Abstract
This study aimed to determine the effects of storage temperature and washing on egg quality and physicochemical properties. A total of 200 eggs (100 washed and 100 unwashed eggs) were obtained from 30-week-old Hy-Line Brown laying hens. The experiment’s main effects were storage temperature (refrigerator and room temperatures) and egg washing (washed and unwashed eggs). The results indicated that eggs stored at refrigerator temperature increased (p < 0.05) in albumen height and Haugh unit at 1 to 4 weeks than those stored at room temperature. Eggs stored at room temperature and unwashed eggs had less (p < 0.05) thiobarbituric acid reactive substances and volatile basic nitrogen at 4 weeks than those subjected to other conditions. These findings suggest that refrigerator temperature improves egg quality, and unwashed eggs and those stored at room temperature potentially inhibits lipid oxidation and protein deterioration in eggs.
Article Highlights
-
Storage temperature and egg washing affect egg quality and physicochemical properties.
-
Unwashed eggs and those stored at refrigerator temperature increase albumen height and Haugh unit values.
-
Washed eggs and those stored at room temperature increase thiobarbituric acid reactive substance and volatile basic nitrogen.
-
Unwashed eggs and storing them at refrigerator temperature have beneficial effects on egg freshness.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Among livestock products, eggs are expected to experience the greatest in average monthly sales (9.5%) compared with their 2022 [1]. Eggs are high-protein products, and they are rich in proteins such as ovalbumin, ovotransferrin, ovomucoid, ovomucin, and lysozyme as well as fatty acids, vitamins, and minerals [2, 3]. Eggs are composed of the eggshell, albumen, and egg yolk. The eggshell accounts for 10.5% of the total egg weight, while the albumen and egg yolk, which are the edible parts, constitute approximately 58.5% and 31.0%, respectively. In addition, they predominantly contain approximately 75% moisture [4]. Owing to their high moisture content, alterations in egg quality may also occur due to various factors such as moisture evaporation and the breathing of eggs during storage.
The major determinants of egg quality are color, weight, eggshell strength, eggshell thickness, albumen properties (height, weight, and pH), the Haugh unit, and moisture content [5, 6]. Egg quality is reportedly influenced by washing conditions (washing methods and washing agents) and temperature (storage temperature and temperature of washing water) [7,8,9]. Egg washing has been found to damage egg cuticles and decrease cuticle integrity, which is responsible for pathogen defense via certain antimicrobial compounds and strong eggshell strength [10, 11]. Despite the reported effects, many countries (e.g. US and Asian countries) allow egg washing for hygiene and consumer safety. However, the European Union prohibits washing of class A eggs based on past reports of increased egg spoilage due to washing [12]. Increasing storage temperature and period tends to decrease albumen height and Haugh unit (HU) values, thus diminishing egg quality. Long-term storage of eggs results in an increase in loss in weight, yolk and albumen pH, air cell size, and a decrease in albumen whiteness [13]. In addition, water evaporation from the egg during long-term storage period is reportedly detrimental to egg quality, and potentially results in up to 5% decreased hatchability per day after 7 days storage [14, 15]. Wash water temperature has been implicated in the reduced spoilage and bacterial contamination of eggs at temperatures lower than the egg temperature [16].
Compared with that in the past, consumer interest in food hygiene has increased. In particular, establishing a hygienic management process and providing eggs of high quality to consumers are imperative because eggs have a relatively brief distribution process between producers and consumers. Therefore, this study aimed to determine the optimal conditions in terms of storage temperature and egg washing, and identify the factors that influence egg quality and physicochemical properties.
2 Materials and methods
2.1 Egg preparation and experimental design
A total of 200 eggs originated from the same flock of 30-week-old Hy-Line Brown commercial laying hens. One hundred washed and 100 unwashed eggs were obtained from a local farm. The washed eggs were basically washed with water through an egg washer in the farm (Seosan farm, Pocheon, Gangwon-do). All hens were raised under normal conditions in consecutive cages. A commercial layer diet was fed to laying hens on an ad libitum basis. The experiment was conducted in 2 × 2 factorial arrangements including two storage temperatures (refrigerator temperature [4–6 °C] and room temperature [20–22 °C]) and egg washing (washed and unwashed eggs). Eggs kept at refrigerator temperature were stored in egg boxes made of paper in the refrigerator and eggs kept at room temperature were stored in egg boxes made of paper. The relative humidity was maintained at 50–60%. Fifty eggs were assigned to each treatment, and the experiment lasted 4 weeks.
