Abstract
The study sought to investigate the impact of a holistic high-power microwave technology during all stages of the extraction on the quality, time of extraction, and degree of deacetylation (DD) of shrimp chitosan (SC) and crab chitosan (KC). The demineralization and deproteinization stages took 7 and 8 min, at 750 and 875 W, respectively. The deacetylation process was conducted at two powers, 875 W and 1250 W, for 10, 15, and 20 min. It only took 25 min at 875 W to successfully prepare chitosan with a high DD and 30 min to reach the maximum DD. The highest DDs by the potentiometric titration method, FTIR, and 1H NMR of SC were 86.6%, 86.7%, and 83%, compared to 83.8%, 82.7%, and 80% for KC, respectively. Extracted SC had 79% solubility, 14.125 kDa, a 46.57% crystallinity index, 705.40% WBC, and 434.60% FBC, against 74.5%, 16.982 kDa, 74.14%, 689.82%, and 413.20% for KC, respectively. The study proved that 30 min of holistic high-power microwave at 875 W produced low-molecular-weight chitosan with relatively high deacetylation and low content of viscosity, crystallinity, and protein residue. The technique can provide a feasible alternative to the commercial production of low-molecular-weight chitosan in less time and energy.
Article Highlights
-
High-power (875 W) microwave irradiation at 15 min-deacetylation produced Chitosan’s maximum deacetylation degree (DD).
-
Holistic microwave irradiation (MW) can successfully produce high DD chitosan, and low molecular weight in 30 min.
-
The chemical structure of MW-extracted chitosan was confirmed by FTIR and 1H NMR and low crystallinity by X-ray.
Similar content being viewed by others
1 Introduction
Generally, optimizing the preparation conditions of biologically active macromolecules is a useful approach to getting the intended product under the least drastic and most favorable conditions for good yield while taking into consideration all the influencing factors [1, 2]. Since a variety of processing agents affect chitosan’s physical and chemical properties, it appears that producing highly deacetylated chitosan in the shortest possible time by a simple and economical method on a commercial scale is a hard task.
Chitosan is a linear amino-polysaccharide derived from the alkaline deacetylation of biopolymer-chitin at high temperatures. During deacetylation, N-acetyl-D-glucosamine units are transformed into D-glucosamine units, including free amino groups that have a positive ionic charge. The free amino groups turn chitosan into a cationic form, with possibly valuable antiviral or antibacterial activities like cationic proteins, being reported as antiviral [3, 4] and antibacterial activities [5, 6]. The positive ionic charge of chitosan allows it to chemically bind to fats, and bile acids, and target the bacterial negative cytoplasmic bacterial membrane. This property is the major factor that makes it a versatile tool for a wide range of applications [7, 8]. Additionally, low molecular weight chitosan has been proven to possess high biological activity, allowing it to be employed in many applications such as pharmaceuticals, medical fields, aquaculture, food technology, and water treatment [9].
Several scientists offered various strategies for the production of chitosan through chitin deacetylation; most of these were based on the use of strong solutions of sodium hydroxide at high temperatures, including alkaline high-temperature treatments at high pressures, ultrasound deacetylation, and enzymatic deacetylation. Chitosan was generally developed using traditional heating methods that required a great deal of time and energy. Many authors are currently dealing with chitin and chitosan extraction time problems [10].
Chitosan extraction takes a lot of time and takes a lot of energy by using conventional methods. Microwave technology is considered to be a great alternative to conventional methods because it allows for faster reactions, shorter reaction times, reduction of energy consumption, and fewer side reactions. Microwave irradiation is gaining traction as a renewable energy source capable of completing chemical transformations in minutes rather than hours or days [10, 11]. Mostly, microwave irradiation was used in the final phase of the chitosan extraction process, converting chitin to chitosan [12]. In addition, the previous studies extracted high molecular chitosan using a low microwave power at the deacetylation step. To get low molecular chitosan the previous methods needed to undergo a subsequent step (depolymerization), extending the time of preparation, and raising the total costs. This study assumes that using a holistic high-power microwave technique to extract chitosan at all stages of the extraction process will result in high-quality chitosan with a high degree of deacetylation (DD) in a short amount of time.
Therefore, this work aimed to obtain chitosan of good quality in a very short time using holistic high-power microwave technology. Developing the process of chitosan extraction via using a holistic high-power microwave technology may lead to new products with probably low molecular weight and upgraded physicochemical properties. Thus, it is possible to obtain highly solubilized chitosan in the least amount of time and at the lowest cost without using the depolymerization stage.
2 Materials and methods
2.1 Raw materials
The shrimp shells (Metapenaeus monoceros (Fabricius)) and crab exoskeletons (Portunus pelagicus) were collected from seafood restaurants in Zagazig, El-Sharkiya Governorate, Egypt. The shrimp shells were collected during the same week while crab exoskeletons samples were collected during the same day. All samples were frozen until further processing. After removing the residual meat, shells were cleaned with tap water and subsequently distilled water, then left to dry at 65 ºC to a constant weight. The dried material was ground and sifted through a 250 µm sieve, then stored in a freezer until use.
2.2 Chemical reagents
All the chemicals and solvents utilized in this investigation were purchased at the highest analytical grade or extra purity. Hydrochloric acid (HCl) and acetic acid (CH3COOH) were purchased from SD Fine-Chem Limited, India. Sodium Hydroxide pellets (NaOH), Acetone (CH3COCH3), Ethyl alcohol (C2H5OH 95%), and commercial chitosan (75%) were purchased from Loba Chemie Pvt. Ltd. for High-Grade Laboratory Reagents and Fine Chemicals Mumbai, India.
2.3 Preparation of chitosan by microwave technique
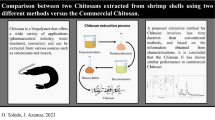
Chitosan is typically produced by acetyl group removal from chitin. The holistic microwave-assisted preparation was carried out in a domestic microwave oven (Smart-Torbito SM-2300MS, China), equipped with a turntable and a power range of 125–1250W. The following sequences were maintained during the extraction of chitosan, as shown in Fig. 1.
2.3.1 Extraction of Chitin
2.3.1.1 Demineralization
Dried shrimp and crab shell powder were first treated with 1N HCl in a ratio of 1:10 (solid: solvent) for 7 min at 750 W. This ratio was derived from surveying the different methods mentioned by El Knidri et al. [10]. The resultant fraction was then filtered, rinsed with distilled water until it achieved a neutral pH, and dried to a constant weight at 65 °C. During the demineralization process, minerals (calcium carbonate and calcium chloride) are removed.
2.3.1.2 Deproteinization
To remove protein, demineralized shrimp and crab shells were deproteinized using an alkaline treatment with 1N NaOH at a ratio of 15 ml of solvent for each gram of the sample at 875 W for 8 min. This ratio was derived from surveying the different methods mentioned by El Knidri et al. [10]. The residue was filtrated, washed thoroughly with distilled water until the pH was neutral, and then dried at 65 °C to a constant weight. The resulting material is known as chitin.
2.3.1.3 Decolorization
The decolorization stage was carried out under condensation, similar to the Soxhlet process for lipid extraction, until the color was eliminated, and a bleached product was obtained. Decolorization was performed by immersing chitin in acetone at a ratio of 15 ml of solvent for each gram of the sample for 2 h in a water bath at the boiling point 57 °C [13] under condensation, followed by stirring at 150 rpm for 1 h to remove oils and pigments. To further bleach and eliminate impurities, chitin was submerged in ethyl alcohol 95% at a ratio of 15 ml of solvent for each gram of the sample for one hour in a water bath at the boiling point 78.4 °C [13] under condensation, followed by one hour of stirring at 150 rpm.
