Abstract
This study describes the preparation of cellulose nanofibers (CNFs) with varying amounts of carboxyl groups from rice straw pulp using the TEMPO/NaBr/NaClO oxidation system. The resulting CNFs were found to be in the form of nanofibers with an average diameter of 6 nm and an average length of 160 nm. To further enhance their properties, the CNFs were grafted with polycaprolactone (PCL) to create CNFs-g-PCL, which was then blended with shape memory polyurethane (SMPU) to produce CNFs-g-PCL/SMPU composites. It was observed that as the carboxyl content in CNFs increased from 0.35 to 1.14 mmol/g, the graft ratio of PCL on CNFs decreased from 24.6 to 10.7%. Consequently, the hydrophobicity of the grafted product (CNFs-g-PCL) also decreased. When 10% CNFs-g-PCL was added to the SMPU matrix, the elastic modulus and tensile stress of the resulting composite were both higher than those of the pure SMPU, increasing by up to 54.4% and 67.3%, respectively. Additionally, the shape retention and shape recovery rates of the composite remained stable after addition of CNFs-g-PCL. In conclusion, incorporating CNFs-g-PCL into SMPU can improve its mechanical properties while maintaining its shape memory properties.
Graphical abstract

Article Highlights
-
1.
Investigated the effect of CNFs with different carboxyl group contents on the grafting of polycaprolactone;
-
2.
Examined the impact of varying amounts of CNFs-g-PCL on the mechanical and shape memory properties of CNFs-g-PCL/SMPU composite films;
-
3.
An appropriate amount of CNFs-g-PCL can enhance the mechanical properties while maintaining the elastic deformation characteristics of SMPU.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
SMPU is a type of shape memory polymer that has been widely studied due to its excellent properties such as a wide range of glass transition temperatures, good biocompatibility, and ease of processing [1,2,3]. However, it also has some drawbacks including low mechanical strength, slow recovery rate, small shape recovery force, and poor chemical durability. These limitations have restricted its application scope [4, 5]. To overcome these limitations, researchers have explored the use of particle fillers to reinforce the polyurethane matrix [6]. Among the various particle fillers developed, nanocellulose stands out as a promising candidate due to its ability to significantly improve the mechanical properties of the composite even at low concentrations [7]. The high surface activity of cellulose nanoparticles allows for strong binding with polyurethane at the molecular level, resulting in enhanced Young’s modulus, tensile strength, storage modulus of the material. Studies have shown that the degree of enhancement is related to the filling ratio of nanocellulose [8, 9]. Therefore, incorporating nanocellulose into SMPU can potentially enhance its mechanical properties and broaden its application scope. Nanocellulose has become an attractive material due to its numerous advantages, including its ability to enhance the mechanical properties of composites [10]. One effective method for preparing nanocellulose is through TEMPO oxidation, which selectively oxidizes the C-6 hydroxyl group in cellulose to a carboxyl group. This process generates electrostatic repulsion between the carboxyl groups, promoting the dispersion of cellulose and reducing agglomeration and sedimentation during high-pressure homogenization [11, 12]. The resulting materials, called CNFs, exhibit single-radical and dispersed characteristics. The carboxyl content of oxidized cellulose is a crucial parameter for CNFs preparation, with two main influencing factors [13]. On one hand, the electrostatic repulsion between carboxyl groups on the surface of cellulose accelerates fiber dissociation; On the other hand, the carboxyl group improves the hydrophilicity of the fiber, significantly increasing the degree of mono-radicalization of nanocellulose, avoiding agglomeration and sedimentation, and forming a well-dispersed suspension in water [14, 15].
Currently, when nanocellulose is used as a filler in shape memory composite, ensuring good shape recovery performance has been the focus and challenge of research. Studies have shown that as the amount of cellulose increases in the dry state, the shape recovery performance of the composite gradually deteriorates, which greatly affects the overall shape memory performance and ductility of the material [16]. Since nanocellulose itself cannot respond to environment changes, the recovery performance of shape memory composites mainly depends on the matrix. However, the microstructure deformation between the matrix and the fiber also plays a significant role in determining the recovery performance. Furthermore, nanocellulose is a highly rigid material with numerous hydroxyl groups on its surface. The hydrophilicity of these hydroxyl groups leads poor compatibility between nanocellulose and the polyurethane matrix, affecting polymer microphase separation and further reducing the shape recovery performance and ductility of the composite [15, 17].
To address the aforementioned issues, researchers have explored the incorporation of modified nanocellulose into SMPU composites. Studies have shown that when modified nanocellulose is used as a filler and the grafted chain structure aligns with that of the polyurethane matrix, a continuous interface can be formed between the two materials, leading to enhanced interfacial binding forces [18]. This effect is particularly pronounced when the grafted molecular weight is sufficiently high, resulting in entanglement between the grafted and the un-grafted chains. In this study, CNFs were prepared by oxidizing straw pulp using the TEMPO oxidation system under alkaline conditions. By controlling the amount of NaClO, CNFs with varying carboxyl content were obtained. These CNFs were then modified by grafting PCL and incorporated into SMPU to create CNFs-g-PCL/SMPU composites. The impact of CNFs-g-PCL on the mechanical properties and shape memory characteristics of the resulting composite was investigated.
2 Experimental parts
2.1 Experimental materials
Rice stalks were taken from Yueyang village, Hunan Province. TEMPO was acquired from Aladdin Chemical Co. Ltd. (Shanghai, China). Other reagents such as sodium bromide (NaBr), sodium hypochlorite (NaClO), sodium hydroxide (NaOH), hydrochloric acid, toluene, acetone, dichloromethane, etc. were purchased from Guangzhou Chemical Reagent Factory (Guangzhou, China). Polyurethane (soft chain segment for PCL) purchased from Shanghai Sanji Chemical Co., Ltd. ε-caprolactone (ε-CL) and stannous octoate (Sn(OCt)2) were purchased from Aladdin Chemical Co., Ltd. Both acetone and toluene need to be dehydrated before use. All solutions in the experiment were prepared from deionized water.