2.2 Egg quality
Egg quality was measured weekly in 10 selected eggs per treatment at 0, 1, 2, 3, and 4 weeks of storage. Eggshell color was determined using an eggshell color fan (Samyangsa, Kangwon-do, Republic of Korea; where 15 = very dark brown and 1 = very light and pale) and egg yolk color was ascertained using the Roche color fan (Hoffman-La Roche Ltd., Basel, Switzerland; where 15 = dark orange and 1 = light pale). The color reader (model CR-10, Konica Minolta Optics Inc., Tokyo, Japan) was used to measure the CIE Lab values for eggshell and egg yolk color. Color reading was expressed as lightness (L*), redness (a*), and yellowness (b*) values. Egg, egg yolk, and eggshell weights were measured using an electronic auto balance (HS-1000A, Hansung Instrument, Gwangmyeong, Republic of Korea). Albumen weight was calculated by subtracting the egg yolk and eggshell weights from the total egg weight. Also, egg yolk, albumen, and eggshell were obtained and weight to calculate the proportions of morphological egg yolk, albumen, and eggshell weight in the egg weight expressed as a percentage of the total egg weight. Eggshell strength was estimated using an eggshell strength tester (FHK, Fujihira Industry Co. Ltd., Tokyo, Japan). Eggshell thickness was measured in three regions (top, middle, and bottom) using a dial pipe gauge (FHK NFN380, Fujihira Industry Co. Ltd., Tokyo, Japan). HU values were calculated from egg weight (W) and albumen height (H), using the following equation as proposed by Eisen et al. [17]: HU = 100 log (H – 1.7 W0.37 + 7.6). Egg yolk moisture was measured in egg yolk using a drying oven (HB-501 M, Hanbeak Science, Bucheon, Republic of Korea) at 105℃ for 24 h. Egg yolk and albumen pH was determined using a pH meter (HI99163, Hanna Instruments, Rhode Island, USA).
2.3 Thiobarbituric acid reactive substance (TBARS)
Ten milliliters of each yolk sample, 25 mL of deionized water, and 15 mL of cold 10% perchloric acid generated by diluting 70% perchloric acid (Samchun Chemicals, Pyeongtaek, Republic of Korea) were added to a conical tube. Samples were shaken for 30 s and filtered using Whatman No.2 filter paper. Thereafter, 5 mL of 0.02 M 2-thiobarbituric acid (TBA; Sigma Aldrich, Darmstadt, Germany) solution and 5 mL of the filtrate were thoroughly mixed and placed in a cool, dark place for 16 h. The blank comprised deionized water and 5 mL of 0.02 M 2-TBA solution. Thereafter, absorbance was measured at 529 nm using a spectrophotometer (Mobi, MicroDigital Co., Ltd., Seongnam, Republic of Korea). The TBA content is presented in mg of malondialdehyde (MDA) per kg of sample (mg MDA/kg). The standard curve used at this time had the following coordinates: x = 0.0011 (r = 0.999) and y = 0.1975, where x = TBA value and y = absorbance.
2.4 Volatile basic nitrogen (VBN)
After adding 5 mL of the yolk sample to a conical tube containing 45 mL of deionized water, the mixture was shaken for 30 s and subsequently filtered through Whatman No.2 filter paper. Thereafter, 1 mL of 0.01 M boric acid solution and three drops of Conway solution, which comprises a mixture of 0.066% bromocresol green (Samchun Chemicals, Pyeongtaek, Republic of Korea) and 0.066% methyl red (Samchun Chemicals, Pyeongtaek, Republic of Korea), were introduced into the inside of a Conway unit. Thereafter, 3 mL of filtrate was added to the outside the Conway unit. Afterwards, 1 mL of 50% K2CO3 (Samchun Chemicals, Pyeongtaek, Republic of Korea) was added to the outside of the Conway unit and subsequently incubated for 120 min at 37 °C. Thereafter, the boric acid solution inside the Conway unit was titrated using 0.01 M sulfuric acid. The resulting VBN value is expressed in mg per 100 g of the sample (mg%).
The following variables were used: (a) which represents the volume of sulfuric acid added in milliliters (mL); (b) which denotes the quantity of sulfuric acid added to the blank sample in mL; and F, which represents the amount of N necessary to react with 1 mL of 0.01 M H2SO4.
