Abstract
To the best of our knowledge for the first time the hydroxyapatite (HA) crystals were synthesized with different doses of HA seed crystals. HA crystals were synthesized from solutions containing the stoichiometric amounts of ammonium dihydrogen phosphate and calcium hydroxide. The various interactions of the NH4H2PO4 concentrations (0.5–5.5 Molar), surfactant {amino tris (methylene phosphonic acid) [N(CH2PO3H2)3] (ATMP)} [ATMP] concentrations (0–100 ppm), and HA seed crystals doses (0–50%) were investigated and their effects on the HA mean diameter and hardness were studied using the Box-Behnken experimental statistical design. Results have shown that no correlations were obtained between the mean diameters of the HA crystals and the obtained HA hardness. HA synthesis in the absence of surfactant resulted in formation of HA rod-like and/or tabular crystals whereas agglomerated crystals were obtained with surfactant. The results indicate that with increasing the % of HA crystal seed dose, an increase in the HA mean diameter was obtained. Moreover, with increase of surfactant concentration, a high increase in HA hardness was achieved. HA crystal particle sizes were ranged from about 11–18 µm whereas the crystallite sizes were ranged from 22 to 46 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Hydroxyapatite (HA, Ca10(PO4)6(OH)2) is the main composition of the human hard tissue, which can be synthesized with a calcium-to-phosphorous ratio of 1.67. Human bones contain 70% of HA [1], encouraging researchers to develop artificial bone grafting and other through chemical synthesis. Nevertheless, compared to the values of human bones, thick synthetic hydroxyapatite powders have shown reduced fracture toughness. The tensile characteristics of HA whiskers have proven to be comparatively high. Conversely, HA needle-like crystals have been synthesized to increase fracture toughness [2,3,4,5].
The hydrothermal method [3, 6, 7], solid-state reaction [8], rapid crystallization (precipitation) [9,10,11], sol–gel [12, 13], sputtering [14], mechanochemical [15, 16], mechanochemical–hydrothermal [16], electrodeposition (electro-crystallization) [17], and micro-emulsion [18] are some of the techniques used to synthesize HA. The precipitating HA material size can be controlled by adjusting the supersaturation ratio via the hydrothermal method. In a hydrothermal container, poly (sodium 4-styrene-sulfonate) (PSS) was added to speed up the production of HA in various morphologies [19]. The hydrothermal method precipitated long HA well-crystalline whiskers with a high aspect ratio of 68–103 and a mean length of 60–116 µm. The chemical reagents used were di-ammonium phosphate, calcium nitrate, and acetamide [20].
When amino tris(methylene phosphonic acid) [ATMP] surfactant was added during the crystallization of calcium sulfate dihydrate, the nucleation rate increased, but the crystal growth rate, mean diameter, and free energy for formation of critical nucleus size and radius decreased [21]. Specific macromolecules and surfactants, such as stearic acid and mono-saccharides were used to regulate the shape and hardness of HA [22, 23].
This work aims to optimize the HA preparation conditions using Box Behnken experimental statistical design and to investigate the effects of amino tris(methylene phosphonic acid) surfactant and addition of different doses of HA seed crystals on HA morphology, mean diameter, and hardness under hydrothermal conditions (i.e. at 140 °C) from ammonium dihydrogen phosphate and calcium hydroxide. Given that ATMP surfactant prevents calcium sulfate dihydrate from crystallizing in an acidic media, this study aims to investigate ATMP's impact on HA at an alkaline medium (pH of 9.1 ± 0.1).
2 Experimental
2.1 Materials and methods
For hydroxyapatite (HA) hydrothermal precipitation, the reactants used were stoichiometric amounts from ammonium dihydrogen phosphate (NH4H2PO4) and calcium hydroxide according to the following chemical reaction:
The surfactant used was Amino tris (methylene phosphonic acid) [N(CH2PO3H2)3] from Pfaltz & Bauer Incorporation (ATMP) of 0.18% concentration and has another name as Nitrilo-tri methyl-phosphonic acid (amino tris).