2.2 Experimental procedure
2.2.1 Preparation of CNFs
To prepare CNFs, the rice straw was cut into small segments of about 10 cm for easy handling. First, the rice straw was thermally treated at 70 °C. Subsequently, NaOH was used to remove most of the lignin and hemicellulose from the straw under the following conditions: NaOH accounted for 12% of the absolute mass of the straw, prepared with a deionized aqueous solution. The ratio of feed to liquid was controlled at 1:6 and cooked in a horizontal rotary pan (KRK 2611, Kumagai Riki Kogyo Co., Ltd., Japan) at 110 °C for 2 h. After pretreatment, rice straw pulp was obtained, washed with deionized water several times, and stored in 4 °C refrigerator. The TEMPO oxidation reaction was carried out in a glass beaker, weighing 10 g rice straw pulp (completely dry) and adding deionized water to make the pulp concentrated to 1% (w/w). Place the beaker in a thermostatic water bath at 25 °C and stir continuously (300 rpm). The dosage of TEMPO and NaBr were 0.015 g/g and 0.1 g/g, respectively. The slurry was continuously stirred with a cantilever agitator to ensure a constant reaction system temperature, and the slurry and reagent were fully mixed. After stirring for 15 min, NaClO was added at one time, and the dosage of NaClO was 2 mmol/g absolute dry slurry, 4 mmol/g absolute dry slurry and 6 mmol/g absolute dry slurry, respectively. After 8 mmol/g dry pulp and 10 mmol/g dry pulp, TEMPO oxidation reaction began. In the reaction process, with the formation of carboxyl groups, the pH of the system decreased. In order to maintain the pH value of the reaction solution at about 10, 0.5 mol/L NaOH standard solution was added to keep the pH at about 10, and the pH decline rate slowed down with the progress of the reaction. When the pH of the system did not change more than ± 0.01 within 15 min, it was considered that the end point of TEMPO oxidation was reached and the reaction was over. Add a lot of deionized water, wash and filter repeatedly, collect the oxidized cellulose on the filter cloth, put it in the refrigerator at 4 °C.
Weigh a certain amount of oxidized cellulose into the beakers, add a certain amount of 0.1 mol/L hydrochloric acid solution, controll the mass fraction of CNFs in the mixture to about 1.2% (w/w), and stir at room temperature for 2 h. After the acidification treatment was completed, it was washed repeatedly and filtered with deion until the filtrate was neutral. Then, the ultrasonic treatment was carried out with an ultrasonic cell shredder. The ultrasonic conditions were as follows: the power was 1200W, the ultrasound was carried out for 30 min, and then centrifuged with a large amount of deionized water, and then dialysis to a pH value of about 7 to obtain TEMPO-oxidized/ultrasound-dispersed nanofibers, which were recorded as CNFs. Keep CNFs in the refrigerator for subsequent processing and analysis.
2.2.2 CNFs surface grafted PCL
PCL was grafted on CNFs with different carboxyl content by ring-opening polymerization, and the effect of different carboxyl content on the grafting reaction was studied. The specific steps are as follows: ε-CL monomer was added for 30 g and ultrasound was carried out for 5 min to ensure that carboxycellulose was dispersed in ε-CL. The suspension was transferred to a dry two-flask, and the reaction system was heated to 110 °C. Stannous octoate (Sn(OCt)2) catalyst was slowly added under the protection of argon gas. The dosage was 2 wt% (relative to the amount of monomer), the reaction time reached more than 24 h. The resultant CNFs-g-PCL was poured into dichloromethane and centrifuged with dichloromethane. Finally, CNFs-g-PCL was obtained by vacuum at 45 °C, and then weighed.
Grafting percentage (GP %) was obtained from Formula 1:
where MCNFs was the mass of cellulose before grafting, and MCNFs-g-PCL was the mass of cellulose after grafting.
2.2.3 Preparation of CNFs-g-PCL/SMPU complex
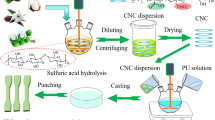
Add the prepared CNFs-g-PCL and shape memory polyurethane (SMPU) into DMF, stir to dissolve it, and prepare a solution with a concentration of 10 wt%. Stir at room temperature for 6 h to allow the CNFs-g-PCL to disperse in the SMPU solution uniformly. Transfer the mixture into a polytetrafluoroethylene mold, place it in a 45 °C oven for 12 h to evaporate the solvent, and then vacuum dry it at 60 °C for 12 h to obtain a composite with various weight fractions of CNFs-g-PCL. The material ratio was reported as CNFs-g-PCL/SMPU-x%, where x was the percentage of CNFs-g-PCL in the composite. The film thickness was measured with a thickness gauge and was approximately 100 μm (Fig. 1).
As a control, unmodified CNFs were used as reinforcements and compounded with SMPU using the same method to prepare CNF/SMPU composites with varying CNF contents. The obtained composite material was recorded as CNFs/SMPU-y%, where y% was the mass fraction of CNFs in the composite, depending on the number of CNFs filled in.
2.2.4 Calculation of oxide pulp yield
The calculation method for the yield of oxidized pulp was as follows: collect the water-insoluble part of oxidized pulp from the filter cloth, wash it several times to remove impurities, carefully transfer it into a self-sealing bag, place it in the refrigerator for 2 days to balance the moisture, weigh it, and perform a moisture test. The calculation of the yield of oxidized pulp was shown in Formula 2:
where W1 represented the mass of the raw material, g; W2 represented the absolute dry mass of the water-insoluble portion of the oxidized cellulose retained by the filter cloth, in grams.
2.2.5 Determination of carboxyl content in oxidized pulp
Before determining the carboxyl content, the CNFs needed to be acidified. The process was as follows: weigh some CNFs in a beaker, add a certain amount of 0.1 mol/L hydrochloric acid solution, control the mass fraction of CNFs in the mixture to around 1.2% (w/w), and stir at room temperature for 2 h. After the acidification treatment was completed, wash and filter the solution repeatedly until the filtrate was neutral. Finally, the CNFs that had been removed from impurities were naturally air-dried and placed in an ISO constant temperature and humidity laboratory for more than 48 h to equalize the moisture content.