2.5 Statistical analysis
All data were analyzed by 2-way ANOVA (analysis of variance) as a completely randomized design using the PROC MIXED procedure of SAS (SAS Institute Inc., Cary, NC). All data were checked for normal distribution and outliers with the UNIVARIATE procedure of SAS [18]. The statistical model used was
where Yijk is the individual observation, μ is the overall mean, Ti is the effect of storage temperature, Wj is the effect of egg washing, TWij is the effect of interaction, and eijk is the random error. The LSMEANS procedure was used to calculate treatment means and the PDIFF option of SAS was used to separate the means when the interaction was significant. Statistical significance was set at p < 0.05.
3 Results and discussion
3.1 Eggshell color
The effects of storage temperature and egg washing on eggshell color are shown in Table 1. Regarding the eggshell color score, interactions (p < 0.05) between storage temperature and egg washing were observed for the eggshell color score at 2 weeks. Therefore, the eggshell color score of unwashed eggs stored at room temperature was lower than that of unwashed eggs stored at refrigerator temperature. The eggshell color score of washed eggs increased (p < 0.05) compared with that of unwashed eggs, regardless of storage temperature. At week 4, the eggshell color score was greater (p < 0.01) for eggs stored at refrigerator temperature than for those stored at room temperature. However, no significant difference in eggshell color fan was observed by egg washing. Significant interaction (p < 0.05) effects of storage temperature and egg washing on eggshell lightness (L* value) were noted at 2 weeks. In addition, washed eggs exhibited significantly less (p < 0.05) eggshell lightness at 4 weeks than washed eggs. The eggshell redness (a* value) at 1 week of washed eggs increased (p < 0.01) compared with that of unwashed eggs. At 1 to 4 weeks, eggs stored at refrigerator temperature increased (p < 0.05) in eggshell redness compared with those stored at room temperature. Regarding the eggshell yellowness (b* value), interactions (p < 0.05) between storage temperature and egg washing were observed for the eggshell yellowness at 4 weeks. At 1–4 weeks, washed eggs increased (p < 0.01) in eggshell yellowness compared with unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.05) eggshell yellowness at 2 weeks than those stored at room temperature. Eggshell color has predominantly been used as a determinant of egg quality. The color values (ΔE ∗ ab values) of brown and white eggs displayed slight washing-induced differences; nevertheless, no significant differences were noted between washed and unwashed eggs [19]. Eggshell pigments include protoporphyrin, zinc porphyrin, zinc biliverdin, and biliverdin [20]; in particular, protoporphyrin IX is the main brown pigment in the cuticle layer. This study found the eggshell color to be higher at refrigerator temperature, exhibiting inconsistency with a previous study that reported no storage temperature-based differences in eggshell color [21]. The eggshell cuticle can be partially damaged by washing water or mechanical abrasion during the washing process [22]. Chlorine, one of the chemicals in washing water, is known to influence eggshell color. Wash water containing > 50 ppm of chlorine has been found to affect eggshell color owing to decomposition of the eggshell surface emanating from chlorine’s effect on specific amino acids, such as glutamic acid and glycine, present in the eggshell’s cuticle layer [23]. Therefore, alterations in eggshell color can be considered to result from the potential effects of mechanical abrasion or chemicals in the washing solution on eggshell pigments during the washing process.