2.3.2 Deacetylation of chitin to chitosan
The decolorized chitin was deacetylated by treating it with 12.5 N NaOH at a ratio of 15 ml of solvent for each gram of the sample at different microwave powers (875W and 1250W) for different irradiation times (10, 15, and 20 min). The alkali fraction was isolated by filtration and washed thoroughly with distilled water until the pH became neutral. Chitosan was obtained and dried to a consistent weight at 55 °C. The DD of chitosan was determined using the potentiometric titration technique for all shrimp and crab samples. The extracted chitosan from shrimp and crab was denoted as SC and KC, respectively, while the commercial chitosan was denoted as CC.
2.4 Chemical analysis of raw materials and chitin
2.4.1 Moisture content (%)
The moisture content of the shells and chitin was determined by the gravimetric method according to [14]. The water mass loss was calculated by drying the sample to a constant weight in an air oven at 105 °C for 24 h. The water mass was determined with the following equation as the difference between the weight of the wet and oven-dried samples per gram.
where, W1 and W2 are the weights of wet and oven-dried samples, respectively.
2.4.2 Ash content (%)
The ash content was determined by sample incineration (0.5 g) at 650 °C for 4 h in a muffle furnace according to [14]. The percentage of ash value was calculated from the ratio between the weight of the residue and that of the sample weight using the following equation:
where, W1 and W2 are the weights (in grams) of the initial sample of chitosan and residue, respectively.
2.4.3 Determination of total protein (%)
The total nitrogen was determined by using the micro Kjeldahl method according to [14]. The protein percentage revealed in this study was calculated by subtracting chitin nitrogen from total nitrogen before and after the deproteinization step and multiplying it by 6.25 [15].
2.5 Determination of chitosan degree of deacetylation (DD) by potentiometric titration method
A weighed amount (0.2 g) of chitosan was dissolved in 0.1 N HCL (20 cm3) and deionized water (25 cm3). After 30 min of stirring, another 25 cm3 of deionized water was added, and the stirring was repeated for another 30 min. The solution was titrated against gradual additions (0.1 ml) of 0.1 N NaOH, allowing it to equilibrate, and recording the pH. This process continued until the deflection points, V1 and V2 (the neuralisation points with 0.1 N NaOH of the free H+ and protonated NH+, respectilvely), were recorded. The DD% was calculated using the equation below [16].
where, M denotes the sample weight. 2.03 is the coefficient derived from the chitin monomer unit molecular weight, while 0.0042 is the coefficient derived from the difference between chitin and chitosan unit molecular weights.
2.6 Characterization of the chitosan with the highest DD
The SC and KC with the highest DD (86.6 and 83.8% respectively) and CC were chosen for the following analyses.
2.6.1 Solubility
In a beaker, 0.2 g of sample was solved in 1% acetic acid (20 ml) for 30 min on a magnetic stirrer at 250 rpm. After 30 min, the solution was filtered onto filter paper and the insoluble fraction was cleaned with distilled water and dried to a constant weight. Chitosan solubility was calculated by the following equation [17].
2.6.2 Water binding capacity (WBC)
A weighed sample (0.5 g) and 10 ml distilled water were placed in a centrifuge tube (50 ml), agitated for 1 min, then shaken for 30 min, and left at room temperature for 12 h. The samples were centrifuged for 25 min at 232 × g. After decanting the supernatant, the tubes were weighed again, and WBC was determined using the following equation [18].
2.6.3 Fat binding capacity (FBC)
A representative sample (0.5 g) of chitosan was mixed with 10 ml olive oil in a 50 ml centrifuge tube for 1 min, held at room temperature for 12 h, then shaken for 30 min. The samples were centrifuged at 232 × g. After discarding the supernatant, the tube was re-weighted. The following equation was used to calculate FBC [19].
2.6.4 Intrinsic viscosity determination
Chitosan samples were dissolved in 1% acetic acid at three different concentrations (0.06, 0.08, and 0.1 g/dl). An Ostwald viscometer measured the flow time for each concentration at room temperature. The intrinsic viscosity was defined by capillary viscometry based on the flow time (t0) of the same volume of solvent or chitosan solution (t) and calculated from the following equations:
Specific viscosity (ηsp) was defined by Eq. (7):
The reduced viscosity (ηred) was obtained by dividing the ηsp to the chitosan concentration (C) in g/dL, Eq. (8):
The inherent viscosity (ηinh), was defined by Eq. (9):
The reduced viscosity and inherent viscosity were plotted versus the chitosan concentration to determine the intrinsic viscosity [20].
2.6.5 Determination of the average molecular weight
By using the Mark–Houwink–Sakurada equation (MHS) Eq. (10), the molecular weight of chitosan was estimated from its intrinsic viscosity ([η]).
where k = 1.81 × 10−3 ml/g and a = 0.93 depending on the kind of solvent and temperature [21].
2.6.6 Fourier transform infrared spectroscopy (FTIR)
FTIR spectra were recorded for chitosans using an FTIR spectrophotometer (4100 Jasco-Japan) with a frequency range of 400–4000 cm−1. The DD% of chitosan could be calculated using the following equation [22].
where A1655 is the absorbance of the amide band at 1655 cm−1. A3450 is the absorbance of the O–H band at 3450 cm−1. The factor (40.1647) is the ratio of A1655/A3450 for completely N-acetylated chitosan. The number 118.883 was presented as a baseline.
2.6.7 Proton nuclear magnetic resonance spectroscopy (1H NMR)
1H NMR (Joil spectrometer, USA) (500 MHz) was used to examine chitosan samples (10 mg) dissolved in 1 ml CD3COOD/D20 (2% v/v) solution. The DD% of chitosan samples was determined using the equation below [23].
where ACH3 = CH3 area in -NHCOCH3 group, AH2-H6 = areas of H2, H3, H4, H5 and H6.
2.6.8 X-ray powder diffraction (XRD)
The chitosan crystallinity was determined by XRD analysis. A PANalyticalX`Pert PRO X-ray machine (Netherland) was used to perform the XRD measurements on powder samples. Cu Kα radiation has been used as an X-ray source (45 kV, 30 mA). The samples were scanned at a 4 min−1 scanning rate and 25 °C from 2θ = 5–40°. Using the following equation [24], the crystalline index (CI%) was determined from the ratio of the crystal phase to the total of the crystal phase and amorphous phase.
where I110 represents the highest intensity of the lattice diffraction pattern at 20°. Iam represents the intensity of amorphous diffraction at 16°.
2.6.9 Scanning electron microscopy (SEM)
SEM was used to examine the surface morphology of chitosan samples using a High-Resolution Field Emission SEM (Quanta FEG 250-Czechoslovakia). A sputter coater (Edwards S150A-BOC Edwards, UK) was used to coat chitosan samples with gold under a vacuum before the examination to improve contrast. At various magnifications and locations, images of the sample surface were captured.
2.7 Statistical analysis
All experiments were carried out in triplicate, except for FTIR, 1H NMR, XRD, and SEM analysis, which were performed on the SC and KC with the highest DD (86.6 and 83.8%, respectively) and CC. Results were obtained from triplicate (n = 3) independent experiments. The statistical significance of differences between means of data for the chemical analysis—solubility, WBC %, FBC %, intrinsic viscosity, and the average molecular weight—was checked by one-way ANOVA with a significance level of p ≤ 0.05. The statistical significance of differences between means of data for the degree of deacetylation (DD) by potentiometric titration method was checked by a two-way ANOVA with a significance level of p ≤ 0.05.