In this study, the determination of carboxyl content was based on headspace gas chromatography (HS-GC). The process was as follows: accurately weigh 0.03 g of air-dried CNFs sample and place it in a headspace reaction vial. Add the pre-prepared reactants (0.05 mol/L NaHCO3 + 0.10 mol/L NaCl) to the reaction vial, seal it, and shake the reaction vial appropriately to disperse the CNFs fibers in the vial. Then place the headspace vial in a headspace autosampler for carboxyl content determination, with three measurements for each sample.
2.2.6 Transmission electron microscopy (TEM) analysis
The particle size and morphology of CNFs with different carboxyl content were characterized by TEM under an electron microscope acceleration voltage of 80 kV. The method was as follows: prepare a suspension of CNFs at a concentration of 0.01% (w/w) and disperse the fibers evenly using ultrasonic vibration in an ultrasonic cleaning machine. Absorb a drop of liquid (20 μL) on a copper mesh (200 mesh), negative staining the CNFs with 3% (w/w) phosphotungstic acid staining reagent, blotting off excess staining reagent with a clean filter paper, and placing them in a desiccator for 12 h. During the test, multiple points were selected for each sample to be observed.
2.2.7 FT-IR analysis
FT-IR is an important tool for studying the chemical composition and structure of materials and their changes. Combine rice straw pulp, freeze-dried CNFs and modified CNFs-g-PCL with KBr powder in a mortar, with a mass ratio of 1:100, and grind to powder. Pour the powder into the tablet press, press it into transparent thin tablets for testing. The wavelength range for the measurement is 4000–400 cm−1.
2.2.8 Surface contact angle analysis
The changes in hydrophobicity of CNFs with different carboxyl content before and after modification were represented by changes in surface contact angle. The contact angle of the grafted samples was measured and compared using a high-precision video contact angle meter (Data Physics OCA 40 Micro, Germany) at room temperature. The specific steps were as follows: grind the sample into powder in a mortar, pour it into a tablet machine, and compress it into small round tablets with smooth surfaces under a pressure of 10 bar. During the test, a drop of ultrapure water (5–10 μL) was placed on the surface of the sample, then recorded the contour image. The contact angle was measured at the intersection point where the base line and the liquid contour form a tangent. Image correction was based on the Young’s-Laplace equation.
2.2.9 Tensile test
The mechanical properties of SMPU and CNFs-g-PCL/SMPU composite membranes were analyzed through tensile experiments. The testing methods were as follows: The tensile properties of pure SMPU membrane and CNFs-g-PCL/SMPU composite membrane were measured using a universal material testing machine. Cut the sample into an I-shaped shape with a total length of 40 mm (gauge length of 20 mm) and a narrowest width of 10 mm. The thickness of the sample can be determined by using a thickness gauge, and the average thickness of 6 points can be used as the thickness of each sample. During the experiment, the force sensor was set to 100 N, and the sample was stretched at a speed of 50 mm/min. Through calculation, the mechanical properties of the sample, such as Young’s modulus, tensile strength, and elongation at break, were obtained to analyze the mechanical properties of the sample. Measure the same sample five times and calculate the average value. Before testing, the samples were placed in a constant temperature and humidity (temperature 25 °C, relative humidity 50%) room for five days continuously.
2.2.10 Shape memory performance test
Because SMPU in this chapter is a hydrophobic, thermo-induced shape memory material, it is possible to complete the shape memory performance testing of SMPU and its composite materials in warm water. The main method was to measure the length of the sample and measure its shape memory performance quantitatively. The method was as follows: cut the sample into a rectangular thin film of size 20 mm × 5 mm, accurately record the initial length L0 of the sample, place the sample in a water bath at a constant temperature (60 °C), hold for one minute to transform the sample into a high elastic state at this temperature, apply an external force at this time, slowly stretch the sample to 200% of its original length under the action of the external force, and then quickly place the stretched sample into a 0 °C mixture of ice and water while maintaining the external force to freeze the stress and accurately measure the results. At this point, the sample length is L1. Remove the external force, the sample will rebound and the length of L2 should be measured at this point. Then put the sample again in a 60 °C water bath and observe its shape regain, measuring the sample’s length L3 at this point. The shape fixation ratio (Rf) and shape recovery ratio (Rr) of the sample can be calculated using Formulas 3 and 4 as parameters for quantitative analysis of the shape memory properties of the sample.
3 Results and discussion
3.1 Analysis of carboxyl content and yield
Under alkaline conditions (pH 10–10.3), rice straw pulp was subjected to TEMPO/NaBr/NaClO oxidation, resulting in the refinement of cellulose and the loosening of fiber structure. This process also led to the generation of a substantial number of carboxyl groups on the surface of the microfibers, in the form of sodium carboxylate. This process causes the cellulose surface to carry the same negative charge, weakening the hydrogen bonding between cellulose filaments under the action of electrostatic repulsion. As a result, the adhesion between cellulose fiber molecules is reduced, which promotes subsequent mechanical treatment of CNFs and reduces energy consumption during processing [19, 20]. The NaClO concentration in the TEMPO/NaBr/NaClO oxidation system significantly influenced the reaction time, carboxyl content, and yield of CNFs. The level of carboxyl content had a profound impact on the ease of fibrillation of CNFs. Figure 2 illustrates how the carboxyl content and CNFs yield vary as the NaClO content rises.
During the catalytic oxidation process using TEMPO, an increase in NaClO dosage led to a corresponding rise in carboxyl content and CNFs yield. This finding is consistent with a previous study by Ryoya Hiraoki et al. [21], which used coniferous wood bleached kraft pulp as the raw material and observed that carboxyl group content increased with NaClO dosage. However, it was also observed that increasing NaClO dosage resulted in a decrease in the yield of oxidized pulp from 92 to 75% due to higher oxidation degrees leading to lower fiber yields. The degradation of cellulose chains and the oxidation reaction to generate carboxylic groups are random processes. As the NaClO dosage increased, the carboxyl group content rose, but the reaction did not cease. It continued to degrade the cellulose chain further, causing some glucose molecular chains that had already formed carboxyl groups at C6 position to detach from the crystal surface and become water-soluble sugars, which were lost during the washing process. Consequently, while carboxyl group content increased, the yield decreased.
