Abstract
Air pollution from industrial areas has become really worrying especially for city dwellers. The plume dispersion emitted from industrial sources is subject to several factors: temperature and emission rate velocity, wind speed and direction, source height, and atmospheric stability. This study aimed to evaluate the applicability of the dispersion coefficients correlated within a Gaussian plume approach to an industrial source in Libya (Mellitah Gas Complex) under low and moderate wind speeds. To this end, we have developed a specific code based on the Gaussian method to study the dispersion of (1) Volatile Organic Compounds (VOCs) from oil storage tanks and condensate storage tanks, and (2) sulfur oxides (SOx) and nitrogen oxides (NOx) emitted by the flaring process through three stacks of 80 m height. The emissions from multisource points and their dispersion have been predicted at calm wind conditions and the flammability and danger-prone toxic zones have been delimited around the studied site. The obtained results reveal that the emissions, particularly generated at low and moderate wind speeds, induce a dispersion with high concentration levels in the area surrounding the industrial site. The VOCs critical concentration region indicates a real risk of flammability at low-speed wind and stable atmospheric condition, from a height of 5 m above the ground. In fact, the VOCs concentration reach the Flammability Inferior Limit value of 0.018 m3 VOCs/m3 and these concentrations, appearing in the form of a plume, extend downstream to approximately 1000 m. The dispersion of NOx and SOx emissions downwind from the stacks are enhanced by wind speed; nevertheless, at 2 m height from the ground, the levels could exceed the limit value of 0.125 mg/m3, especially under the condition of unstable and very unstable atmospheric classes. From our findings, we recommend continuous monitoring campaigns inside and around the complex of Mellitah to ensure an environmentally secure zone that respects safety and health guidelines. Furthermore, enhanced simulations based on hourly weather conditions for extended area would be of great interest to accurately assess the air quality index in the region.
Article highlights
-
Air pollution and plume dispersion emitted from multiple sources in Mellitah oil and gas complex using a Gaussian plume approach.
-
Dispersion of VOCs, SOx and NOx emitted by the flaring process at various atmospheric stability classes under low and moderate wind speeds.
-
Delineation of the flammability and danger-prone toxic zones around the studied site.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The industrial revolution and the huge technological progress that followed are closely related to the discovery of oil and gas and their almost universal use in different industrial sectors. However, pollution accompanying oil and gas extraction and processing is one of the most serious threats facing humanity today. In petroleum industry activities, diverse pollutants, including Volatile Organic Compounds (VOCs), toxic sulfur oxides (SOx), and nitrogen oxides (NOx), are released into the atmosphere. These pollutants can disperse over relatively long distances, leading to contamination of extensive areas. Prolonged exposure to dangerous gases, such as hydrocarbons and toxic substances, can result in severe respiratory issues and, in extreme cases, even death [1]. Additionally, improper handling and storage of chemicals at these facilities may cause spills, posing additional threats to human safety and to the environment. Hence, the primary objective of studying flares and oil tanks is generally to identify potential risks of leaks and explosions. To mitigate such risks, it is essential to implement effective regulations and safety protocols, thereby reducing the impact of hazardous or inflammable emissions [2,3,4,5]. One of the effective solutions has often been the flaring of volatile organic compounds using a combustion control process. Also, flaring of hazardous gas allows to dilute emissions with air to non-toxic concentrations. In this context, regular extensive analysis studies and monitoring programs are conducted, particularly in areas surrounding industrial plants, to assess air quality and protect the environment of neighboring agglomerations [6,7,8,9,10,11,12].