Table 1 shows the Box Behnken design of the experimental runs. HA synthesis parameters are 0.5 M, 3.0 M and 5.5 M NH4H2PO4 concentration, 0%, 25% and 50% HA seed crystals dose and 0 ppm, 50 ppm and 100 ppm ATMP surfactant concentration. The mole ratio of Ca(OH)2 to NH4H2PO4 was kept constant at 1.67, reaction temperature was 90 °C for 1 h, hydrothermal temperature was 140 °C for 3 h and the pH of the slurry was adjusted to be 9.1 ± 0.1. After the required reaction and hydrothermal times, the suspension was filtered and washed with water and ethyl alcohol at the end. Finally, HA cake was dried in vacuum oven at 60 °C for 24 h.
The same previous procedure was used for synthesis of HA seed crystals with applied the variables at level − 1 as in Table 1 as well as the slurry was filtered and the wet cake was weighted for taking 25% and 50% of the wet HA wet cake as HA seed crystals.
For sintering HA dry samples, the samples were ground, which are isostatic pressing using 25 mm die (ICL, Macro/ Micro KBr Die). The die is pre lubricated with two drops of oleic acid and the obtained pellets are sintered. The applied sintering technique follows the following 4 steps [24]:
-
1.
Temperature is increased from about (25 °C) room temperature to 800 °C at 2 °C per minute heating rate and maintained at 800 °C for 2 h.
-
2.
Temperature is raised from 800° to 900 °C at 2 °C per minute heating rate and kept at 900 °C for 1 h.
-
3.
Temperature is increased from 900° to 1200 °C at 2 °C per minute heating rate and maintained at 1200 °C for 1 h.
-
4.
Temperature is naturally decreased from 1200 °C to room temperature.
2.2 Particle size analysis
The particle size analysis was carried out using Coulter Laser Diffraction Analyzer model LS230. About 0.5 g of HA slurry was mixed with pure water in 25 ml tube and then was subjected to sonication (CREST Sonicator). X-ray Crystallography analyses were also performed for all the HA samples using (BRUKER X-Ray Diffractometer). Crystallite sizes (Lc) of the HA samples were calculated applying the following Scherrer’s equation:
where k is the shape coefficient, λ is the wave length, β is the full width at half maximum (FWHM) of HA phase, and θ is the diffraction angle [15].
The hydroxyapatite particles were characterized by Scanning Electron Microscopy SEM (LEO 1455VP). Transmission Electron Microscope (TEM, JEOL-JM1230) was used for observation of the particle’s morphology.
2.3 Hardness measurement
Vickers hardness of the obtained HA material is conducted after pressing and sintering of the pellets. All the pellets were subjected to a Vickers micro hardness tester (Micromet 3, Buehler LTD., Lake Bluff, IL). The pellets were indented on their polished surfaces. The Vicker’s hardness of the pellets (HV) was calculated according to Eq. (3), where, L is the indentation load in Newtons, 2a, the average diagonal length of the indentation in meters [25]. From 5 to 8 indentations were done on each specimen using a load of 1 kg with a 10 s loading time. Then average hardness value for each pellet was calculated.
3 Results and discussion
3.1 Effects of synthesis conditions on HA mean diameter and micro hardness
The results of HA mean diameter, crystallite size and micro hardness of the 15 tests are given in Table 2.
3.1.1 Effect of synthesis conditions on HA mean diameter
Precipitation of hydroxyapatite is a very rapid crystallization. Crystallization is the result of three stages: supersaturation followed by nucleation then crystal growth. Supersaturation stage occurs when particle aggregates (clusters) of various sizes are formed. Then the clusters will grow in size till exceeding the critical nucleus size and formation of large crystals.