3.2 Analysis of surface morphology features
CNFs were synthesized using the TEMPO oxidation/ultrasonic dispersion method. The resulting CNFs displayed variations in particle size and aspect ratio due to differences in oxidation degrees. To examine the surface morphology and size of CNFs, we employed TEM for characterization and analysis, as depicted in Fig. 3. The corresponding carboxyl content for each sample (a–e) was 0.35 mmol/g, 0.47 mmol/g, 0.71 mmol/g, 0.83 mmol/g, and 1.14 mmol/g, respectively.
As observed from Fig. 3, CNFs with varying carboxyl contents exhibited good dispersibility and had a rod-like nanocrystal morphology. Despite the differences in carboxyl contents, the overall morphology of CNFs remained consistent. It was noted that when the carboxyl group content was lower, the repulsion force between nanocellulose particles was weaker, potentially leading to particle aggregation. In contrast, an increase in carboxyl groups content strengthened the electrostatic repulsion between crystals, resulting in improved dispersion between fibers [22].
To determine the particle size and distribution of CNFs, 100 particles were randomly selected from the TEM images for statistical analysis. This allowed us to obtain the length and diameter distribution of CNFs with varying carboxyl content, as depicted in Fig. 4. The corresponding carboxyl content for each sample (a–e) was 0.35 mmol/g, 0.47 mmol/g, 0.71 mmol/g, 0.83 mmol/g, and 1. 14 mmol/g, respectively.
a1, b1, c1, d1, e1 Length distribution of CNFs with carboxyl contents of 0.35 mmol/g (a1), 0.47 mmol/g (b1), 0.71 mmol/g (c1), 0.83 mmol/g (d1), and 1.14 mmol/g (e1); a2, b2, c2, d2, e2 Width distribution of CNFs with carboxyl contents of 0.35 mmol/g (a2), 0.47 mmol/g (b2), 0.71 mmol/g (c2), 0.83 mmol/g (d2), and 1.14 mmol/g (e2)
As shown in Fig. 4, the diameter and length of CNFs with varying carboxyl content were not significantly different, with diameters mainly ranging from 4 to 8 nm and lengths mainly between 120 and 220 nm. This can be attributed to the β-elimination reaction and degradation of amorphous regions, which reduce the polymerization degree of cellulose to a certain range. Additionally, there is a linear relationship between fiber length and polymerization degree [23]. However, previous studies have demonstrated that the length (degree of polymerization) of CNFs is influenced by the oxidation and ultrasonic conditions employed [23, 23]. During these processes, cellulose undergoes random degradation and oxidation. While TEMPO introduces carboxyl groups on the surface of the amorphous region of cellulose, it also causes the glucoside bond in this region to cleave, resulting in shortened cellulose molecular chains and reduced macromolecule degradation into small units. This results in a decrease in polymerization degree, which subsequently leads to a reduction in fiber length. As the amount of NaClO increases, so does the carboxyl content, leading to an increased degree of cellulose degradation. Ultimately, the degree of polymerization stabilizes, and the fiber length did not change significantly.
3.3 Chemical structure analysis of CNFs and CNFs-g-PCL
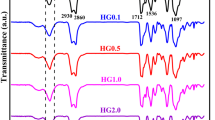
The FT-IR scanning of rice straw pulp and CNFs with varying carboxylic contents was conducted to analyze the changes in chemical groups within the CNFs molecules, as shown in Fig. 5a. As observed form the figure, both the straw pulp and CNFs with different amounts of carboxylic groups exhibited strong absorption peaks at 3351 cm−1, 2904 cm−1,1620 cm−1, and 1427 cm−1, which represented stretching and bending vibrations in the O–H bond, C–H bond, and O–H–C–H bonds, respectively. This indicated that the TEMPO oxidation process did not destroy the structure of cellulose. Following the TEMPO oxidation, the spectral band of the hydrogen bonding in the hydroxy stretch vibrational range of CNFs shifted to a lower wavenumber direction, and the characteristic peak strength increased, suggesting that both the intermolecular and intramolecular hydrogen bonds in CNFs had both been strengthened, which may be related to the oxidation of alcohol-OH groups in CNFs molecules. The carboxylic group in the product existed as -COONa. After being acidified, the carboxyl group exists as –COOH, which occurred at a wavelength of 1730 cm−1. This peak indicated that cellulose was oxidized, and as the amount of the carboxyl group increased, the intensity of the oscillation of the-COOH at this wavelength also increased. This result was consistent with that of Carlsson et al. [24]. As shown in Fig. 5b, before grafting, the carboxylated nanocelluloses showed strong absorption peaks at 3351 cm−1, 2904 cm−1, 1730 cm−1, 1620 cm−1, and 1427 cm−1, corresponding to the stretching vibration of O–H, the stretching vibration of C–H, the carbonyl (C=O) stretch in –COOH, the bending vibration of O–H, and the bending vibration of C–H, respectively. The broad peak at 3351 cm−1 indicates strong hydrogen bonding between the carboxylated nanocellulose molecules. After grafting, a strong absorption peak appeared at 3605 cm−1, which is characteristic of free hydroxyl groups, and there was still an absorption peak for hydroxyl groups at 3200 cm−1. The absorption peak for –COOH at 1730 cm−1 decreased, and the stretching vibration peak for C–H at 2900 cm−1 significantly increased, with the appearance of a characteristic absorption peak for C=O at 1610 cm−1. This may be due to the consumption of some hydroxyl groups during the reaction of nanocellulose grafted with caprolactone with the hydroxyl groups on the cellulose surface, which disrupted some hydrogen bonds. Some of the caprolactone might have reacted with the hydroxyl groups in the carboxylic acid, leading to the decrease in the characteristic absorption peak for –COOH at 1730 cm−1. On the other hand, the grafting of caprolactone onto nanocellulose introduced –CH2 groups, which significantly increased the stretching vibration peak for C–H at 2900 cm−1 [25].