3 Results and discussion
3.1 Chemical analysis of raw materials
The proximate chemical composition of fresh shrimp and crab wastes is presented in Table 1. The values in Table 1 report the mean ± SD of three separate experiments. The moisture content of the shrimp and crab shells was 11.39% and 5.37%, respectively. Chemical analysis revealed that the waste of the shrimp shell retained more water than the waste of the crab shell, in agreement with the results reported by Olafadehan et al. [25]. The crab waste was found to have high ash content (56.08%) compared with the shrimp waste, which was 35.02%. Hamdi et al. [26] found 53.8% ash in blue crab shells, and Zhang et al. [27] obtained an ash content in shrimp shells of more than 30%. The crude protein obtained showed that the shrimp and crab shell waste were rich in this component at 38.49% and 36.24%, respectively. Rødde et al. [28] found that the protein content varied between 33 and 40%, and the ash content varied between 32 and 38% of the dry weight of the shrimp shells. The composition difference may be due to the used species.
3.2 Chemical analysis of chitin
Eliminating all organic and mineral content in the raw materials is the purpose of any extraction process. The produced chitin quality depends on the extraction conditions such as acid and alkali concentrations and the reaction time with samples [29, 30]. To judge whether there are any differences in moisture, ash, and protein content between shrimp and crab shells and chitin prepared under the used method. The ingredients were measured in the produced chitin. The results are shown in Table 1. The values in Table 1 report the mean ± SD of three separate experiments. The moisture content of the extracted chitin from shrimp and crab shells was 8.85% and 9.30%, respectively. The high moisture content may be due to the remaining water in the sample, which was utilized during the deproteinization step, as previously stated by da Silva et al. [31]. The content of ash and protein indicates the effectiveness of the demineralization and deproteinization stages. Compared to the precursor, high reductions in the ash and protein content of extracted chitin were observed. After 7 min of microwave irradiation (MW), the ash content dropped to around 0.99% for shrimp chitin and 1.69% for crab chitin. Samar et al. [32] obtained similar results.
Under the selected conditions of demineralization (high MW power (750 W) and 1N HCl), the demineralization of shrimp chitin reached 99.1% in 7 min against 98% in 10 min in a similar study using lower microwave power and HCl 2.5 M [33]. The fact that Chitin is tightly linked to proteins necessitates a more rigorous treatment to eliminate all associated proteins [34]. Deproteination at room temperature should be done at 4% NaOH for 21 h to get a low protein content [35]. In this study, only 8 min of microwave technique was enough to minimize the protein residue in shrimp chitin to 2.83% and crab chitin to 3.11%. These percentages were lower than those obtained by Olafadehan et al. [25], using the traditional heat technique. The deproteinization extent in this study using 875 W and 1 N NaOH for 8 min. reached 97.2 and 96.9%, for shrimp and crab chitins, respectively, excelling the level (88%) obtained by Knidri et al. [33] for shrimp chitin, using MW technique at 650 W and 5% NaOH in 6 min. Marei et al. [36] used the traditional heating approach to achieve maximum deproteinization at 90 °C for 8 h, with a percentage of removal of only 90%. Microwave irradiation produces effective internal heating by direct coupling of microwave energy with the molecules of biomass, which explains the mechanism by which microwave technology reduces reaction time. The high-frequency microwave energizes the starting materials (i.e., amorphous carbon and other carbon sources) to a in a short time. The energized mineral particles alongside the energized water molecules will help extract minerals in a short time. The same concept applies for deproteinization and deacetylation. The heating mechanism of microwave irradiation can be explained by two main processes, namely, dipolar polarization and ionic conduction. When the dipolar molecules or ion molecules of the samples are irradiated by microwave, they are sensitive to the electric field and lead to the alignment of the dipolar or ions with the electric field by rotation. During this process, the dipolar molecules or ion molecules are influenced by oscillation and molecules friction, resulting in the rapid heating of the samples under microwave irradiation with a fast-processing time [37]. Moreover, there is evidence that microwave irradiation can improve the regioselectivity and stereoselectivity aspects of vital importance in synthesizing bioactive compounds [38].
Preliminary studies were done to obtain chitin using the conventional heating method and the results are shown in Table 1. It took us 3 h in the demineralization step and 6 h in the deproteinization step to reach a removal ratio close to the ratio that we obtained for both shrimp and crab chitin, which took only 15 min by microwave technology in this study. Thus, in this study, holistic high-power microwave technology assisted in reducing the time it takes, as well as chemicals, with a high percentage of demineralization and deproteinization to obtain pure chitin. Finally, the new technique has a consensus time of 15 min. for demineralization and deproteination versus 9 h for the conventional heating method. This will greatly reduce the preparatory costs, and the new technique will be highly cost-effective.
During the preliminary study, we estimated the extracted yield percentage (%) based on the dry weight throughout the extraction processes. Crustacean shells consist mainly of minerals, protein, and chitin. Using the holistic high-power microwave technology used in the current study, we found that the yield percentage (%) of shrimp shells was 42% minerals, 28% protein, and 30% chitin, against 73%, 12%, and 15% for crab crustaceans, respectively. In general, crustacean shells consist of 30–40% proteins, 30–50% minerals, and 20–30% chitin, but these percentages vary depending on the source, or even the species, from which chitin is isolated. For example, Crangon crangon shrimp waste consists of 10–38% proteins, 31–44% minerals, and 24–46% chitin [10]. This demonstrates that using the holistic high-power microwave technology in this study, enabled the obtention of precise results comparable to previous studies, requiring longer time and higher temperatures. The 15 min-deacetylation process led to chitosan production amounting to 25% and 11% of the starting shell waste and 83.3% and 73.3% of chitin of shrimp and crab, respectively.