For this purpose, many on-site studies are conducted using a simple Gaussian Plume Model (GPM) to estimate the concentrations of air pollutants/toxics downwind from single or multiple pollution sources [13, 14]. For instance, the study of Abdul-Wahab et al. [15] revealed that geographical and meteorological conditions significantly impact the SOx plume, particularly at lower wind speeds and in mountainous regions. In such cases, SOx tend to accumulate near the stack location, resulting in a peak load. In another study, Broomandi et al. [16] investigated the impact of the explosion of a large amount of ammonium nitrate stored at the Port of Beirut, Lebanon, which occurred on August 2020, on air quality. ALOHA and HYSPLIT models were employed to estimate the concentrations of NO2 and NO and their exposure times in the high-risk zone. The results highlight the environmental risks associated with old factories, emphasizing the need for regular safety monitoring in industrial zones and neighboring areas to prevent future incidents. Fawole et al. [17] have conducted a study employing ADMS 5 and AERMOD software to analyze how the dispersion and ground-level concentration of pollutants from gas flares in the Niger Delta are influenced by prevailing meteorological conditions, fuel composition and flare size. These authors recommended to mix the different flow rates into a single overall flow rate which should be evacuated from a single exhaust, thereby enhancing the buoyancy of the plume as it exits the stack.
Although flaring is the common method applied in the Libyan oil and Gas installations for safe discharge of excess hydrocarbons, only few studies have been conducted on emissions control, site monitoring, or crisis management in the event of an accident [2, 18]. In the current case study, a specific calculation code based on the GPM has been developed to analyze the dispersion of emissions from Mellitah Oil and Gas Complex, the biggest oil Company in Libya. The study is particularly conducted at prevailing low and moderate wind conditions. Within this wind speed margin, the turbulence parameterization model recommended by Essa [19] was adopted. The plume rise is expressed using Moses and Carson model [20,21,22,23,24] which takes into account the Pasquill class stability. Accordingly, the current “worst-case” study of multisource points and their dispersion, namely, the VOCs from oil and condensate storage tanks, sulfur oxide (SOx) and nitrogen oxides (NOx) emitted by the flaring process, is essential to establish a protection plan against the risks of flammability and pollution in the area surrounding the Mellitah Oil and Gas Complex.
2 Materials and methods
2.1 Problem position
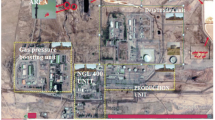
The Mellitah complex is one of the biggest gas treatment plants in the world. It stretches on the Mediterranean shore in Libya (Fig. 1). The complex consists essentially of two plants; the first is for treatment of Oil and condensate production from Wafa field while the second plant treats the Gas and condensate provided by Sabratha Offshore Platform. The complex includes five storage tanks of oil: 3 tanks (T1, T2 and T3 in Fig. 1) with a capacity of almost 100,162 m3each and two buffer tanks with a capacity of 45,152 m3 each (T4 and T5 in Fig. 1). Furthermore, the complex includes three condensate storage tanks with a capacity of almost 44,516 m3 each (T6, T7 and T8 in Fig. 1).
Despite the tank insulation designed to limit the penetration of external heat, a small amount of hydrocarbon will be evaporated and vented to the atmosphere [25]. Boil-off gas (BOG) from oil and condensate storage tanks is often calculated by taking into account all heat ingress to the liquid; however, its assessment is often based on the static boil-off rate (BOR) value suggested by the storage tank vendors [26].
Pressure in storage tanks battery is controlled by gas emission through venting VOCs with a rate, estimated as a function of oil or condensate throughput, working losses and standing losses [27]. In the present study, simulation of dispersion of VOCs emissions from storage tanks was based on the supposition that the boil-off rate (BOR) results are about 0.015 wt‰ per day at an 80% tank fill. In fact, considering the high capacity of the storage oil tank T1, T2 and T3, they were classified as high sources of VOCs emissions with an estimated rate value of 1 ton/day each. Moreover, buffer tanks T4, T5, T6, T7 and T8 would approximately emit half of the previous value, i.e., 0.5 ton/day each.
Furthermore, the residue gas from the processing is flared through three stacks of 80 m height, designed by F1, F2 and F3 in Fig. 1, with a discharge temperature of 341 K. Completion gases flaring also causes the release of more than 2000 tons/year of nitrogen oxides (NOx) and sulfur oxide (SOx) and 2.5 M tons of carbon dioxide/year [18]. The details about the different emission sources in the current study are summarized in Table 1.
