh N, Tamadon A. Application of bioactive glasses in various dental fields. Biomater Res. 2022;26:31.
Acknowledgements
The authors of this work wish to express their gratitude to the authorities of the National Research Centre, Cairo, Egypt for their financial support through research project No. 13020218 (2023).
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
F. H. ElBatal, M. A. Marzouk, M. A. Azooz, and H. A. Elbatal contributed to executing data analysis, writing manuscript, drawing figures, and manuscript revision.
Corresponding author
Ethics declarations
Consent for publication
All authors approved the version of the manuscript to be published.
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
ElBatal, F.H., Marzouk, M.A., Azooz, M.A. et al. Preparation of some selective glass–ceramics from nano-silica within the Li2O–SiO2 system with ZrO2 for future dental applications. Discov Appl Sci 6, 156 (2024). https://doi.org/10.1007/s42452-024-05785-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05785-6