3.3 Results of chitosan DD experiment by potentiometric titration method
In this study, high-watt holistic microwave technology for chitosan extraction during demineralization, deproteinization, and deacetylation steps was investigated. This was done to obtain highly deacetylated chitosan in minutes rather than days or even hours, to reduce the effort and energy used by traditional methods. The ash and protein levels in the shells and the resulting chitin were determined to evaluate the effect of microwave power at 750 W and 875 W during demineralization and deproteinization stages for 7 and 8 min, respectively. Section 3. 1. contains the discussion of results. As a result of using microwave-aided extraction, deacetylation time was significantly reduced, as was energy consumption. The impact of 10, 15, and 20-min periods at 875 W and 1250 W microwave irradiation on the chitosan DD during the deacetylation step were investigated. The results are shown in Fig. 2. The values in Fig. 2 report the mean of three separate experiments. The results show that chitosan with a high DD has been extracted at 875 W for 10 min with a DD% of 80.3% and 78.9% for SC and KC, respectively. It confirms that chitosan was successfully prepared in 25 min using comprehensive microwave technology with a high DD of 875 W. Microwave-assisted chitosan extraction was studied only during the deacetylation step of chitin to chitosan [12, 39]. Knidri et al. [33] and El Knidri et al. [40] studied the influence of microwave irradiation during all stages of chitosan extraction at a maximum power of 650 W. In an experiment, El Knidri et al. [40] obtained chitosan with a DD of 74.79% in 24 min via the microwave irradiation method at 650 W. Hence, the optimal condition of this study (875 W, 25 min) yielded a similar quantity of chitosan but with a higher DD. At 875 W, the maximum DD of chitosan was achieved at 15 min, recording 86.6% and 83.8% for SC and KC, respectively. The maximum DD reached by Knidri et al. [33] was 82.8% at 650 W for 14 min during the deacetylation step. He et al. [41] reported that chitosan with DD between 55 and 70% is considered low; 70–85% means medium; 85–95% means high; and 95–100% means ultrahigh chitosan. Subsequently, results have shown that microwave radiation at 875 W may be employed for chitosan extraction with a high DD as a simple, effective, and speedy heating approach in just 30 min. A decrease in the DD was observed at 875 W for 20 min, which was 73.2% and 71.7% for SC and KC, respectively. The obtained findings may be attributed to the thermal degradation of chitosan or concentration of NaOH due to microwave heating at higher powers or over longer periods. Consistent with Al Sagheer et al. [12], they reported that extending the period after exceeding the optimum time causes chitosan degradation and loss into the reaction solvent. Thus, heating polar solvents forces their constituting particles to rotate with the field and lose energy during collisions. This may be subsequently reflected on the degree deacetylation. The DD at 1250 W for 10, 15, and 20 min were 72.4%, 69.5%, and 65.7% for SC against 71.0%, 70.2%, and 65.0% for KC, respectively. It is clear that not only with the increase in time, the DD decreases, but also with the increase in the power of the microwave, the DD decreases. We note that the DD at 875 W for 10 min is higher than at 1250 W, as well as at 15 and 20 min. This could be due to the effect of the rapid increase in temperature achieved via microwave heating. Whatever, chitosan with a higher DD was obtained by microwave heating for 30 min at 875 W, as opposed to conventional heating methods, which required a much longer time of 7–8 h to prepare chitosan. For example, under deacetylation conditions of 50% NaOH at 100 °C for 8 h [36], have been obtaining lower DD (74%) for shrimp chitosan. As a result of this experience, the SC and KC with the highest DD (86.6 and 83.8%, respectively) and CC were chosen for the rest of the analyses.
Means of the degree of deacetylation (DD%) of extracted shrimp and crab chitosan as assayed by the potentiometric titration method. The statistical significance of differences between means of data for (DD) was checked by a two-way ANOVA with a significance level of p ≤ 0.05. The data is presented as the mean ± SE. The DD at 875 W for 10, 15, and 20 min were 80.30 ± 2.3%b, 86.60 ± 1.7%a, and 73.20 ± 3.2% c for shrimp chitosan (SC) against 78.90 ± 4% b, 83.80 ± 0.58% a, and 71.70 ± 2.6% c for crab chitosan (KC), respectively. The DD at 1250 W for 10, 15, and 20 min were 72.40 ± 1.5 c, 69.50 ± 2.5% d, and 65.70 ± 1.5% e for SC against 71.00 ± 3.1% c, 70.20 ± 2.1% c, and 65.00 ± 2.1%.d for KC, respectively. The LSD at 0.05 for Watt, Time, and Compare Means were 3.9, 4.8, and 1.5 for SC against 4.6, 5.7, and 1.8 for KC, respectively. Shrimp chitosan and crab chitosan at 875 W with a 15-min deacetylation time showed a significant difference at the level of significance (p ≤ 0.05)
When we used the conventional heating method to deacetylate chitin, it took us 4 h of heating at 100 °C in addition to 2 h of shaking on the shaker device to reach a deacetylation point of 83.8% for shrimp chitosan and 84.5% for crab chitosan. While using holistic high-power microwave technology, it took us only 15 min to achieve a similar deacetylation rate at 875 W. Even though the current study and Knidri et al. [33] had the same total extraction time, the current study has a higher percentage of demineralization, deproteinization, and deacetylation than Knidri et al. [33] and the conventional heating method in the study conducted by Marei et al. [36]. On an industrial scale, conventional chemical procedures are considered the most common methods for chitosan recovery from crustacean shells. They use strong acids and bases to achieve the desired results. Moreover, classical chemical treatments are hazardous, energy-consuming, and environmentally unfriendly. A holistic high-power microwave at 875 W for 30 min can be a common chemical reaction technique since it provides an approach for extracting highly clean, economical, simple, effective, and convenient speedy DD chitosan.
3.4 Characterization of the chitosan with the highest DD
3.4.1 Solubility
Chitosan solubility is one of the essential properties that must be understood and regulated [42]. The findings in Table 2 indicate that the obtained chitosan has a higher solubility than commercial chitosan in 1% acetic acid, registering 79% and 74.5% for SC and KC, respectively, against only 68.5% for CC. The obtained solubility ratio may also confirm the degree of deacetylation for shrimp and crab chitosan. The removal of acetyl groups from chitin determines chitosan solubility. Where the presence of the hydrophobic acetyl group may cause insolubility due to more hydrogen bonds between it and the 3,6–OH on the same or adjacent chitin chains by the following bonds ─NH⋯O═C and ─OH⋯O═C. On the other hand, the high removal of the acetyl group leads to the presence of free primary amino groups, and chitosan becomes an electrolyte with a high density of positive charges (R–NH3+ groups) in acidic solutions. As a result, chitosan becomes able to dissolve in diluted acidic aqueous solutions. The low molecular weight of the resultant chitosan might have an impact on its solubility. Because an increase in molecular weight results in an increase in molar mass for a given amount of solvent, it is predicted that a polymer will have a smaller solubility range and a lower solubility concentration with the increasing molecular weight. But the lower the molecular weight, the greater the attraction between molecules, and thus the higher the solubility. According to Divya et al. [43], the solubility of chitosan in acetic acid is a sign of its purity. Kumari et al. [18] found that the solubility of chitosan from crab was 60%, with a DD% of 70%, when using 40% KOH at 90 ºC for six hours. This confirms that the microwave method used in this study led to better solubility in less time and less energy when compared to conventional heating methods.
3.4.2 WBC analysis
WBC is one of the functional properties of chitosan and expresses the tendency of water to bind to hydrophilic substances [44, 45]. As indicated in Table 2, the WBC of CC, SC, and KC was 673.76%, 705.40%, and 689.82%, respectively. When compared to commercial chitosan, the isolated chitosan from shrimp and crab had a greater WBC. The present findings support those of earlier researchers, who discovered that WBC varied amongst crawfish chitosan samples, ranging from 660.6 to 745.4% [46]. According to Kucukgulmez et al. [47], Metapenaeusstebbingi chitosan has a WBC of 712.99%. Furthermore, the study conducted by Cho et al. [48] submitted that commercial chitosan from shrimp and crab shells had a WBC of 458% to 805%. The differences in results may be explained by Huang and Tsai [49], with hydrostatic pressure that degraded chitosan into far fewer molecules, increasingly its surface area, thus allowing hydrogen bonds to form with water molecules. The contact angles of chitosan were reduced as the DD was raised, implying that chitosan’s hydrophilicity had risen.