3.2 Egg yolk color
The effects of storage temperature and egg washing on egg yolk color are presented in Table 2. No significant interaction between storage temperature and egg washing was observed for egg yolk color score and egg yolk lightness (L* value). At 1–2 weeks, washed eggs decreased (p < 0.05) in egg yolk color score compared with unwashed eggs. However, the egg yolk color score at 3 week of washed eggs increased (p < 0.05) compared with that of unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.05) egg yolk color score at 2 weeks than those stored at room temperature. At week 2, washed eggs decreased (p < 0.01) in egg yolk lightness compared with unwashed eggs. Eggs stored at refrigerator temperature had less (p < 0.01) egg yolk lightness at 1 to 4 weeks than those stored at room temperature. Interactions (p < 0.05) between storage temperature and egg washing were observed for egg yolk redness (a* value) and yellowness (b* value) at 2 weeks. At 1–4 weeks, eggs stored at room temperature had greater (p < 0.05) egg yolk redness and yellowness than those stored at refrigerator temperature. Carotenoids constitute the main pigments of egg yolk, whose color is affected by the types and concentrations of carotenoids included in feed (grass or herbs) [24]. In addition, egg yolk color was associated with the ratio of yellow and red carotenoids in the feed [25]. The yellowness is associated with the concentration of yellow carotenoids, such as lutein, violaxanthin, zeaxanthin, and cryptoxanthin, obtained from feed [25]. Washing is known to influence cuticle damage in eggs, leading to the easy penetration of external factors into the inner side. At this time, the lipids in the eggshell membrane or egg yolk potentially produce lipid-derived radicals and hydroperoxide via oxidation; in addition, the radicals affect carotenoid composition [26, 27]. Therefore, egg washing might have influenced changes in egg yolk color by affecting the carotenoid content of egg yolk. In addition, the increasing mass of the yolk by diffusion of water from the protein into the yolk, thus changing its color and shape index [28]. This study found egg yolk to have a higher lightness value when stored at room temperature; however, Suk and Kwon [6] reported that egg yolk color was not significantly altered by storage temperature (3℃ or 10℃). In addition, the lightness of egg yolk observed a decreasing trend with increasing storage period in our experiment. Previous study also found that lightness of egg yolk decreased with increasing storage period [29]. The possible reason for these observation may be associated with Maillard reaction occurred during storage by increasing brown or black melanoids, which decreased the lightness of egg yolk. Therefore, increase of storage period may have impact to decrease the lightness of egg yolk color.
3.3 Egg, yolk, albumen, and eggshell weights
The effects of storage temperature and egg washing on egg weight are shown in Table 3 and the proportions of morphological elements in the egg weight are presented in Table 4. Regarding the egg weight, interactions (p < 0.05) between storage temperature and egg washing were observed for the egg weight at 2 to 3 weeks. At 1 to 4 weeks, washed eggs decreased (p < 0.01) in egg weight compared with unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.01) egg weight at 4 weeks than those stored at room temperature. In the egg yolk weight, the egg yolk weight at 2 weeks of washed eggs increased (p < 0.05) compared with that of unwashed eggs. Eggs stored at refrigerator temperature had less (p < 0.05) egg weight at 1 or 4 weeks than those stored at room temperature. In the albumen weight, interactions (p < 0.05) between storage temperature and egg washing were observed for the albumen weight at 3 weeks. At 1 to 4 weeks, washed eggs decreased (p < 0.01) in albumen weight compared with unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.01) albumen weight at 3–4 weeks than those stored at room temperature. Regarding the eggshell weight, washed eggs decreased (p < 0.01) in eggshell weight at 2–3 weeks compared with unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.05) eggshell weight at 2–4 weeks than those stored at room temperature. No significant interaction between storage temperature and egg washing was observed for proportions of morphological yolk, albumen, and eggshell in the egg weight. However, washed eggs increased (p < 0.01) in proportions of morphological egg yolk in the egg weight at 1–2 weeks than unwashed eggs. Eggs stored at room temperature had greater (p < 0.05) proportions of morphological egg yolk in the egg weight at 1, 3, or 4 weeks than those stored at refrigerator temperature. In contrast, washed eggs exhibited significantly less (p < 0.05) proportions of morphological albumen in the egg weight at 1–2 weeks than unwashed eggs. At 1 or 4 weeks, eggs stored at refrigerator temperature had greater (p < 0.05) proportions of morphological albumen in the egg weight than those stored at room temperature. Washed eggs increased (p < 0.05) in proportions of morphological eggshell in the egg weight at 1 week than unwashed eggs. However, proportions of morphological eggshell in the egg weight at 2 weeks of washed eggs decreased (p < 0.05) compared with that of unwashed eggs. Eggs stored at refrigerator temperature had greater (p < 0.05) proportions of morphological eggshell in the egg weight at 2 weeks than those stored at room temperature. In our experiment, egg weight did not obtain clear measurement results for egg washing and storage by week. In general, egg, eggshell, albumen, and yolk weight may vary depending on the random selection in the analysis. Therefore, in order to overcome this problem, measuring the proportions of morphological elements in the egg weight can obtain accurate results. Resultantly, the proportions of morphological yolk and albumen in the egg weight were negative tendency under both storage temperature and egg washing. Thus, proportions of morphological elements in the egg weight displayed no significant interaction between storage temperature and egg washing. Nonetheless, further research is warranted, as this study’s results are potentially limited by inconsistencies with previous findings.