The numerical simulations were performed for the physical area of 3000 m (ox), 1000 m (oy) and 200 m (oz) for VOCs dispersion and 2000 m (ox), 600 m (oy) and 200 m (oz) for hazardous gas as depicted in Fig. 1. The x-axis is parallel to the direction of the wind; the y-axis is oriented in the lateral direction to the wind, while the z-axis is the vertical axis taking its origin at the ground surface. The general wind direction and speed for each sampling period is given by the wind rose graph illustrated in Fig. 2, with measured data provided by the Libyan Meteorological Authority [28]. The wind rose graph gives a schematic view of the speed ranges and frequency over a year they blow in different directions. It appears that the prevailing wind directions are eastward with a peed range from 5 to 11 m/s and at less frequency in the range between 0.5 and 5 m/s.
Annual wind rose plot by the Libyan National Meteorological Center [28]
Generally, the affected area by the hazardous gas clouds is usually more important for low wind speeds [29, 30]. Such conditions are, therefore, crucial to model in an appropriate way, and to understand both the nature and the frequency of these low wind speed conditions. Relying on simulation studies under the scenario cases illustrated in Table 2, it would be possible to evaluate the hazards and assess the risks [31,32,33].
2.2 Pollutants dispersion modelling
The Gaussian plume dispersion model is used to determine a realistic description of dispersion. This model represents an analytical solution to the diffusion equation for ideal circumstances to the contaminant concentrations while traveling through advection due to the wind. To describe the plume specification and calculate its concentration downwind of a source assuming steady state conditions, this model introduces some diffusion parameters which correlate the plume dispersion in the atmosphere. The coefficients of dispersion are the Gaussian distribution function standard deviation, responsible for distributing the plume in the vertical and horizontal (z and y) axes. The different meteorological conditions normally result in different plume shapes and characteristics.
The Gaussian plume model is derived from the gas dispersion fundamental equation [13]. It is frequently used as the basis for modelling emissions from continuous point sources of emissions and assuming a constant meteorological condition, which eliminates time dependency of concentration. Moreover, the GPM Model neglects turbulence in the wind direction focusing only on advection transfer in longitudinal direction [13]. The concentration field is expressed by the following normal distribution equation:
where x0 and y0 are the coordinates of the source emission in the horizontal plane, Qm denotes the source rate emission (g/s), U is the mean wind speed (m/s), x is the upstream distance, y is the crosswind distance (m), z is the vertical distance above the ground (m), σy and σz are respectively the horizontal and vertical dispersion parameters (m) and zi is the mixing height of the layer between the surface and the base of the inversion layer. H is the effective stack height (m). H is given by: H = z0 + ∆h, where z0 is the physical stack height (m) and ∆h is the plume rise expressed using Moses and Carson model [20,21,22,23,24] which takes into account the combination of the gases momentum and buoyancy causing the gases to be lifted higher in the atmosphere. The height rise is expressed as a function of the Pasquill class stability and the heat flux Qh of the flue gas as per the following equations:
stability classes A, B and C:
stability class D:
stability classes E and F:
with
where ρ is the density of the emitted gas, Ds is the stack diameter, Wgas is the exit velocity of the stack and Cp is the specific heat, Tgas and Ta are, respectively, the temperature at the exit of the stack and ambient temperatures.
In the present study, rather than a single source, we have eight storage tanks and three flares, which means we have multisource of contaminants and each source has its own corresponding emission rate and position. The total mass of the deposited contaminant in the studied area is simply the sum of the contributions of the different sources.
3 Results
The GPM was used in this study to estimate the pollution emission and dispersion of VOCs and flare gases from the Mellitah Gas Complex. Firstly, a comparison was conducted to choose the most suitable model among the different GPM models. Then, the chosen model was used to study the pollution dispersion from the different sources over the studied area.