3.4.3 FBC analysis
Chitosan can be used as a functional food and a nutritional component with higher FBC levels [45]. According to the FBC results, chitosan has a high ability to bind or absorb fat. Chitosan has three types of reactive functional groups: the amino group and primary and secondary hydroxyl groups at the C-2, C-3, and C-6 positions, respectively. It can form stable covalent bonds via several reactions, such as etherification, esterification, and reductive amination reactions. As shown in Table 2, the FBC of CC was 387.82%, whereas the FBC value of SC and KC was 434.60% and 413.20%, respectively. The FBC has a similar pattern to the WBC. It is due to chitosan with a low molecular weight is more effective than chitosan with a high molecular weight at binding fat. This conclusion is consistent with the findings of Huang and Tsai [49]. Chitosan has an FBC ranging from 314 to 535% [48]. Marei et al. [36] reported that the FBC of shrimp chitosan is around 326%, but Rasweefali et al. [45] discovered that the FBC of chitosan samples ranges from 722.00 to 819.17%. The difference in results between this study and earlier studies might be attributed to the type of oil used in the trial. A higher WBC value than FBC can be ascribed to the fact that water has a higher polarity than oil, causing greater water attachment to the chitosan chain’s amine and hydroxyl groups [49]. The low molecular weight chitosan produced in this way can be used in many excellent applications, based on its enhanced solubility and functional characteristics when compared to integral chitosan, according to Gonçalves et al. [50].
3.4.4 The intrinsic viscosity and average molecular weight analysis
The data in (Fig. 3) represents the evolution of a linear fit of the reduced and inherent viscosity as a function of chitosan concentration, which was used to calculate the intrinsic viscosity, as presented in Table 2. The intrinsic viscosity decreased as the DD of chitosan increased. The intrinsic viscosity of SC and KC was 13.418 and 15.987, respectively, compared to 39.672 for the CC, corresponding to DD values of 86.6%, 83.8%%, and 74.6%, respectively. This demonstrates how chitosan deacetylation affects the viscosity and flow parameters of the solutions [51].
We obtained the viscosimetric average molecular weight by substituting the intrinsic viscosity value in Eq. (10), which is approximately 14.125 kDa for SC with 86.6% DD and 16.982 kDa for KC with 83.8% DD while increasing to 44.66 kDa for CC with 74.6% DD, as shown in Table 2. These results are similar to those found by El Knidri et al. [40]. The variance in molecular weight is generally influenced by the DD and the different chitosan sources. Furthermore, numerous parameters in the extraction of chitosan, such as concentration of used alkali, reaction duration, and prior chitin treatment, may influence the molecular weight of chitosan [40] and particle size [47]. The molecular weight of chitosan extracted with high-power microwave technology (875 W) was at a low rate of 14.125 kDa, which is much lower than that obtained by Knidri et al. [33] with low microwave power (650 W), which was on the order of 165 kDa. This may be due to the effect of the high power of microwave radiation on the chitosan chain. Thus, the holistic high-power microwave technology used here caused the production of low molecular-weight chitosan. El-Sayed et al. [52] reported that the inhibitory activities of molecular weight chitosan between 10 and 100 kDa were as efficient as the antibiotics Flomox and Kluacid against Bacillus cereus. Also, Chang et al. [53] mentioned that the DPPH antioxidant activity was higher in chitosan with a molecular weight of less than 72 kDa with a reduction potential of up to 90% than in chitosan with a molecular weight of 300 kDa. So, the low molecular weight chitosan produced in this study can be considered a possible alternative for antimicrobial agents or additives in pharmaceutical compositions and medical applications.
3.4.5 FTIR spectrum analysis
To comprehensively define the structure of chitosan, the FTIR chitosan spectrum was registered. The FTIR chitosan spectrums are exhibited in Fig. 4. The extent of the absorption peaks of the chitosan derived from the tested species with FTIR yielded ranges similar to those obtained in the studies conducted earlier by Kaya et al. [54] and Kim [55] as presented in Table 3. CC, SC, and KC had given absorption peaks of 3352.45 cm−1, 3451.33 cm−1, and 3450.71 cm−1, respectively, which correspond to the stretching vibrations of the hydroxyl group –OH. While, the distinctive absorption peak, which corresponds to the –CH stretching regions of CC, SC, and KC was at 2881.56 cm−1, 2880.15 cm−1, and 2878.06 cm−1, respectively. SC, KC, and CC absorption peaks in the FTIR spectrum match those achieved by [33, 56, 57]. Amide I (C=O in −NHCOCH3) and amide II (amine -NH2) were ascribed to absorption bands between 1620 and 1660 cm−1 and 1590–1610 cm−1, respectively. Whereas the distinctive bands of saccharide structure seem to be at roughly 1155 cm−1 to 1165 cm−1 (the C–O–C glycosidic linkage), in the chitosan, which is consistent with Kim [55]. This confirms the preservation of the structure of the obtained product. Yang et al. [58] obtained similar results. Pyranose ring vibrations are observed at 890 cm−1 to 900 cm−1 for CC, SC, and KC. These results agree with the results found previously by Apriyanti et al. [59] and Boudouaia et al. [60]. A slight FTIR spectra variation between prepared chitosan and commercial chitosan may be due to the different molecular weights of the prepared and standard chitosan; the prepared is generally lower in molecular weight than the standard. This corresponds to Saeed et al. [61]. Mourya et al. [62] and Suryani et al. [63] also indicated that the chemical structure of low molecular weight chitosan is similar to that of native chitosan except for the difference caused by the depolymerization and the decrease of the acetyle group in low molecular weight chitosan compared to native chitosan. The DD% was estimated using the intensity of the amide I band (at 1655 cm−1) and the OH band (at 3450 cm−1), according to Eq. (11). The outcomes are shown in Table 5. In this study, high-power microwave technology induced a higher deacetylated degree (86.7%) than a previous study by Knidri et al. [33] using a lower-power microwave (650 W), which recorded 82.84%.
3.4.6 1H NMR analysis
The most effective approach to determining the structure of chitosan is 1H NMR [33]. So, the chemical composition of chitosan samples was confirmed by the 1H NMR spectrum, as shown in Fig. 5. The summary of the chemical shifts of all chitosan samples is given in Table 4. The chemical shift of the C1 proton of the glucosamine unit in CC, SC, and KC appears at 4.88, 4.88, and 4.92 ppm, respectively. The chemical shift of the C2 proton appears at roughly around 3.18–3.24 ppm, while the peak from 3.74 to 4.34 ppm is assigned to C3–C6 protons of the glucosamine unit and N-acetylglucosamine unit (Protons of the pyranose ring) [55]. The peaks at 1.98, 2.02, and 1.97 ppm for CC, SC, and KC, respectively refer to the methyl protons in the acetamide group. The obtained results are consistent with the results found previously by Kim [55] and Leke-Aladekoba [64]. The area of the methyl group signal (at 1.95–2.09 ppm), and the sum of the H2, H3, H4, H5, and H6 areas (3.18 to 4.34 ppm) were used to compute the DD%, according to Eq. (12). The DD% for SC, KC, and CC samples employed by 1H NMR are presented in Table 5.