3.4 Eggshell strength and eggshell thickness
The effects of storage temperature and egg washing on eggshell strength and thickness are shown in Table 5. No significant interactions were noted between storage temperature and egg washing on eggshell strength and thickness. As regards the main effects of storage temperature, eggs stored at refrigerator temperature had greater (p < 0.05) eggshell strength at 2 weeks than those stored at room temperature. However, egg washing did not have any significant impact on eggshell strength. Regarding the main effects of egg washing and storage temperature, no significant differences in eggshell thickness were observed. Eggshell contains calcium carbonate (thin layer of hydroxyapatite in the inner cuticle), which accounts for approximately 9–12% of the total egg weight, as well as cuticle protein, a spongy layer with pores, and a mammillary layer [20, 30]. Egg washing has been found to damage egg cuticles, which are involved in pathogen defense, resulting in Salmonella Typhimurium penetration of eggshells and its survival in egg albumen [10]. Egg washing is potentially responsible for damage to the cuticle layer; therefore, external bacteria can easily enter the egg. Moreover, the egg’s moisture content potentially decreases owing to the exposed respiratory pores and alleviation of surface antimicrobial proteins [31]. Furthermore, damage to the cuticle layer of eggs may depend on the processing method and chemicals or temperature used during the egg washing process [32]. In our experiment, eggs stored at room temperature decreased in eggshell strength at 2 weeks than those stored at refrigerator temperature. The possible reason for these observation may be associated with temperature caused by increased damage to cuticle layer due to continuous room temperature storage. However, these results were not found in other weeks. It is suggested that the negative effect of eggshell strength may not be directly caused by temperature change.
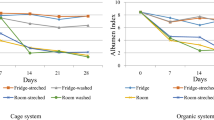
3.5 Albumen height and Haugh unit (HU)
The effects of storage temperature and egg washing on albumen height and HU are presented in Table 6. No significant interaction between storage temperature and egg washing was observed for albumen height and HU. As regards the main effects of egg washing, unwashed eggs had a greater (p < 0.05) albumen height at 3 weeks than washed eggs. Eggs stored at refrigerator temperature had greater (p < 0.01) albumen height and HU values at 1 to 4 weeks than those stored at room temperature. Similar patterns were observed for eggshell thickness, albumen height, and HU, whereby higher values were yielded at refrigerator temperature. As the laying age of chickens increases, egg quality decreases, with particular reductions in the albumen height and HU values [33]. Egg quality can be determined based on HU value, and eggs with HU values of > 72, 71–60, 59–31, and < 31 are classified into grades AA, A, B, and C, respectively, by the United States Department of Agriculture [34]. Although the HU value decreased with increasing storage period, all the HU values observed in this study indicated grade AA, regardless of storage temperature and egg washing. Therefore, processes such as not washing eggs and storing them at refrigerator temperature can maintain egg quality for a longer period; in addition, this study’s findings suggest that albumen height and HU values are suitable egg quality factors.
3.6 Egg yolk moisture, egg yolk pH, and albumen pH
The effects of storage temperature and egg washing on egg yolk moisture, egg yolk pH, and albumen pH are shown in Table 7. No significant interactions were observed between storage temperature and egg washing on egg yolk moisture. Regarding the main effects of storage temperature, eggs stored at room temperature had greater (p < 0.01) egg yolk moisture at 2–4 weeks than those stored at refrigerator temperature. Interactions (p < 0.05) between storage temperature and egg washing were observed for albumen pH at 1 or 4 weeks and egg yolk pH at 3 weeks. Washed eggs had greater (p < 0.01) albumen pH values at 2 or 4 weeks than unwashed eggs. In addition, eggs stored at room temperature exhibited significantly greater (p < 0.01) albumen pH values at 1 to 4 weeks than those stored at refrigerator temperature. Washed eggs increased (p < 0.01) in egg yolk pH at 2 weeks than unwashed eggs. However, washed eggs had less (p < 0.01) albumen pH values at 3 weeks than unwashed eggs. Eggs stored at room temperature had greater (p < 0.01) egg yolk pH at 2 weeks than those stored at refrigerator temperature. However, eggs stored at refrigerator temperature increased (p < 0.05) in egg yolk pH at 4 weeks compared with those stored at room temperature. Albumen pH increased significantly with increasing storage temperature [8]. Previous studies have reported that a rise in albumen pH may affect the carbonic acid-bicarbonate buffer system by releasing carbon dioxide during storage [35]. Other studies have also suggested that albumen pH is related to carbon dioxide loss through eggshell pores [36, 37]. In other words, a low albumen pH can be considered a safe condition that does not cause the release of carbon dioxide via eggshell pores. However, the albumen and yolk pH measurements yielded conflicting results depending on the egg washing and storage temperature conditions, respectively. As regards the main effects of egg washing, unwashed eggs had higher (p < 0.01) egg yolk pH values at 3 weeks than washed eggs. Eggs stored at refrigerator temperature had higher (p < 0.05) egg yolk pH values at 4 weeks than those stored at room temperature. Other studies have found the pH changes of albumen and yolk to be affected by refrigerator temperature [38, 39].