Results of HA crystals sizes at different ATMP surfactant doses are given in Table 3. In addition, the experimental design results are given in the 3-D Plot of the experimental data as shown in Fig. 1. This Figure shows that, with increasing NH4H2PO4 and/or ATMP surfactant concentration, no significant effect on HA mean diameter. On the other hand, with increasing the HA crystal seed dose %, high increase in mean diameters at all studied levels ranging from about 34–37% at high levels. Figure 2 shows that, the average values of HA mean diameters are 12.4 µm, 13.9 µm and 17.0 µm at 0%, 25% and 50% HA seed crystals dose, respectively. The average % increases in HA mean diameter are 12% with 25% HA seed dose and 37% with 50% HA seed dose. Figure 3A, B shows the particle size distribution with and HA seed crystals doses.
3.1.2 Effect of synthesis conditions on HA micro hardness
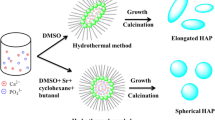
The experimental design results are displayed in a 3-D plot, as shown in Fig. 4. The obtained HA micro hardness values ranged from 120 to 484 kg/mm2, meanwhile the hardness of human bone is 50 kg/mm2 [25]. These findings demonstrate that HA micro hardness was raised from 16 to 69% at high NH4H2PO4 concentration. Furthermore, a significant increase in HA micro hardness ranged from 170 to 311% was observed at high ATMP surfactant concentration (Table 4, Fig. 5). The HA mean diameter and HA micro hardness did not correlate, according to the data. Better fine particle agglomeration (Fig. 6A) is the mechanism proposed to explain the micro hardness increase upon surfactant addition. These results indicate high hardness, low pore size and high shrinkage after pressing and sintering. Conversely, lower hardness values were measured of tabular HA crystals prepared in the absence of the surfactant (Fig. 6B). Additionally, the surface's adsorbed surfactant affects the HA compactness during pressing, affecting their micro hardness. The addition of more HA seed crystals validate this, as the micro hardness dropped by a range of 3–12%. The average percentage increase in HA micro hardness upon increasing the concentration of NH4H2PO4 was 41%, while the percentage increase with adding 100 ppm surfactant was 234%. Conversely, the average percentage drop in HA micro hardness that occurred when the HA seed dose was increased was 7%. There are two possible explanations for how ATMP surfactant affects the agglomeration of nano HA aggregates: (i) Instead of generating tiny nuclei, the negative charged phosphonic groups can chelate with the Ca2+ cations in the solution, increasing HA clumping, (ii) The positive charged crystal faces of HA can bond to the negative phosphonic groups.
The P and C sites, two distinct types of sites found on the crystal surface of the primitive unit cell of hydroxyapatite, have been reported. P sites [on the (a, b) crystal face] lack calcium ions or positive charge, C sites [on the (a, c) or (b, c) crystal face] are rich in calcium ions or positive charge. Therefore, the negatively charged phosphonic groups will be preferentially adsorbed onto the (a, c) or (b, c) crystal faces, namely, {100} and {010} facets of the nuclei, leading to modify the nucleation and crystal growth process of HA crystals [26]. These particles exhibited a higher degree of hardness upon pressing and sintering than surfactant-free tabular, rod, and cubic crystals.
3.2 Characterization of precipitated HA particles
The precipitated HA powder was characterized using XRD, SEM and Laser Size Distribution.
X-ray diffraction pattern (Fig. 7) was conducted on the 15 samples. The precipitated phase was confirmed as hydroxyapatite [JCPDS # 00-009-0432]. The crystallite size was ranged from 22 to 46 nm as shown in Table 2. Samples of 5, 9 and 11 have low intense peaks as they do not contain ATMP surfactant. Despite sample 7 does not contain the surfactant, relatively high intense peaks are obtained due to applying higher concentrations of reactants.
SEM photomicrographs of the crystallized hydroxyapatite powder samples are previously shown (Fig. 6). It is clear that, the crystallized hydroxyapatite powders from all the Tests without surfactant are agglomerated particles of different sizes. However, with surfactant addition, tabular, rectangular, rods and elongated HA crystals are obtained.