3.4 The effect of different carboxyl contents on the thermal stability of nanocelluloses before and after modification
The thermal stability of nanocelluloses with different carboxyl contents and their modified products was analyzed by TG, and the TG and DTG curves are shown in Fig. 6. As can be seen from Fig. 6, when the temperature was raised to around 100 °C, there was a slight decrease in the weight of all samples, which was due to the evaporation of moisture present in the samples. The surface chemical modification by grafting PCL altered the thermal stability of the nanocelluloses. The initial thermal degradation temperatures for nanocelluloses with carboxyl contents of 0.35 mmol/g, 0.47 mmol/g, 0.71 mmol/g, 0.83 mmol/g, and 1.14 mmol/g were 285 °C, 282 °C, 289 °C, 270 °C, and 262 °C, respectively. After grafting, the corresponding initial thermal degradation temperatures were 257 °C, 261 °C, 268 °C, 265 °C, and 259 °C, respectively. Before grafting, nanocelluloses with lower carboxyl content had relatively higher initial decomposition temperatures. However, after grafting, those with lower carboxyl content exhibited lower initial degradation temperatures. This is because nanocelluloses with lower carboxyl content had higher grafting rates, and the PCL in the modified products could act as a ‘mediator’ during the thermal degradation process of cellulose [26]. Modified products with higher PCL content showed a greater reduction in initial degradation temperature. From the DTG graph, it is evident that compared to the ungrafted nanocelluloses, the maximum thermal degradation temperature (Tmax) of the modified products was significantly reduced.
3.5 Effect of nanocelluloses before and after modification on the dynamic thermal mechanical properties of composites
Through dynamic thermomechanical property analysis, the changes in storage modulus (E′) and loss factor (tanδ) of composites with temperature were studied, and the results are shown in Fig. 7. Figure 7a shows the change in E′ of the composites with temperature. It can be seen that as the temperature increases, the motion of the matrix molecular chains intensifies, and the storage modulus of the sample gradually decreases. The variation of storage modulus with temperature indicates that the composite is prone to deformation at high temperatures, while it has the ability to resist deformation at low temperatures [27]. After adding CNFs and CNFs-g-PCL with a mass fraction of 5% to SMPU, the storage modulus of the material was improved, but the enhancement effect of CNFs-g-PCL on SMPU was significantly better than that of CNFs/SMPU. In addition, the compatibility of the blend system can be characterized by observing its glass transition temperature (Tg). If the blend has only one glass transition temperature, it can be considered a compatible homogeneous system from a macroscopic thermodynamic perspective. If there are two or more glass transition temperatures, and they show a trend of approaching each other, this indicates that the components of the blend are partially compatible, and the determination of the degree of compatibility is related to the shift in the glass transition temperatures of the components. Figure 7b shows the loss factor (tanδ) versus temperature curves for CNFs/SMPU, SMPU, and CNFs-g-PCL/SMPU. The peak value of the Tan δ curve corresponds to the glass transition temperature (Tg) of the sample. Both SMPU and CNFs-g-PCL/SMPU have only one glass transition temperature, at − 21 °C and 2 °C respectively, while CNFs/SMPU shows two glass transition temperatures, at − 18 °C and 80 °C. Therefore, it is indicated that the interfacial compatibility of CNFs-g-PCL with the SMPU matrix is better than that of CNFs, and CNFs-g-PCL can be more uniformly dispersed in the matrix.
3.6 Effect of hydrophobicity of CNFs with different carboxyl content before and after modification
The effect of carboxyl content on the hydrophobic properties of CNFs before and after modification was analyzed using static contact angle analysis, as shown in Fig. 8. The contact angles of modified CNFs with carboxyl contents of 0.35 mmol/g, 0.47 mmol/g, 0.71 mmol/g, 0.83 mmol/g, and 1.14 mmol/g are represented by a to e, respectively, while f represents the contact angle of CNFs before grafting. As observed from Fig. 8, the initial contact angle of CNFs was 26.6°, indicating strong hydrophilicity due to rapid absorption of water droplets on the sample surface. However, after the grafting reaction, the surface absorption rate of water droplets decreased, and the surface contact angle increased for modified CNFs with different carboxyl contents. This increase in contact angle is attributed to the hydrophobic PCL coating on the CNFs-g-PCL surface. The contact angles of the modified products of CNFs with carboxyl contents of 0.35 mmol/g, 0.47 mmol/g, 0.71 mmol/g, 0.83 mmol/g, and 1.14 mmol/g were found to be 110.5°, 99.1°, 94.3°, 91.6°, and 74.2°, respectively. Notably, the contact angles of the modified products with lower carboxyl content were larger than those with higher carboxyl content, suggesting that more hydrophobic PCL was grafted onto the surface of the CNFs with lower carboxyl content [28], which is similar to the measured grafting percentage (GP) of nanocelluloses with varying carboxyl contents (Table 1).
The analysis of CNFs with varying carboxyl contents and grafting PCL for ring opening polymerization revealed that CNFs with high carboxyl content offer fewer reactive sites for monomer opening polymerization due to the oxidation of more hydroxyl groups to carboxyl groups. This results in lower graft chain content and density in the obtained grafted products. In contrast, CNFs with low carboxyl content exhibit high graft density and yield. The modified products of these CNFs display greater contact angles and hydrophobic properties.
The lower the carboxyl content of CNFs, the higher the grafting PCL density. However, further investigation is needed to determine if CNFs with higher grafting PCL density have a better impact on the performance of composite materials. To study the effect of CNFs-g-PCL with varying grafting contents on the mechanical properties and shape memory properties of SMPU, the obtained CNFs-g-PCL was combined with SMPU to create a composite with stable mechanical and deformation properties. The influence of CNFs-g-PCL with different carboxyl content and grafting efficiency on the properties of SMPU composite was examined.
3.7 Mechanical properties of CNFs-g-PCL/SMPU composite membranes
To investigate the effect of adding CNFs-g-PCL on the mechanical properties of the composite, CNFs with an appropriate carboxyl content and CNFs-g-PCL were selected and mixed with SMPU separately. A series of CNFs/SMPU and CNFs-g-PCL/SMPU composites with varying CNFs contents were prepared using the solution casting method (CNFs/SMPU-x% or CNFs-g-PCL/SMPU-y%, where x represents the percentage content of CNFs in the composite, and y represents the percentage content of CNFs-g-PCL in the composite). These composites were stretched to study the changes in Young’s modulus, elongation at break, and tensile strength during the stretching process as a function of the additive content (Fig. 9).