3.7 Thiobarbituric acid reactive substance (TBARS) and volatile basic nitrogen (VBN)
The effects of storage temperature and egg washing on TBARS and VBN are presented in Table 8. Interactions (p < 0.01) between storage temperature and egg washing were observed for TBARS values at 4 weeks and VBN values at 3 weeks. Regarding the main effects of egg washing, washed eggs decreased (p < 0.05) in TBARS values at 1 week than unwashed eggs. However, washed eggs had greater (p < 0.05) TBARS at 2–4 weeks than unwashed eggs. Eggs stored at room temperature had greater (p < 0.05) TBARS values at 2 or 4 weeks than those stored at refrigerator temperature. However, eggs stored at room temperature decreased (p < 0.01) in TBARS values at 3 weeks compared with those stored at refrigerator temperature. Washed eggs had greater (p < 0.05) VBN values at 2 weeks than unwashed eggs. But, washed eggs increased (p < 0.05) in VBN values at 4 weeks than unwashed eggs. Eggs stored at room temperature had greater (p < 0.01) VBN values at 2 or 4 weeks than those stored at refrigerator temperature. TBARS, which are the secondary products of lipid oxidation, are represented by the MDA content. Eggs are prone to freshness deterioration when stored under inappropriate conditions because they are rich in protein content. Since proteins decompose into organic nitrogen with low-molecular weight through amino acids as the putrefaction process, they are used to measure the putrefaction of protein through measuring for degree of VBN [40]. The VBN of animal products is currently limited to 20 mg% according to the Food Code of South Korea [41]. Although TBARS and VBN values increased with egg washing and room temperature, the values observed in this study were safe levels. Therefore, storage temperature and egg washing did not affect lipid peroxidation or protein putrefaction in eggs.
4 Conclusion
In conclusion, egg quality and physicochemical properties were affected by storage temperature and egg washing. In particular, eggs stored at refrigerator temperature had superior albumen height and HU values. In addition, unwashed eggs and those stored at room temperature had decreased TBARS and VBN values. Further studies are required to elucidate the effects of specific conditions, such as wash-water temperature and different washing agents, on egg quality and physicochemical properties.
Data availability
The data presented in this study are available on request from the corresponding author.
References
Korean Rural Economic Institute. Trends for rural and agricultural economics in spring. Available online: https://krei.re.kr (accessed on 12 July 2023).
Razi SM, Fahim H, Amirabadi S, et al. An overview of the functional properties of egg white proteins and their application in the food industry. Food Hydrocoll. 2023;135:108183. https://doi.org/10.1016/j.foodhyd.2022.108183.
Palmieri N, Stefanoni W, Latterini F, et al. Factors influencing Italian consumers’ willingness to pay for eggs enriched with omega-3-fatty acids. Foods. 2022;11:545. https://doi.org/10.3390/foods11040545.
Lee JC, Kim SH, Sun CW, et al. Comparison of principle components and internal quality of eggs by age of laying hens and weight standard. Korean J Poult Sci. 2013;40:49–55. https://doi.org/10.5536/KJPS.2013.40.1.049.
Wengerska K, Batkowska J, Drabik K. The eggshell defect as a factor affecting the egg quality after storage. Poult Sci. 2023;102:102749. https://doi.org/10.1016/j.psj.2023.102749.
Suk YO, Kwon JT. Effects of egg storage, storage temperature, and insemination of hens on egg quality. Korean J Poult Sci. 2004;31:203–12.
Sabrani M, Payne CG. Effect of oiling on internal quality of eggs stored at 28 and 12° G. Br Poult Sci. 1978;19:567–71. https://doi.org/10.1080/00071667808416515.