Results of HA crystals size distributions of 15 samples are given in Fig. 8 and Table 2. More data about mean diameters, median, mode, d10 and d90 of the first 12 samples are given In Table 3.
4 Conclusions
Using a statistical experimental design, the results of 15 hydroxyapatite hydrothermal precipitation tests suggest that adding amino tris(methylene phosphonic acid) [N(CH2PO3H2)3] (ATMP) surfactant aids in the formation of agglomerated, tough hydroxyapatite particles. The HA rod-like or tabular crystals formed when surfactant was absent during synthesis. No discernible relationship was found between the hardness and the average crystal diameters. The statistical experimental design analysis findings show that an increase in the HA crystal seed dose percentage was associated with a corresponding rise in the HA mean diameter. Furthermore, a significant increase in HA hardness was seen with a rise in surfactant concentration. The diameters of the crystallites varied from 22 to 46 nm, whereas the HA crystal particle sizes varied from roughly 11 µm to 18 µm.
Availability of data and materials
The data will be available by authors upon request.
References
Gong JK, Arnold JS, Cohn SH. Composition of trabecular and cortical bone. Anat Rec. 1964;149(3):325–31. https://doi.org/10.1002/ar.1091490303.
Hench LL. Bioceramics: from concept to clinic. J Am Ceram Soc. 1991;74(7):1487. https://doi.org/10.1111/j.1151-2916.1991.tb07132.x.
Ioku K, Yamauchi S, Fujimori H, Goto S, Yoshimura M. Hydrothermal preparation of fibrous apatite and apatite sheet. Solid State Ionics. 2002;151:147. https://doi.org/10.1016/S0167-2738(02)00593-3.
Cuneyt Tas A. Molten salt synthesis of calcium hydroxyapatite whiskers. J Am Ceram Soc. 2001;84(2):295. https://doi.org/10.1111/j.1151-2916.2001.tb00653.x.
Branda F, Costantini A, Luciani G, Rosso F, Peluso G, Barbarisi A. Hydroxyapatite coating of polyelectrolite hydrogels by means of the biomimetic method. Mater Sci Eng C. 2003;23:376. https://doi.org/10.1016/S0928-4931(02)00288-6.
Liu J, Ye X, Wang H, Zhu M, Wang B, Yan H. The influence of pH and temperature on the morphology of hydroxyapatite synthesized by hydrothermal method. Ceram Int. 2002;121:59. https://doi.org/10.1016/S0272-8842(02)00210-9.
Abdel-Aal EA, Dawood R, Ewais EM, Kandeel WA, Shemis MA, Abdel-Ghafar HM. Synthesis of agglomerated nano spheres hydroxyapatite particles with performance as anti-viral material. Int J Mater Technol Innov. 2021;1:1–17. https://doi.org/10.21608/ijmti.2021.181066.
Ramachandra Rao R, Roopa HN, Kannan TS. Solid State synthesis and Thermal stability of HAp and Hap-beta-TCP composite ceramic powders. J Mater Sci Mater Med. 1997;8:511. https://doi.org/10.1023/a:1018586412270.
Wang P, Li C, Gong H, Jiang X, Wang H, Li K. Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 2010;23(2):315. https://doi.org/10.1016/j.powtec.2010.05.023.
Afshar A, Ghorbani M, Ehsani N, Saeri MR, Sorrell CC. Some important factors in the wet precipitation process of hydroxyapatite. Mater Des. 2003;24:197. https://doi.org/10.1016/S0261-3069(03)00003-7.
Dorozhkina EI, Dorozhkin SV. Application of the turbidity measurements to study in situ crystallization of calcium phosphates. Colloids Surf. 2002;203:237. https://doi.org/10.1016/S0927-7757(01)01108-6.
Bezzi G, Celotti G, Landi E, La Torretta TMG, Sopyan I, Tampieri A. A novel sol–gel technique for hydroxyapatite preparation. Mater Chem Phys. 2003;78:816. https://doi.org/10.1016/S0254-0584(02)00392-9.