As shown in Table 2, the test results showed that the Young’s modulus of the pure SMPU film was 1.80 Mpa, with a tensile strength of 15.29 Mpa and an elongation at break of 870%. When CNFs-g-PCL was added to the SMPU film at concentrations of 0.5%, 0.8%, 2%, 5%, and 10%, the corresponding Young’s modulus values for the CNFs-g-PCL/SMPU composite were 2.35 Mpa, 2.31 Mpa, 2.84 Mpa, 2.36 Mpa, and 2.40 Mpa, respectively. The tensile strength was 23.78 Mpa, 22.33 Mpa, 22.82 Mpa, 17.14 Mpa and 15.67 Mpa, while the elongation at break values were 1163%, 1225%, 1068%, 884%, and 727%, respectively.
As shown in Fig. 9, the CNFs-g-PCL/SMPU composites exhibited an increasing trend in elastic modulus, tensile strength, and elongation at break as the content of CNFs-g-PCL increased, followed by a decreasing trend. The elastic modulus and tensile stress of the CNFs-g-PCL/SMPU-2 composite membrane increased by 57.8%, from 1.80 to 2.84 Mpa. The CNFs-g-PCL/SMPU-0.8 composite membrane reached its maximum values for tensile strength and elongation at break, with tensile strength increasing by 49.2% (from 15.29 to 22.82 Mpa) and elongation at break increasing by 40.8% (from 870 to 1225%). After reaching their maximum values, the elastic modulus, tensile strength, and elongation at break all decreased, with tensile strength and elongation at break declining at a faster Decision in Process rate. Notably, the elastic modulus, tensile strength, and elongation at break of the CNFs-g-PCL/SMPU-10 composite decreased significantly, but they still increased by 33.3%, 28.4%, and decreased slightly (by 4.9%) compared to pure SMPU, respectively.
When the addition amount of CNFs was increased to 5% and 10%, the dispersion in the SMPU matrix deteriorated, leading to significant agglomeration and an uneven distribution of CNFs/SMPU composite. The experimental results were not reliable enough to justify further testing. On the other hand, although the dispersion of CNFs-g-PCL in the CNFs-g-PCL/SMPU composite was better than that of CNFs, increasing the mass concentration of CNFs-g-PCL compromised the compactness of the composite, resulting in poor dispersion of CNFs-g-PCL in the SMPU matrix, weakening the interfacial interactions between the two materials. Additionally, the presence of CNFs-g-PCL acted as an inhibitor between the SMPU molecules, increasing their distance and reducing intermolecular forces. However, it was challenging to counteract the interaction between CNFs-g-PCL and SMPU molecules. Furthermore, the PCL chain on the surface of CNFs-g-PCL improved the dispersion of CNFs, resulting in higher elastic modulus, tensile stress, and elongation at break compared to pure SMPU at high levels of addition (5% and 10%).
The aforementioned analysis revealed that incorporation of CNF-g-PCL into the SMPU matrix resulted in a more pronounced enhancement in mechanical properties compared to the addition of CNFs alone, with only a minor impact on material ductility. Even at high loading levels (10%), the CNFs-g-PCL/SMPU composite exhibited higher elastic modulus and tensile stress than pure SMPU, albeit with a slight reduction in elongation at break.
The mechanical properties of composite polymers are largely determined by the reinforcing phase, and the interfacial adhesion strength between CNFs-g-PCL and SMPU plays a crucial role in affecting these properties. When external forces are transferred from the matrix to the reinforcing phase, weak bonding interfaces can lead to failure in force transfer and poor mechanical performance [29]. Therefore, the mechanical properties of CNFs-g-PCL/SMPU composites depend on factors such as the addition amount of CNFs-g-PCL and the grafting rate (degree of modification) of CNFs. Additionally, due to the low grafting rate of CNFs, the PCL content in CNFs-g-PCL is relatively low, necessitating an appropriately high filling amount of CNFs-g-PCL. Even at a filling amount of 10%, the CNFs-g-PCL/SMPU composite membrane still exhibits higher elastic modulus and tensile stress than pure SMPU, with only a slightly decrease in elongation at break. Based on this result, a series of CNFs-g-PCL/SMPU-y (y represents the carboxyl content before modification, mmol/g) composite membranes were prepared through solution casting. Their mechanical properties are shown in Fig. 10 below.
As shown in Fig. 10, our analysis revealed that the elastic modulus (up to 54.4%) and tensile stress (up to 67.3%) of the CNFs-g-PCL/SMPU composite membrane with lower carboxyl content (0. 35 mmol/g, 0. 47 mmol/g, 0. 71 mmol/g) were higher than those of pure SMPU membrane. The elongation at break of the CNFs-g-PCL/SMPU composite membrane was also higher than that of the pure SMPU membrane, except when the carboxyl content reaches 0.83 mmol/g and 1.14 mmol/g, where the elongation at break was lower than that of the pure SMPU membrane.
As the carboxyl content in CNFs-g-PCL/SMPU composite increased, the elastic modulus of the composite also increased, while the tensile strength and elongation at break decreased. This is because when the addition amount was 10%, the rigid CNFs had a greater impact on the elastic modulus of the composite, leading to a significant increase in its elastic modulus. However, as the content of carboxyl groups increased, the grafting rate of CNFs-g-PCL decreased. This meant that at the same addition level, CNFs-g-PCL with high carboxyl content had lower caprolactone content and higher CNFs content, resulting in a more pronounced increase in elastic modulus. On the other hand, the low caprolactone content made it more challenging to form a low crystalline state on the surface of CNFs, which was not conducive to forming a good adhesive interface between the matrix and filler, thereby reducing surface adhesion. In addition, the increase in carboxyl content decreased the contact angle of CNFs-g-PCL, leading to reduced compatibility between CNFs-g-PCL and polyurethane, resulting in decreased tensile strength and elongation at break.