3.4.7 XRD analysis
Microwave-irradiated shrimp and crab chitosan diffractograms are depicted in Fig. 6. It demonstrates the existence of two distinctive crystalline peaks at 2θ = 9.17°, 9.75°, and 9.37° for CC, SC, and KC, respectively, and at 2θ = 20.18°, 19.49°, and 19.66° for CC, SC, and KC, respectively. Gbenebor et al. [65] and Ibitoye et al. [66] found similar findings. Generally, numerous chitosan XRD patterns exhibit two distinct peaks that are often located around 2θ = 9–11° and 2θ = 19–20° [33, 67]. The obtained results concur highly with results given by Jampafuang et al. [68], who found that peaks at roughly 2θ = 9–11° refer to amine I “–NH–CO–CH3” of chitosan, and peaks at roughly 2θ = 19–20° refer to amine II “–NH2” of chitosan. According to the obtained findings, chitosan produced using the microwave technique has a lower crystallinity index than commercial chitosan. The X-ray data represents an average of molecules arranged in a periodic crystal lattice. In addition, the technique is not easily applicable to oligosaccharides. Due to the difficulty of obtaining single crystals from oligosaccharides [69], this confirms the preservation of the structure of the obtained chitosan. The CI% for SC and KC was 46.57% and 74.14%, respectively, but increased to 76.34% in CC. The crystallinity index (CI%) is shown in Table 2. Rasweefali et al. [45] obtained similar results in terms of CI% values. The degree of crystallinity of SC in this study attained a lower value (46.57%) than that obtained by Knidri et al. [33], at (61.20%), probably due to the higher deacetylation degree in the first case. In the same trend, the CI% for the commercial sample CC is greatly higher (76.34%), than the prepared sample, being associated with lower DD (74.6%).
3.4.8 SEM analysis
The surface morphology of the obtained chitosan was examined using SEM. At lower magnification (800X), the commercial and produced chitosan appeared to feature layers of flakes, a lamellar organization, and a dense structure as shown in Fig. 7. The obtained results concur highly with results given by El Knidri et al. [40] and Knidri et al. [33]. While the surface morphologies of commercial and produced chitosan appear to have a smooth surface at higher magnification (24000X). The surface morphology of the obtained SC with a high DD appears smoother than the surface morphology of the obtained KC with a low DD and the surface morphology of the CC appears to have a rough surface. The larger amount of free amino groups (-NH2) in high deacetylated chitosan might explain these results compared to low deacetylated chitosan. The surface roughness in low deacetylated chitosan returns to the presence of monomers containing the acetamide group (acetylated monomer “Glu-NHCOCH3”) in the chitosan chain. As the number of free amino groups increases, the surface of chitosan becomes smoother. The obtained results are consistent with the results found previously by Hussain et al. [21] and Yang et al. [58].
4 Conclusions
This study identified the optimal conditions for microwave-assisted extraction of chitosan during the demineralization, deproteinization, and deacetylation steps of shrimp and crab shell waste. Chitosan has been successfully prepared under optimal conditions of high-power microwave technology in 30 min with a higher deacetylated degree (86.7%) at 875 W. The molecular weight and the crystallinity index (CI%) were both relatively lower in the new preparation (SC), recording 14.125 kDa and 46.57% (CI), respectively. The physicochemical features of the produced chitosan measured by X-ray, FTIR, and 1H NMR findings, confirmed the high degree of deacetylation and preserved chemical characteristic structure of chitosan. When compared to a previous study using low-power microwave technology, these results are impressive and produce a new product of chitosan. In the current study, the use of microwave-assisted heating for just 30 min improved chitosan production and properties and reduced energy and time consumption, compared to previous microwave methods and conventional heating methods. As a result, the current study’s enhancing chitosan extraction can provide a clean, convenient, appealing, and feasible alternative to commercial production. The preparation process is quick and simple, and the resulting new product is specified with a relatively high deacetylation extent, low molecular weight, low viscosity, low crystallinity, and low protein residue. So, the low molecular weight chitosan produced in this way can be used as a possible alternative antimicrobial agent or additive in pharmaceutical compositions and medical applications. The authors seek to apply this method in an expanded manner to extract chitosan from its different sources in future experimental or application works.
Data availability
Any data associated with this work are available on request.
References
Sitohy M, Taha S, Osman A, Abdel-Hamid M, Hamed A, Abdelbacki A (2020) Antiviral action of native and methylated lactoferrin and β-lactoglobulin against potato virus Y (PVY) infected into potato plants grown in an open field. Antibiotics 9:430. https://doi.org/10.3390/antibiotics9070430
Sitohy M, Mahgoub S, Osman A (2011) Controlling psychrotrophic bacteria in raw buffalo milk preserved at 4 C with esterified legume proteins. LWT-Food Sci Technol 44:1697–1702. https://doi.org/10.1016/j.lwt.2011.03.008
Chobert JM, Sitohy M, Billaude S, Dalgalarrondo M, Haertlé T (2007) Anticytomegaloviral activity of esterified milk proteins and L-Polylysines. J Mol Microbiol Biotechnol 13:255–258
Sitohy M, Taha S, Abdel-Hamid M, Abdelbacki A, Hamed A, Osman A (2021) Protecting potato plants against PVX and PVY viral infections by the application of native and chemically modified legume proteins. J Plant Dis Prot 128:1101–1114. https://doi.org/10.1007/S41348-021-00448-9
Osman A, El-Didamony G, Sitohy M, Khalifa M, Enan G (2016) Soybean glycinin basic subunit inhibits methicillin resistant-vancomycin intermediate Staphylococcus aureus (MRSA-VISA) in vitro. Int J Appl Res Nat Prod 9:17–26
Sitohy M, Mahgoub S, Osman A, El-Masry R, Al-Gaby A (2013) Extent and mode of action of cationic legume proteins against Listeria monocytogenes and Salmonella Enteritidis. Probiotics Antimicrob Proteins 5:195–205. https://doi.org/10.1007/s12602-013-9134-2
Chattopadhyay K, Xavier KM, Balange A, Layana P, Nayak BB (2019) Chitosan gel addition in pre-emulsified fish mince-Effect on quality parameters of sausages under refrigerated storage. LWT 110:283–291. https://doi.org/10.1016/j.lwt.2019.04.081
Sabu S, Ashita T, Stephy S (2020) Chitosan and lemon peel extract coating on quality and shelf life of yellowfin tuna (Thunnus albacares) meat stored under refrigerated condition. Indian J Fish 67:114–122. https://doi.org/10.21077/ijf.2019.67.1.91361-15
Minh NC, Van Hoa N, Trung TS (2020) Preparation, properties, and application of low-molecular-weight chitosan. In: Handbook of Chitin and Chitosan, Elsevier, Amsterdam, vol 1, pp 453–471. https://doi.org/10.1016/B978-0-12-817970-3.00015-8
El Knidri H, Belaabed R, Addaou A, Laajeb A, Lahsini A (2018) Extraction, chemical modification and characterization of chitin and chitosan. Int J Biol Macromol 120:1181–1189. https://doi.org/10.1016/j.ijbiomac.2018.08.139
Omara NA, Elsebaie EM, Kassab HE, Salama AA (2019) Production of chitosan from shrimp shells by microwave technique and its use in minced beef preservation. Slov Vet Res 56:773–780. https://doi.org/10.26873/SVR-818-2019
Al Sagheer FA, Al-Sughayer MA, Muslim S, Elsabee MZ (2009) Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohyd Polym 77:410–419. https://doi.org/10.1016/j.carbpol.2009.01.032
Joshi DR, Adhikari N (2019) An overview on common organic solvents and their toxicity. J Pharm Res Int 28(3):1–18. https://doi.org/10.9734/jpri/2019/v28i330203
AOAC (2000) Association of Official Analytical Chemists. Official Methods of Analysis, seven tenth ed.