3.1 Comparison of dispersion coefficient models for duty gas dispersion estimation in moderate and low winds
The effective quantification of the concentrations standard deviation in the lateral and vertical directions σy and σz were estimated, in the range of 100 m to 10,000 m from the emission source, by empirical correlations [34]. The standard deviations for the compared models are resumed in Table 3, 4, 5 and 6:
However, Essa parameterization model is reported to perform much better turbulent diffusion coefficient than the standard Briggs model with a relatively low wind speed (< 2.5 m/s) [19]. Comparisons were made between the different used models for splitting σy and σz [34], to appreciate the relative difference between them. To this end, we applied the Gaussian plume solution using different dispersion parameter versions of an emission corresponding to the scenario number 1, wherein oil and condensate storage tanks release a total nominal mass rate of 5.5 tons VOCs/hour into the atmosphere from eight sources. Simulations were conducted with 1 m/s wind speed in very unstable atmosphere, i.e., Pasquill Stability Class A. Figure 3 shows the VOCs distribution in the particular horizontal plane at 2 m from the ground level (level near the height of a person and that may cause a potential danger), based on various parameterized models.
3.2 Wind velocity and stability effects on VOCs dispersion
The effect of wind velocity on the spatial distribution of the VOCs pollutants in the horizontal section at 2 m height is depicted in Fig. 4a. It can be noticed that the plume pollutant concentration in the prevailed wind direction spreads further whenever the wind speed increases.
Figure 4b illustrates VOCs distribution as a function of stability with an imposed wind velocity of 2 m/s. An unstable air condition leads to a more pronounced dispersion with greater values of σy(x) and σz(x); however, stable air leads to smaller values for the estimated vertical and horizontal dispersion coefficients. Hence, stable conditions show a longer but thinner plume shape as illustrated in Fig. 4b for scenarios 6 and 7, leading to a more confined and concentrated plume. This is the opposite to unstable conditions (scenario 5) in which case the plume is more widely dispersed.
3.3 Flare gases dispersion
In order to assess the potential emissions of SOx and NOx from the active flares in Mellitah Complex, the spatial distribution of these gasses’ concentrations have been evaluated in two unfavorable conditions of stability and wind velocity, namely scenario 8 and scenario 9 as defined in Table 1.
The SOx concentrations for Scenarios 8 and 9 are shown in Fig. 5a and b, respectively. The results are reproduced by fixing the emission rate equal to 1184.55 tons SOx/yr, which corresponds to the nominal total emission of hazardous gases of three flares measured in 2013, for two level heights from the ground: 2 m (corresponding to the height of person) and 80 m (corresponding to the height of the stacks).
As for the NOx emissions, the dispersions given as a function of distance downwind of the three flares, in the meteorological conditions corresponding respectively to weak velocity wind of 1 m/s and a very unstable atmospheric condition (scenario 8) and a wind velocity of 3 m/s in slightly unstable atmospheric condition (scenario 9), are depicted in Fig. 6a and b. The concentration distributions are given at two level heights from the ground (2 m and 80 m) to evaluate the air pollution impact on the environment of the industrial complex based on the maximum emission rate of 1302.36 tons/yr reported in 2013.
In both cases of Figs. 5a and 6a, interaction between low wind speed and high stability, clearly leads to accumulation of NOx and SOx gases in the ground levels (Figs. 5a and 6a at 2 m height), not far away from the source.
4 Discussion
4.1 Comparison of dispersion coefficient models for duty gas dispersion estimation in moderate and low winds
The achieved results of simulations based on various parameterized models show that Brookhaven National Laboratory parameterization model (Fig. 3e) gives a very weak dispersion compared to the other models. Also, it was noticed that the maximum concentration found by Brookhaven National Laboratory model were in the immediate vicinity of the storage tanks. Noteworthy, it appears that Essa and Pasquill–Turner (Fig. 3c and d, respectively) parameterization models give almost the same fields’ concentrations. Furthermore, Briggs model in urban conditions (Fig. 3a) yielded the closest values compared to Pasquill–Turner and Essa parameterization models. In all the studied cases, the maximum concentration does not reach VOC slower flammability limit which is, at least, (if 100% butane) of 0.018 volume ratio [36, 37]. This finding could be comparable with the research results of Saikomol et al. [5] where the emission of butane is predominant.