3.8 Shape memory properties of CNFs-g-PCL/SMPU composites
Figure 11 illustrates the shape memory properties of CNFs-g-PCL/SMPU composites with varying carboxyl content (10% CNFs-g-PCL) as compared to pure SMPU.
The shape memory process of composite materials involves heating SMPU to a high elastic state, applying external forces to deform SMPU and alter its initial shape, followed by rapid cooling. During this process, the polymer chains in SMPU transition from the high elastic state to either a glassy or crystalline state, where any uncompleted reversible deformation is “frozen” into the molecular chain as internal stress. This phenomenon demonstrates the sample’s shape-fixing properties. Subsequently, when the SMPU is heated again to a high elastic state, and the movement of polymer chain segments intensifies. Driven by the internal stress, the unfinished reversible deformation is completed, showcasing the sample’s shape recovery performance [30].
Figure 11 illustrates the shape retention and recovery rates of CNFs-g-PCL/SMPU composite membranes when the CNFs-g-PCL addition was 10%. Rf represents the material’s ability to fix instantaneous deformation, while Rr signifies its ability to return to its original shape. The Rf and Rr values for pure SMPU membranes were 86% and 93%, respectively. The shape fixity of SMPU is influenced by the strength of the material in its glassy state. A higher strength in the glassy state results in a lower tendency for deformation and smaller deformation occurrences. The greater the reversible deformation that occurs due to internal stress freezing, the better the material’s ability to fix or freeze the deformation, leading to a higher rate of shape fixation [9, 30].
When the CNFs-g-PCL addition was 10%, the Rf of the composite material ranged from 83 to 90%, which was not significantly different from that of pure SMPU. Based on the mechanical property analysis, it is believed that the formation of a co-continuous phase between CNFs-g-PCL and the matrix interface can significantly improve the elastic modulus of the material. During the tensile process, there was no irreversible deformation, and all deformations were reversible, indicating that the shape fixation rate of the composite material changed mildly. The Rf of the composite material fluctuated within 90% to 95%, which was consistent with that of pure SMPU. This is because CNFs-g-PCL can form a co-continuous phase with the matrix, facilitating micro-phase separation in the composite and having little effect on the Rr variation of the material. The elastic deformation properties of the composite material were not significantly altered and were well maintained.
The incorporation of PCL onto the modified surface of CNFs with varying carboxyl content resulted in a well-dispersed grafted modified CNFs within polyurethane, forming a co-continuous phase with the soft segment of polymer. Even at a high addition level of 10%, the shape fixation and recovery rates of the composite materials remained relatively unchanged. The mechanical property analysis revealed that the elastic modulus, tensile stress, and elongation at break of the composite material were significantly enhanced. Therefore, it is feasible to enhance the mechanical properties of polyurethane while preserving its elastic deformation properties through modification.
4 Conclusions
The study successfully prepared a series of CNFs with varying carboxyl content using rice straw pulp as the raw material through TEMPO oxidation and ultrasound dispersion. The structure and morphology of both the rice straw pulp and CNFs were studied using TEM and FT-IR, revealing rod-shaped nanocrystalline cellulose morphology with an average diameter of 6 nm and length of 160 nm. FT-IR analysis showed that the TEMPO oxidation process increased hydrogen bonding but did not alter the chemical structure of the CNFs. The relationship between NaClO usage and carboxyl content in the CNFs was investigated, showing a direct proportional relationship. This suggests that by adjusting the amount of oxidant used, CNFs with different carboxyl contents can be produced. The CNFs were then grafted onto caprolactone to prepare CNFs-g-PCL. As carboxyl content increased, grafting efficiency and PCL content decreased. Contact angle measurements showed that CNFs with lower carboxyl content had a more hydrophobic surface and were easier to graft with PCL. When added to composite materials at the same concentration, CNFs-g-PCL enhanced mechanical properties compared to pure CNFs while maintaining ductility. Even at high loadings (10%), CNFs-g-PCL exhibited superior elastic modulus, tensile strength, and elongation compared to pure SMPU. The shape retention and shape recovery properties of the composite materials remained unchanged. Overall, CNFs-g-PCL has great potential for enhancing the mechanical properties of polyurethane while maintaining its elastic deformation characteristics.
Data availability
All data generated or analyzed in the study are included in this manuscript.
References
Aranguren MI, Marcovich NE, Salgueiro W, et al. Effect of the nano-cellulose content on the properties of reinforced polyurethanes. A study using mechanical tests and positron anihilation spectroscopy. Polym Test. 2013;32:115–22. https://doi.org/10.1016/j.polymertesting.2012.08.014.
Luo H, Hu J, Zhu Y. Path-dependent and selective multi-shape recovery of a polyurethane/cellulose-whisker nanocomposite. Mater Lett. 2012;89:172–5. https://doi.org/10.1016/j.matlet.2012.08.098.
Shi S, Shen DY, Xu T, et al. Thermal, optical, interfacial and mechanical properties of titanium dioxide/shape memory polyurethane nanocomposites. Compos Sci Technol. 2018;164:17–23. https://doi.org/10.1016/j.compscitech.2018.05.022.
Vishwakarma J, Jaiswal S, Bharti P, et al. Competing and decisive roles of 1d/2d/3d sp(2)-carbons in controlling the shape switching, contact sliding, and functional properties of polymers. Mater Today Chem. 2022. https://doi.org/10.1016/j.mtchem.2022.100960.
Wang TJ, Zhao J, Weng CX, et al. A bidirectionally reversible light-responsive actuator based on shape memory polyurethane bilayer. Compos Part A Appl Sci Manuf. 2021. https://doi.org/10.1016/j.compositesa.2021.106322.
Pebdani MH, Sabetvand R. Mechanical properties of carbon nanotube reinforced polyurethane matrix using computational method: a molecular dynamics study. Phys Scr. 2022. https://doi.org/10.1088/1402-4896/ac6cae.