Xie J, Xie W, Yu J, Xin R, Shi Z, Song L, Yang X (2021) Extraction of chitin from shrimp shell by successive two-step fermentation of Exiguobacterium profundum and Lactobacillus acidophilus. Front Microbiol 12:677126. https://doi.org/10.3389/fmicb.2021.677126
Czechowska-Biskup R, Jarosińska D, Rokita B, Ulański P, Rosiak JM (2012) Determination of degree of deacetylation of chitosan-comparison of methods. Prog Chem Appl Chitin Deriv 17:5–20
Agarwal M, Agarwal MK, Shrivastav N, Pandey S, Gaur P (2018) A simple and effective method for preparation of chitosan from chitin. Int J Life Sci Sci Res 41:1721–1728. https://doi.org/10.21276/ijlssr.2018.4.2.18
Kumari S, Annamareddy SHK, Abanti S, Rath PK (2017) Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int J Biol Macromol 104:1697–1705. https://doi.org/10.1016/j.ijbiomac.2017.04.119
Ocloo FCK, Quayson ET, Adu-Gyamfi A, Quarcoo EA, Asare D, Serfor-Armah Y, Woode BK (2011) Physicochemical and functional characteristics of radiation-processed shrimp chitosan. Radiat Phys Chem 80:837–841. https://doi.org/10.1016/j.radphyschem.2011.03.005
Norzita Y, Norhashidah T, Maznah M (2013) Determination of viscosity-average molecular weight of chitosan using intrinsic viscosity measurement. J Nuclear Relat Technol 10:39–44
Maji TK, Hussain MR, Iman M (2013) Determination of degree of deacetylation of chitosan and their effect on the release behavior of essential oil from chitosan and chitosan-gelatin complex microcapsules. Int J Adv Eng Appl. 6:4–12
Rout SK (2001) Physicochemical, functional and spectroscopic analysis of crawfish chitin and chitosan as affected by process modification. PhD Thesis, Louisiana State University and Agricultural & Mechanical College. https://digitalcommons.lsu.edu/gradschool_disstheses/432
Alvarenga ES, de Oliveira CP, Bellato CR (2010) An approach to understanding the deacetylation degree of chitosan. Carbohyd Polym 80:1155–1160. https://doi.org/10.1016/j.carbpol.2010.01.037
Liu S, Sun J, Yu L, Zhang C, Bi J, Zhu F, Yang Q (2012) Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules 17:4604–4611. https://doi.org/10.3390/molecules17044604
Olafadehan OA, Amoo KO, Ajayi TO, Bello VE (2021) Extraction and characterization of chitin and chitosan from Callinectes amnicola and Penaeus notialis shell wastes. J Chem Eng Mater Sci 12:1–30. https://doi.org/10.5897/JCEMS2020.0353
Hamdi M, Nasri R, Dridi N, Li S, Nasri M (2020) Development of novel high-selective extraction approach of carotenoproteins from blue crab (Portunus segnis) shells, contribution to the qualitative analysis of bioactive compounds by HR-ESI-MS. Food Chem 302:125334. https://doi.org/10.1016/j.foodchem.2019.125334
Zhang P, Hu H, Tang H, Yang Y, Liu H, Lu Q, Yao H (2019) In-depth experimental study of pyrolysis characteristics of raw and cooking treated shrimp shell samples. Renew Energy 139:730–738. https://doi.org/10.1016/j.renene.2019.02.119
Rødde RH, Einbu A, Vårum KM (2008) A seasonal study of the chemical composition and chitin quality of shrimp shells obtained from northern shrimp (Pandalus borealis). Carbohyd Polym 71:388–393. https://doi.org/10.1016/j.carbpol.2007.06.006
Hahn T, Tafi E, Paul A, Salvia R, Falabella P, Zibek S (2020) Current state of chitin purification and chitosan production from insects. J Chem Technol Biotechnol 95:2775–2795. https://doi.org/10.1002/jctb.6533
Pighinelli L, Broquá J, Zanin BG, Flach AM, Mallmann C, Taborda FGD, Dias RJSP (2019) Methods of chitin production a short review. Am J Biomed Sci Res 3:307–314. https://doi.org/10.34297/AJBSR.2019.03.000682
da Silva Lucas AJ, Oreste EQ, Costa HLG, López HM, Saad CDM, Prentice C (2021) Extraction, physicochemical characterization, and morphological properties of chitin and chitosan from cuticles of edible insects. Food Chem 343:128550. https://doi.org/10.1016/j.foodchem.2020.128550
Samar MM, El-Kalyoubi MH, Khalaf MM, Abd El-Razik MM (2013) Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann Agric Sci 58:33–41. https://doi.org/10.1016/j.foodchem.2020.128550
Knidri HE, Dahmani J, Addaou A, Laajeb A, Lahsini A (2019) Rapid and efficient extraction of chitin and chitosan for scale-up production: effect of process parameters on deacetylation degree and molecular weight. Int J Biol Macromol 139:1092–1102. https://doi.org/10.1016/j.ijbiomac.2019.08.079
Campana-Filho SP, Britto DD, Curti E, Abreu FR, Cardoso MB, Battisti MV, Lavall RL (2007) Extraction, structures and properties of alpha-and beta-chitin. Quim Nova 30:644–650. https://doi.org/10.1590/S0100-40422007000300026
Lertsutthiwong P, How NC, Chandrkrachang S, Stevens WF (2002) Effect of chemical treatment on the characteristics of shrimp Chitosan. J Metals Mater Min 12:11–18
Marei NH, Abd El-Samie E, Salah T, Saad GR, Elwahy AH (2016) Isolation and characterization of chitosan from different local insects in Egypt. Int J Biol Macromol 82:871–877. https://doi.org/10.1016/j.ijbiomac.2015.10.024
Seekaew Y, Arayawut O, Timsorn K, Wongchoosuk C (2019) Synthesis, characterization, and applications of graphene and derivatives. Carbon-based nanofillers and their rubber nanocomposites. Elsevier, Amsterdam, pp 259–283. https://doi.org/10.1016/B978-0-12-813248-7.00009-2
Albuquerque HM, Pinto DC, Silva AM (2021) Microwave irradiation: alternative heating process for the synthesis of biologically applicable chromones, quinolones, and their precursors. Molecules 26(20):6293. https://doi.org/10.3390/molecules26206293
Santos VP, Maia P, Alencar NDS, Farias L, Andrade RFS, Souza D, Campos-Takaki GM (2019) Recovery of chitin and chitosan from shrimp waste with microwave technique and versatile application. Arq Inst Biol 86:e0982018. https://doi.org/10.1590/1808-1657000982018
El Knidri H, El Khalfaouy R, Laajeb A, Addaou A, Lahsini A (2016) Eco-friendly extraction and characterization of chitin and chitosan from the shrimp shell waste via microwave irradiation. Process Saf Environ Prot 104:395–405. https://doi.org/10.1016/j.psep.2016.09.020
He X, Li K, Xing R, Liu S, Hu L, Li P (2016) The production of fully deacetylated chitosan by compression method. Egypt J Aquat Res 42:75–81. https://doi.org/10.1016/j.ejar.2015.09.003
Akpan EI, Gbenebor OP, Adeosun SO, Cletus O (2020) Solubility, degree of acetylation, and distribution of acetyl groups in chitosan. Handbook of Chitin and Chitosan. Elsevier, Amsterdam, pp 131–164. https://doi.org/10.1016/B978-0-12-817970-3.00005-5
Divya K, Rebello S, Jisha MS (2014) A simple and effective method for extraction of high purity chitosan from shrimp shell waste. In: Proceedings of the international conference on advances in applied science and environmental engineering—ASEE. https://doi.org/10.15224/978-1-63248-004-0-93
Mohan K, Ganesan AR, Muralisankar T, Jayakumar R, Sathishkumar P, Uthayakumar V, Revathi N (2020) Recent insights into the extraction, characterization, and bioactivities of chitin and chitosan from insects. Trends Food Sci Technol 105:17–42. https://doi.org/10.1016/j.tifs.2020.08.016