4.2 Wind velocity and stability effects on VOCs dispersion
The increase of wind velocity resulted in further spread of the plume pollutant concentration due to the effect of advection of the emitted VOCs. Moreover, the concentration decreases downwind in all of our results but it is clearly remarkable that the flammability limit of 0.018 m3 VOCs per m3 of air was not achieved in any case. The scenario 7 corresponding to a weak wind speed (2 m/s) in class F, is an unfavorable scenario giving a maximum surface concentration value of 0.012 m3 VOCs/m3 of air at 1500 m downwind distance from the emission sources. Pan et al. [38] have reported that more VOCs can be enriched near the storage tank in downwind direction at constant wind speed and F atmospheric stability. This tendency was detailed in Fig. 7 where the VOCs concentration profiles were given at different levels from the ground, as a function of the distance downwind of the gas emitted in the meteorological conditions corresponding to scenario 7 (U = 2 m/s, stability F). The concentration curves are given at 2 m, 5 m, 10 m and above storage tanks level at 15 m (Fig. 7). It is worth mentioning that high risk of flammability increases at 10 m and 15 m from the ground. Indeed, the VOCs concentration is in the flammability range of 0.018– 0.095 m3 VOCs/m3 of air. The red-colored area in Fig. 8 represents the delineation area above the lower flammability limit of 0.018 m3 VOCs/m3, at 10 m height and at the top of the tanks (15 m above the ground). These critical concentrations extend downstream to approximately 1200 m. A danger area within a zone of less than 10 m, where VOCs are up to 0.02 m3 VOCs/m3 in the case of an accidental spill scenario reported by Howari [39]. Saikomol et al. [5] have recommended additional mitigation measures to control VOCs emissions when they found that pentane concentrations could exceed 30.325 mg/m3 within an area of 6 km × 6 km around storage tanks of petroleum refinery. Furthermore, occupational exposure to VOCs emitted by processing plants (even at low concentrations) can adversely affect the health of workers engaged in various steps of crude oil processing [40, 41].
4.3 Flare gases dispersion
Several oil and gas installations in Libya, like Mellitah Complex, use Flaring to ensure a safe disposal of the produced hydrocarbons unwanted products. However, this procedure may lead to massive emissions of hazardous gases including CO, SOx, and NO, among others. The degree to which SOx and NOx emissions harm human health depends essentially on the ground-level concentrations and exposure duration [42]. Table 7 summarizes the air quality guidelines for SO2 and NOx concentrations [1].
The comparison between SOx concentrations shown in Fig. 5a and b, indicates that in the case of scenario 8, corresponding to a very low wind velocity of 1 m/s and a very unstable air condition, a guideline violation is more pronounced in such a way that the limits of 0.350 mg and 0.500 mg are exceeded in the flares upstream and cover regions defined by the contours in Fig. 5a. With low to moderate wind speeds (U < 3 m/s) and unstable condition (scenario 9), the guideline of 0.125 mg/m3 limit would be violated to an extent of 2 km from the flares, at the height level of 2 m (Fig. 5b). In the study of Alnahdi et al. [43] for an oil refinery site, the maximum monthly average concentration of SO2 was 1.3 mg/m3 and the plumes dispersed 5 km downwind.
Moreover, comparison between NOx concentrations shown in Fig. 6a and b reveals that wind speed enhances mass transport and stability contributes to the formation of a finer plume. Furthermore, it seems that at 2 m level height, the limit value of 0.125 mg/m3 is exceeded within the area delineated in Fig. 6a and b.
5 Conclusions and recommendations
The distribution of pollutants in the horizontal planes intersecting multiple sources of Mellitah industrial complex was determined by a Gaussian approach in order to simulate pollutants dispersion, from eight storage tanks and three flaring stacks, at a dominant low wind speed. The main conclusions observed for the shape of the VOCs, SOx and NOx plumes are:
The plume spreads in transverse directions by turbulent diffusion. The distribution is characterized by source flow rate emission, wind speed, and horizontal and vertical standard deviation. The increase in wind speed enhanced mass transport. Wind carries the pollutant downstream from the source and the plume stretches in the direction of the wind. Standard deviations increase with distance from the source under the effect of mixing by atmospheric turbulence. As the plume extends, the focus on the axis oy and oz decreases;
VOCs critical concentration region indicates a real risk of flammability at low-speed wind and stable atmospheric condition, from a height of 5 m above the ground level. VOCs concentration reach the Flammability Inferior Limit value of 0.018 m3 VOCs/m3 and these concentrations, appearing in the form of a plume, extend downstream to approximately 1000 m.