Liu YC, Chen TH, Wu YC. Effects of egg washing and storage temperature on the quality of eggshell cuticle and eggs. Food Chem. 2016;211:687–93. https://doi.org/10.1016/j.foodchem.2016.05.056.
Jones DR, Ward GE, Regmi P, et al. Impact of egg handling and conditions during extended storage on egg quality. Poult Sci. 2018;97:716–23. https://doi.org/10.3382/ps/pex351.
Gole VC, Chousalkar KK, Roberts JR. Effect of egg washing and correlation between eggshell characteristics and egg penetration by various Salmonella Typhimurium strains. PLoS ONE. 2014;9:e90987. https://doi.org/10.1371/journal.pone.0090987.
Wellman-Labadie O, Picman J, Hincke MT. Antimicrobial activity of the Anseriform outer eggshell and cuticle. Comp Biochem Physiol B Biochem Mol Biol. 2008;149:640–9. https://doi.org/10.1016/j.cbpb.2008.01.001.
Hutchison ML, Gittins J, Walker A, et al. An assessment of the microbiological risks involved with egg washing under commercial conditions. J Foof Prot. 2004;67:4–11. https://doi.org/10.4315/0362-028X-67.1.4.
Tabidi MH. Impact of storage period and quality on composition of table egg. Adv Environ Biol. 2011;5:856–61.
Mayes FJ, Takeballi MA. Storage of the eggs of the fowl (Gallus domesticus) before incubation: a review. Worlds Poult Sci J. 1984;40:131–40. https://doi.org/10.1079/WPS19840011.
Samli HE, Agma A, Senkoylu N. Effects of storage time and temperature on egg quality in old laying hens. J Appl Poult Res. 2005;14:548–53. https://doi.org/10.1093/japr/14.3.548.
Lucore LA, Jones FT, Anderson KE, et al. Internal and external bacterial counts from shells of eggs washed in a commercial-type processor at various wash-water temperatures. J Food Prot. 1997;60:1324–8. https://doi.org/10.4315/0362-028X-60.11.1324.
Eisen EJ, Bohren BB, McKean HE. The haugh unit as a measure of egg albumen quality. Polult Sci. 1962;41:1461–8.
Steel RGD, Torrie JH, Dckey DA. Principles and procedures of statistics: a biometrical approach. 3rd ed. New York: McGraw Hill Book Co.; 1997.
Leleu S, Messens W, De Reu K. Effect of egg washing on the cuticle quality of brown and white table eggs. J Food Prot. 2011;74:1649–54. https://doi.org/10.4315/0362-028X.JFP-11-013.
Samiullah S, Roberts JR. The eggshell cuticle of the laying hen. Worlds Poult Sci J. 2014;70:693–708. https://doi.org/10.1017/S0043933914000786.
Feddern V, Prá MCD, Mores R. Egg quality assessment at different storage conditions, seasons and laying hen strains. Ciênc Agrotec. 2017;41:322–33. https://doi.org/10.1590/1413-70542017413002317.
Burley RW, Vadehra DV. The egg shell and shell membranes: properties and synthesis. In: The avian egg chemistry and biology. Wiley: New York, USA; 1989, pp. 25–64.
Song IS. Utilization and processing of egg product. II. Storage of Egg. Curr Poult. 1983;7:104–10.
Hammershøj M, Johansen NF. Review: the effect of grass and herbs in organic egg production on egg fatty acid composition, egg yolk colour and sensory properties. Livest Sci. 2016;194:37–43. https://doi.org/10.1016/j.livsci.2016.11.001.
Grashorn M. Feed additives for influencing chicken meat and egg yolk color. In: Carle R, Schweiggert RM, editors. Handbook on Natural Pigments in Food and Beverage. Sawston: Woodhead Publishing; 2016. p. 283–302.
Han C, Chen Y, Shi L, et al. Advances in eggshell membrane separation and solubilization technologies. Front Vet Sci. 2023;10:1116126. https://doi.org/10.3389/fvets.2023.1116126.
Morgan JN, Armstrong DJ. Quantification of cholesterol oxidation products in egg yolk powder spray-dried with direct heating. J Food Sci. 1992;57:43–5. https://doi.org/10.1111/j.1365-2621.1992.tb05420.x.