Rasweefali MK, Sabu S, Sunooj KV, Sasidharan A, Xavier KM (2021) Consequences of chemical deacetylation on physicochemical, structural and functional characteristics of chitosan extracted from deep-sea mud shrimp. Carbohydr Polym Technol Appl 2:100032. https://doi.org/10.1016/j.carpta.2020.100032
Fernandez-Kim SO (2004) Physicochemical and functional properties of crawfish chitosan as affected by different processing protocols. https://digitalcommons.lsu.edu/gradschool_theses/1338
Kucukgulmez A, Celik M, Yanar Y, Sen D, Polat H, Kadak AE (2011) Physicochemical characterization of chitosan extracted from Metapenaeusstebbingi shells. Food Chem 126:1144–1148. https://doi.org/10.1016/j.foodchem.2010.11.148
Cho YI, No HK, Meyers SP (1998) Physicochemical characteristics and functional properties of various commercial chitin and chitosan products. J Agric Food Chem 46:3839–3843. https://doi.org/10.1021/jf971047f
Huang YL, Tsai YH (2020) Extraction of chitosan from squid pen waste by high hydrostatic pressure: effects on physicochemical properties and antioxidant activities of chitosan. Int J Biol Macromol 160:677–687. https://doi.org/10.1016/j.ijbiomac.2020.05.252
Gonçalves C, Ferreira N, Lourenço L (2021) Production of low molecular weight chitosan and chitooligosaccharides (COS): a review. Polymers 13:2466. https://doi.org/10.3390/polym13152466
Costa CN, Teixeira VG, Delpech MC, Souza JVS, Costa MA (2015) Viscometric study of chitosan solutions in acetic acid/sodium acetate and acetic acid/sodium chloride. Carbohyd Polym 133:245–250. https://doi.org/10.1016/j.carbpol.2015.06.094
El-Sayed ST, Ali AM, Omar NI (2019) A comparative evaluation of antimicrobial activity of chitooligosaccharides with broad spectrum antibiotics on growth of some pathogenic microorganisms. Biocatal Agric Biotechnol 22:101382. https://doi.org/10.1016/j.bcab.2019.101382
Chang SH, Wu CH, Tsai GJ (2018) Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohyd Polym 181:1026–1032. https://doi.org/10.1016/j.carbpol.2017.11.047
Kaya M, Baran T, Asan-Ozusaglam M, Cakmak YS, Tozak KO, Mol A, Sezen G (2015) Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). J Biotechnol Bioprocess Eng 20:168–179. https://doi.org/10.1007/s12257-014-0391-z
Kim SK (2010) Chitin, chitosan, oligosaccharides and their derivatives: biological activities and applications. CRC Press, Boca Raton. https://doi.org/10.1201/EBK1439816035
Sudatta BP, Sugumar V, Varma R, Nigariga P (2020) Extraction, characterization and antimicrobial activity of chitosan from pen shell, Pinna bicolor. Int J Biol Macromol 163:423–430. https://doi.org/10.1016/j.ijbiomac.2020.06.291
Weißpflog J, Vehlow D, Müller M, Kohn B, Scheler U, Boye S, Schwarz S (2021) Characterization of chitosan with different degree of deacetylation and equal viscosity in dissolved and solid state–Insights by various complimentary methods. Int J Biol Macromol 171:242–261. https://doi.org/10.1016/j.ijbiomac.2021.01.010
Yang J, Shen M, Luo Y, Wu T, Wen H, Xie J (2021) Construction and characterization of Mesonachinensis polysaccharide-chitosan hydrogels, role of chitosan deacetylation degree. Carbohyd Polym 257:117608. https://doi.org/10.1016/j.carbpol.2020.117608
Apriyanti DT, Susanto H, Rokhati N (2018) Influence of microwave irradiation on extraction of chitosan from shrimp shell waste. Reaktor 18:45–50. https://doi.org/10.14710/reaktor.18.1.45-50
Boudouaia N, Bengharez Z, Jellali S (2019) Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: application for Eriochrome black T removal from aqueous solutions. Appl Water Sci 9:1–12. https://doi.org/10.1007/s13201-019-0967-z
Saeed A, Zahid S, Sajid M, Ud Din S, Alam MK, Chaudhary FA, Abutayyem H (2022) Physico-mechanical properties of commercially available tissue conditioner modified with synthesized chitosan oligosaccharide. Polymers 14(6):1233. https://doi.org/10.3390/polym14061233
Mourya VK, Inamdar NN, Choudhari YM (2011) Chitooligosaccharides: synthesis, characterization and applications. Polym Sci, Ser A 53:583–612. https://doi.org/10.1134/S0965545X11070066
Suryani S, Chaerunisaa AY, Joni IM, Ruslin R, Ramadhan LOAN, Wardhana YW, Sabarwati SH (2022) Production of low molecular weight chitosan using a combination of weak acid and ultrasonication methods. Polymers 14(16):3417. https://doi.org/10.3390/polym14163417
Leke-Aladekoba AA (2018) Comparison of extraction methods and characterisation of chitin and chitosan with antimicrobial and antioxidant properties from black soldier fly (Hermetiaillucens) meal. http://hdl.handle.net/10222/75013
Gbenebor OP, Adeosun SO, Lawal GI, Jun S, Olaleye SA (2017) Acetylation, crystalline and morphological properties of structural polysaccharide from shrimp exoskeleton. Eng Sci Technol Int J 20:1155–1165. https://doi.org/10.1016/j.jestch.2017.05.002
Ibitoye EB, Lokman IH, Hezmee MNM, Goh YM, Zuki ABZ, Jimoh AA (2018) Extraction and physicochemical characterization of chitin and chitosan isolated from house cricket. Biomed Mater 13:025009. https://doi.org/10.1088/1748-605X/aa9dde
De Queiroz ARSCM, Lia Fook BRP, de Oliveira Lima VA, de Farias Rached RÍ, Lima EPN, da Silva Lima RJ, Lia Fook MV (2017) Preparation and characterization of chitosan obtained from shells of shrimp (Litopenaeus vannamei Boone). Mar Drugs 15:141. https://doi.org/10.3390/md15050141
Jampafuang Y, Tongta A, Waiprib Y (2019) Impact of crystalline structural differences between α-and β-Chitosan on Their nanoparticle formation via ionic gelation and superoxide radical scavenging activities. Polymers 11:2010. https://doi.org/10.3390/polym11122010
Cunha RA, Soares TA, Rusu VH, Pontes FJ, Franca EF, Lins RD (2012) The molecular structure and conformational dynamics of chitosan polymers: an integrated perspective from experiments and computational simulations. Complex World Polysacch. https://doi.org/10.5772/51803
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
MS and AA conceptualized the research concept. AE has done the data curation; Formal analysis and investigation. AA has achieved the Funding acquisition, revised the manuscript, and administered the project. MS has supervised the Investigation and validated the methodology. RS has contributed to the Methodology; Project administration; Resources. AS has contributed to Supervision, validation, and Visualization. AE has written the draft manuscript and MS has written the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ewais, A., Saber, R.A., Abdel Ghany, A. et al. High quality, low molecular weight shrimp and crab chitosans obtained by short-time holistic high-power microwave technology. SN Appl. Sci. 5, 365 (2023). https://doi.org/10.1007/s42452-023-05602-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05602-6