Dispersion of NOx and SOx emissions downwind from the stacks are enhanced by wind speed, but levels at 2 m height from the ground can exceed the limit value of 0.125 mg/m3 essentially for SOx emissions under the condition low wind speed (< 2 m/s) and unstable to very unstable atmospheric classes. Nevertheless, concentrations of NOx and SOx emissions at the proximity of the ground evidence a reduced contaminated region because of the height of the stacks which corresponds to 80 m.
In the light of these results, we recommend continuous monitoring campaigns inside and around the complex of Mellitah to ensure an environmentally secure zone that respects WHO standards. Furthermore, more accurate simulations (based on daily/hourly weather conditions) over an extended area would be of great interest to accurately assess the air quality index in the region.
Data availability
The datasets used and/or analyzed for this study are available from the corresponding authors upon request.
References
WHO. Lignes directrices OMS relatives à la qualité de l’air: particules (PM2.5 et PM10), ozone, dioxyde d’azote, dioxyde de soufre et monoxyde de carbone. Résumé d’orientation [WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. Executive summary]. Genève, Organisation mondiale de la Santé, 2021. Licence: CC BY-NC-SA 3.0 IGO.
Danna ABM, Kallel A, Rouis MJ. Evaluation of air pollutants and dispersion patterns for the adjacent areas of Mellitah Gas Complex, Libya (recent advances in geo-environmental engineering, geomechanics and geotechnics, and geohazards). Cham: Springer; 2019. p. 69–73. https://doi.org/10.1007/978-3-030-01665-4_17.
Chen C, McCabe DC, Fleischman LE, Cohan DS. Black carbon emissions and associated health impacts of gas flaring in the United States. Atmosphere. 2022;13:385. https://doi.org/10.3390/atmos13030385.
McDonald-Buller E, McGaughey G, Grant J, Shah T, Kimura Y, Yarwood G. Emissions and air quality implications of upstream and midstream oil and gas operations in Mexico. Atmosphere. 2021;12:1696. https://doi.org/10.3390/atmos12121696.
Saikomol S, Thepanondh S, Laowagul W. Emission losses and dispersion of volatile organic compounds from tank farm of petroleum refinery complex. J Environ Health Sci Eng. 2019;17:561–70. https://doi.org/10.1007/s40201-019-00370-1.
Ciobanu C, Tudor P, Istrate I-A, Voicu G. Assessment of environmental pollution in cement plant areas in Romania by co-processing waste in clinker kilns. Energies. 2022;15:2656. https://doi.org/10.3390/en15072656.
Fathey Fayek Tadros A. Environmental aspects of petroleum storage in above ground tank. E3S Web Conf. 2020;166:01006. https://doi.org/10.1051/e3sconf/202016601006.
Giovannini L, Ferrero E, Karl T, Rotach MW, Staquet C, Trini Castelli S, Zardi D. Atmospheric pollutant dispersion over complex terrain: challenges and needs for improving air quality measurements and modeling. Atmosphere. 2020;11:646. https://doi.org/10.3390/atmos11060646.
Holliman J, Schade GW. Comparing permitted emissions to atmospheric observations of hydrocarbons in the eagle ford shale suggests permit violations. Energies. 2021;14:780. https://doi.org/10.3390/en14030780.
Li X, Chen G, Zhang R, Zhu H, Xu C. Simulation and assessment of gas dispersion above sea from a subsea release: a CFD-based approach. Int J Naval Archit Ocean Eng. 2019;11:353–63. https://doi.org/10.1016/j.ijnaoe.2018.07.002.
Shen L, Xiang P, Liang S, Chen W, Wang M, Lu S, Wang Z. Sources profiles of volatile organic compounds (VOCs) measured in a typical industrial process in Wuhan, Central China. Atmosphere. 2018;9:297. https://doi.org/10.3390/atmos9080297.