Drabik K, Prochniak T, Kasperek K. The use of the dynamics of changes in table eggs during storage to predict the age of eggs based on selected quality traits. Animals. 2021;11:3192. https://doi.org/10.3390/ani11113192.
Luo W, Xue H, Xiong C, Li J, Tu Y, Zhao Y. Effects of temperature on quality of preserved eggs during storage. Poult Sci. 2020;99:3144–57. https://doi.org/10.1016/j.psj.2020.01.020.
Dennis JE, Xiao SQ, Agarwal M. Microstructure of matrix and mineral components of eggshells from white leghorn chickens (Gallus gallus). J Morphol. 1996;228:287–306. https://doi.org/10.1002/(SICI)1097-4687(199606)228:3%3c287::AID-JMOR2%3e3.0.CO;2-%23.
Kulshreshtha G, Benavides-Reyes C, Rodriguez-Navarro AB. Impact of different layer housing systems on eggshell cuticle quality and salmonella adherence in table eggs. Foods. 2021;10:2559. https://doi.org/10.3390/foods10112559.
Chousalkar KK, Roberts JR, Sexton M. Effects of egg shell quality and washing on Salmonella Infantis penetration. Int J Food Microbiol. 2013;165:77–83. https://doi.org/10.1016/j.ijfoodmicro.2013.05.002.
Samiullah S, Omar AS, Roberts J. Effect of production system and flock age on eggshell and egg internal quality measurements. Poult Sci. 2017;96:246–58. https://doi.org/10.3382/ps/pew289.
United States Department of Agricultural Service. United States standards, grades, and weight classes for shell eggs. Available online: https://www.fas.usda.gov (accessed on 20 July 2023).
Shin D, Narciso-Gaytán C, Regenstein JM, et al. Effect of various refrigeration temperatures on quality of shell eggs. J Sci Food Agric. 2012;92:1341–5. https://doi.org/10.1002/jsfa.4699.
Caner C, Yüceer M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult Sci. 2015;94:1665–77. https://doi.org/10.3382/ps/pev102.
Biladeau AM, Keener KM. The effects of edible coatings on chicken egg quality under refrigerated storage. Poult Sci. 2009;88:1266–74. https://doi.org/10.3382/ps.2008-00295.
Ahn DU, Sell JL, Jo C. Effect of dietary conjugated linoleic acid on the quality characteristics of chicken eggs during refrigerated storage. Poult Sci. 1999;78:922–8. https://doi.org/10.1093/ps/78.6.922.
Jones DR, Musgrove MT. Effects of extended storage on egg quality factors. Poult Sci. 2005;84:1774–7. https://doi.org/10.1093/ps/84.11.1774.
Kim YJ, Moon HJ, Song BR, et al. Evaluation of shelf life of non-pasteurized egg yolks, egg whites, and whole egg liquid products in Korea. J Food Hyg Saf. 2019;34:94–9. https://doi.org/10.13103/JFHS.2019.34.1.94.
Korea Ministry of Food and Drug Safety 2021 Ministry of food and drug safety, food leecode, "chapter 4-standard and specifications for long shelf-life foods: 17. Processed meat products and packaged meats". Available online: https://mfds.go.kr (accessed on 29 June 2023).
Funding
This research was supported by "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001).
Author information
Authors and Affiliations
Contributions
Conceptualization, Y.B.K, S.Y.L., and J.H.K.; formal analysis, Y.B.K., K.H.Y., W.T.L., S.H.P., H.L.Y., N.Y.C., and S.Y.J.; data curation, Y.B.K., K.H.Y., S.H.P., and J.S.C.; investigation, Y.B.K, S.Y.L., and J.H.K.; writing—original draft preparation, Y.B.K., S.Y.L.; writing—review and editing, J.S.C., and J.H.K.; supervision, J.S.C., and J.H.K. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The present study was conducted in accordance with the ethical standard for animal welfare and the use of animals prepared and with the approval of the Institutional Animal Care and the Use Committee at Chungbuk National University, Cheongju, Republic of Korea (CBNUA-2126-23-01).
Consent for publication
All authors agree to the content of paper for publication.
Competing interests
The authors have no competing interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, Y.B., Lee, S.Y., Yum, K.H. et al. Effects of storage temperature and egg washing on egg quality and physicochemical properties. Discov Appl Sci 6, 111 (2024). https://doi.org/10.1007/s42452-024-05760-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05760-1