Ivdre A, Mucci V, Stefani PM et al. Nanocellulose reinforced polyurethane obtained from hydroxylated soybean oil. In: BALTIC POLYMER SYMPOSIUM 2015, Baltic Polymer Symposium. 2016. https://doi.org/10.1088/1757-899X/111/1/012011.
Du WN, Zhang ZJ, Yin CL, et al. Preparation of shape memory polyurethane/modified cellulose nanocrystals composites with balanced comprehensive performances. Polym Adv Technol. 2021;32:4710–20. https://doi.org/10.1002/pat.5464.
Memis NK, Kaplan S. Temperature and moisture responsive nanocomposite treated polyester fabric for smart bagging recovery. Indian J Fibre Text Res. 2021;46:293–302.
Gorbunova M, Grunin L, Morris RH, et al. Nanocellulose-based thermoplastic polyurethane biocomposites with shape memory effect. J Compos Sci. 2023. https://doi.org/10.3390/jcs7040168.
Khansary MA, Pouresmaeel-Selakjani P, Aroon MA, et al. A molecular scale analysis of tempo-oxidation of native cellulose molecules. Heliyon. 2020. https://doi.org/10.1016/j.heliyon.2020.e05776.
Okahashi K, Takeuchi M, Zhou YX, et al. Nanocellulose-containing cellulose ether composite films prepared from aqueous mixtures by casting and drying method. Cellulose. 2021;28:6373–87. https://doi.org/10.1007/s10570-021-03897-5.
Isogai A, Hanninen T, Fujisawa S, et al. Review: catalytic oxidation of cellulose with nitroxyl radicals under cob for aqueous conditions. Prog Polym Sci. 2018;86:122–48. https://doi.org/10.1016/j.progpolymsci.2018.07.007.
Isogai A. Preparation and characterization of tempo-oxidized cellulose nanonetworks, nanofibers, and nanocrystals. In: Abstracts of Papers of the American Chemical Society, vol 257; 2019
Zhou YX, Saito T, Bergstrom L, et al. Acid-free preparation of cellulose nanocrystals by tempo oxidation and subsequent cavitation. Biomacromolecules. 2018;19:633–9. https://doi.org/10.1021/acs.biomac.7b01730.
Khadivi P, Salami-Kalajahi M, Roghani-Mamaqani H. Evaluation of in vitro cytotoxicity and properties of polydimethylsiloxane-based polyurethane/crystalline nanocellulose bionanocomposites. J Biomed Mater Res A. 2019;107:1771–8. https://doi.org/10.1002/jbm.a.36696.
Chung YC, Khiem ND, Choi JW, et al. Polyurethane membrane functionalization with the grafted cellulose derivatives to control water vapor permeability. Fiber Polym. 2015;16:492–502. https://doi.org/10.1007/s12221-015-0492-0.
Goo S, Yook S, Park SY, et al. Mechanical properties and soiling resistance of paper with polyurethane coating reinforced with cellulose nanomaterials. BioResources. 2019;14:8973–86. https://doi.org/10.15376/biores.14.4.8973-8986.
Tianhong L. Preparation and properties of nanocellulose/collagen composites; 2014.
Chen T. Study on Nanocellulose Modification and Its Reinforcement of Environmentally Responsive Composite; 2015.
Hiraoki R, Ono Y, Saito T, et al. Molecular mass and molecular-mass distribution of tempo-oxidized celluloses and tempo-oxidized cellulose nanofibrils. Biomacromolecules. 2015;16:675–81. https://doi.org/10.1021/bm501857c.
Wu J, Zhu W, Shi X, et al. Acid-free preparation and characterization of kelp (Laminaria japonica) nanocelluloses and their application in pickering emulsions. Carbohydr Polym. 2020. https://doi.org/10.1016/j.carbpol.2020.115999.
Shinoda R, Saito T, Okita Y, et al. Relationship between length and degree of polymerization of tempo-oxidized cellulose nanofibrils. Biomacromolecules. 2012;13:842–9. https://doi.org/10.1021/bm2017542.
Carlsson DO, Lindh J, Nyholm L, et al. Cooxidant-free tempo-mediated oxidation of highly crystalline nanocellulose in water. RSC Adv. 2014;4:52289–98. https://doi.org/10.1039/c4ra11182f.
Huang Q, Huang J, Chang PR. Polycaprolactone grafting of cellulose nanocrystals in ionic liquid [bmim]cl. Wuhan Univ J Nat Sci. 2014;19:117–22. https://doi.org/10.1007/s11859-014-0987-3.
Shen DK, Gu S. The mechanism for thermal decomposition of cellulose and its main products. Bioresour Technol. 2010;101:6879. https://doi.org/10.1016/j.biortech.2010.04.002.
Cao XD, Habibi Y, Lucia LA. One-pot polymerization, surface grafting, and processing of waterborne polyurethane-cellulose nanocrystal nanocomposites. J Mater Chem. 2009;19:7137–45. https://doi.org/10.1039/b910517d.
Qiao X, Wang Z, Sun K. Renewable rice straw cellulose nanofibril reinforced poly(ε-caprolactone) composite films. Mater Chem Phys. 2022. https://doi.org/10.1016/j.matchemphys.2022.126879.
Shojaeiarani J, Bajwa DS, Hartman K. Esterified cellulose nanocrystals as reinforcement in poly(lactic acid) nanocomposites. Cellulose. 2019;26:2349–62. https://doi.org/10.1007/s10570-018-02237-4.
Meng H, Hu JL. A brief review of stimulus-active polymers responsive to thermal, light, magnetic, electric, and water/solvent stimuli. J Intell Mater Syst Struct. 2010;21:859–85. https://doi.org/10.1177/1045389X10369718.
Funding
This work was supported by the National Key Research and Development Program (2021YFE0104500), the National Natural Science Foundation of China (22078114).
Author information
Authors and Affiliations
Contributions
Xiaohong Liu and Shiyu Fu wrote the main manuscript text and Rui Liu prepared the figures. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, X., Basta, A.H., Liu, R. et al. Preparation of shape memory polyurethane composite materials by grafting PCL onto CNFs with different carboxyl content. Discov Appl Sci 6, 148 (2024). https://doi.org/10.1007/s42452-024-05752-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05752-1