Toja-Silva F, Pregel-Hoderlein C, Chen J. On the urban geometry generalization for CFD simulation of gas dispersion from chimneys: comparison with Gaussian plume model. J Wind Eng Ind Aerodyn. 2018;177:1–18. https://doi.org/10.1016/j.jweia.2018.04.003.
Arya SP. Air pollution meteorology and dispersion. New York: Oxford University Press; 1999.
Snoun H, Krichen M, Chérif H. A comprehensive review of Gaussian atmospheric dispersion models: current usage and future perspectives. Euro-Mediterr J Environ Integr. 2023;8:219–42. https://doi.org/10.1007/s41207-023-00354-6.
Abdul-Wahab S, Fadlallah S, Al-Rashdi M. Evaluation of the impact of ground-level concentrations of SO2, NOx, CO, and PM10 emitted from a steel melting plant on Muscat, Oman. Sustain Cities Soc. 2018;38:675–83. https://doi.org/10.1016/j.scs.2018.01.048.
Broomandi P, Jahanbakhshi A, Nikfal A, Kim JR, Karaca F. Impact assessment of Beirut explosion on local and regional air quality. Air Qual Atmos Health. 2021;14:1911–29. https://doi.org/10.1007/s11869-021-01066-y.
Fawole OG, Cai X, Abiye OE, MacKenzie AR. Dispersion of gas flaring emissions in the Niger delta: impact of prevailing meteorological conditions and flare characteristics. Environ Pollut. 2019;246:284–93. https://doi.org/10.1016/j.envpol.2018.12.021.
Hasan M, Gambri A, Shibani A, Abukshim M. SOx emission and pollution control at Mellitah gas plant. Clean Technol Environ Policy. 2008;11:133–5. https://doi.org/10.1007/s10098-008-0168-1.
Essa KSM, Mubarak F, Khadra SA. Comparison of some sigma schemes for estimation of air pollutant dispersion in moderate and low winds. Atmos Sci Lett. 2005;6:90–6. https://doi.org/10.1002/asl.94.
Cheremisinoff P. Air pollution control and design for industry. Routledge; 2018. https://doi.org/10.1201/9781315137063.
Moses H, Carson JE. Stack design parameters influencing plume rise. J Air Pollut Control Assoc. 1968;18:454–7. https://doi.org/10.1080/00022470.1968.10469155.
Spellman FR. Essentials of environmental engineering. Bernan Press; 2020.
Turner DB. Workbook of atmospheric dispersion estimates: an introduction to dispersion modeling. 2nd ed. CRC Press; 2020.
Weiner R, Matthews R. Environmental engineering. Butterworth-Heinemann; 2003.
Office of Fossil Energy. Natural Gas flaring and venting: state and federal regulatory overview, trends, and impacts. Washington: US Department of Energy; 2019.
Khan MS, Qyyum MA, Ali W, Wazwaz A, Ansari KB, Lee M. Energy saving through efficient BOG prediction and impact of static boil-off-rate in full containment-type LNG storage tank. Energies. 2020;13:5578. https://doi.org/10.3390/en13215578.
U.S. EPA. AP-42 compilation of air pollutant emission factors, vol. I. 5th Ed, Chapter 7: Liquid Storage Tanks US Environmental Protection Agency Research, Triangle Park, NC; 2020. https://www3.epa.gov/ttn/chief/ap42/ch07/final/ch07s01.pdf
LNCM. Windrose—Zwara metorological station 1961–2003. Climatic Atlas Project (CAP). Climatological department. Libyan National Meteoroligal Center, LNMC; 2006.
Deaves DM, Lines IG. The nature and frequency of low wind speed conditions. J Wind Eng Ind Aerodyn. 1998;73:1–29. https://doi.org/10.1016/S0167-6105(97)00278-X.
Woodward JL, Pitbaldo R. LNG risk based safety: modeling and consequence analysis. Wiley; 2010.