Weng W, Han G, Du P, Shen G. The effect of citric acid addition on the formation of sol–gel derived hydroxyapatite. Mater Chem Phys. 2002;74:92. https://doi.org/10.1016/S0254-0584(01)00399-6.
Yamashita K, Arashi T, Kitagaki K, Yamada S, Umegaki T. Preparation of apatite thin films through sputtering from calcium phosphate glasses. J Am Ceram Soc. 1994;77(9):2401. https://doi.org/10.1111/j.1151-2916.1994.tb04611.x.
Silva CC, Pinheiro AG, Miranda MAR, Góes JC, Sombra ASB. Structural properties of hydroxyapatite obtained by mechanosynthesis. Solid State Sci. 2003;5:553. https://doi.org/10.1016/S1293-2558(03)00035-9.
Nakamura S, Isobe T, Senna M. Hydroxyapatite nano sol prepared via a mechanochemical route. J Nanopart Res. 2001;3:57. https://doi.org/10.1023/A:1011407814795.
Abdel-Aal EA, El-Midany AA, El-Shall H. Mechanochemical-hydrothermal preparation of nano-crystallite hydroxyapatite using statistical design. Mater Chem Phys. 2008;112:202. https://doi.org/10.1016/j.matchemphys.2008.05.053.
Lim GK, Wang J, Ng SC, Chew CH, Gan LM. Processing of hydroxyapatite via microemulsion and emulsion routes. Biomaterials. 1997;18:1433. https://doi.org/10.1016/s0142-9612(97)00081-1.
Xiao X, Liu R, Liu F, Zheng X, Zhu D. Effect of poly(sodium 4-styrene-sulfonate) on the crystal growth of hydroxyapatite prepared by hydrothermal method. Mater Chem Phys. 2010;120:603. https://doi.org/10.1016/j.matchemphys.2009.12.004.
Zhang H, Darvell BW. Synthesis and characterization of hydroxyapatite Whiskers by hydrothermal homogeneous precipitation using acetamide. Acta Biomater. 2010;6:3216. https://doi.org/10.1016/j.actbio.2010.02.011.
El-Shall H, Rashad MM, Abdel-Aal EA. Effect of phosphonate additive on crystallization of gypsum in phosphoric and sulfuric acid medium. Crystal Res Technol. 2002;37:1264. https://doi.org/10.1002/crat.200290001.
Abdel-Aal EA, Abdel-Ghafar HM, El-Sayed D, Ewais EM. Synthesis of high hardness hydroxyapatite particles using surfactant assisted hydrothermal method. Int J Mater Technol Innov. 2022;2(1):35–50. https://doi.org/10.21608/ijmti.2022.115060.1044.
Yan L, Li Y, Deng ZX, Zhuang J, Sun X. Surfactant-assisted hydrothermal synthesis of hydroxyapatite nanorods. Int J Inorg Mater. 2001;3:633. https://doi.org/10.1016/S1466-6049(01)00164-.
Muralitharn G, Ramesh S. The effects of sintering temperature on the properties of hydroxyapatite. Ceram Int. 2002;26:221. https://doi.org/10.1016/S0272-8842(99)00046-2.
Ramrakhiani M, Deepti P, Murty TS. Micro-indentation hardness studies on human bones. Acta Anat. 1979;103:358. https://doi.org/10.1159/000145035.
Zhang H, Wang Y, Yan Y, Li S. Precipitation of biocompatible hydroxyapatite Whiskers from moderately acid solution. Ceram Int. 2003;29:413. https://doi.org/10.1016/S0272-8842(02)00153-0.
Acknowledgements
Not applicable.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that there is no any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdel-Aal, E.A., El-Sayed, D. & Abdel-Ghafar, H.M. Hydrothermal synthesis of hydroxyapatite crystals with and without seed crystals and surfactant. Discov Appl Sci 6, 143 (2024). https://doi.org/10.1007/s42452-024-05754-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05754-z