Węsierski T, Piec R, Majder-Łopatka M, Król B, Gawroński W, Kwiatkowski M. Hazards generated by an LNG road tanker leak: field investigation of vapour propagation under class B conditions of atmospheric stability. Energies. 2021;14:8483. https://doi.org/10.3390/en14248483.
Vallero DA. Chapter 3—pollutant transformation. In: Vallero DA, editor. Air pollution calculations. Elsevier; 2019. p. 45–72.
Yang Z, Yao Q, Buser MD, Alfieri JG, Li H, Torrents A, McConnell LL, Downey PM, Hapeman CJ. Modification and validation of the Gaussian plume model (GPM) to predict ammonia and particulate matter dispersion. Atmos Pollut Res. 2020;11:1063–72. https://doi.org/10.1016/j.apr.2020.03.012.
Perkins R, Soulhac L, Mejean P, Rios I. Modélisation de la dispersion des émissions atmosphériques d’un site industriel. Vers un guide de l’utilisateur. Phase 1: état de l’art. Phase 2: évaluation des modèles LMFA, Ecole Centrale de Lyon. Rapport RECORD; 2005.
Thoman DC, O’Kula KR, Laul JC, Davis MW, Knecht KD. Comparison of ALOHA and EPIcode for safety analysis applications. J Chem Health Saf. 2006;13:20–33. https://doi.org/10.1016/j.jchas.2006.02.003.
Crowl DA. Understanding explosions. Wiley; 2010.
Zabetakis MG. Flammability characteristics of combustible gases and vapors. Washington: Bureau of Mines; 1965.
Pan H-J, Zhou J-B, Chen L-C, Yang J-F, Lu X-Y. Study on leakage and diffusion law of “large breathing” loss of gasoline storage tank based on PHAST. In: Proceedings of the 2018 international conference on energy development and environmental protection (EDEP 2018), Atlantis Press; 2018. pp 134–140. https://doi.org/10.2991/edep-18.2018.22
Howari FM. Evaporation losses and dispersion of volatile organic compounds from tank farms. Environ Monit Assess. 2015;187:273. https://doi.org/10.1007/s10661-015-4456-z.
Rajabi H, Hadi Mosleh M, Mandal P, Lea-Langton A, Sedighi M. Emissions of volatile organic compounds from crude oil processing—global emission inventory and environmental release. Sci Total Environ. 2020;727:138654. https://doi.org/10.1016/j.scitotenv.2020.138654.
Johnston JE, Lim E, Roh H. Impact of upstream oil extraction and environmental public health: a review of the evidence. Sci Total Environ. 2019;657:187–99. https://doi.org/10.1016/j.scitotenv.2018.11.483.
Hertel O, Johnson MS, Goodsite ME. Air pollution sources, statistics, and health effects: introduction air pollution sources, statistics and health effects, Springer; 2020. pp. 1–3. https://doi.org/10.1007/978-1-0716-0596-7_911
Alnahdi A, Elkamel A, Shaik MA, Al-Sobhi SA, Erenay FS. Optimal production planning and pollution control in petroleum refineries using mathematical programming and dispersion models. Sustainability. 2019. https://doi.org/10.3390/su11143771.
Acknowledgements
This work is part of a doctoral thesis by Abdulhamid B. M. Danna. We thank the Ministry of Higher Edu-cation and Scientific Research, Tunisia, for its support. Last, authors would like to pay their gratitude and respects to their Professor Dr. Mohamed Jamel Rouis. After helping to initiate this study, Dr. Mohamed Jamel Rouis passed away on 23 November 2017.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study design. ABMD: Investigation, Writing—original draft, Formal analysis. MH, HD: Visualization, Validation, Formal analysis, Writing—original draft. AK: Methodology, Data curation, Writing—review and editing. MB: Methodology, Software, Data curation, Validation, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Danna, A.B.M., Haddar, M., Djemel, H. et al. Preliminary assessment of volatile organic compounds and hazardous gases dispersion at low winds: case study of Mellitah Gas Complex, Libya. Discov Appl Sci 6, 84 (2024). https://doi.org/10.1007/s42452-024-05730-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-024-05730-7