Abstract
The present research activity's major objective is to improve the performance and emission characteristics of diesel engines that burn pyro oil and cashew nut shell nanoparticles. This work identifies the nanoparticles that should be added to cashew nut shell pyro oil to allow it to be utilized efficiently in compression ignition engines. In this experiment, the two nanoparticles, Al2O3 and TiO2, were mixed with cashew nut shell pyro oil. For testing purposes, nanoparticles were combined with cashew nut shell pyro oil at various concentrations, including 150 and 200 ppm. The criterion for picking the best combination was stability. To improve the optimal performance of the nanoparticle-blended oil, the cashew nut shell pyro oil was emulsified. The nanoparticles, which also act as an enhancing agent, speed up the combustion process. The combination of pyro oil, cashew nut shell emulsion, and nanoparticles improved performance and reduced emissions. The brake thermal efficiency values for 200 ppm Al2O3 and TiO2 mixed emulsified cashew nut shell pyro oil were 29.6% and 29.4%, respectively, whereas the values for diesel and cashew nut shell pyro oil were 31.6% and 29.8%. When comparing cashew nut shell pyro oil to oil with 200 ppm Al2O3 and TiO2, the smoke value was found to be reduced by 58.8% and 56.7%, respectively. It was discovered that cashew nutshell pyro oil with 200 ppm Al2O3 and TiO2 had 35.3% and 34.96% reduced hydrocarbon emissions respectively.
Article Highlights
-
This paper used Al2O3 and TiO2 nanoparticles mixed in the Cashew Nut Shells oil. The emulsion of the fuel as at rated power output, performance results of CPO200 Al2O3 showed greater than BTE, lower BSFC, and also lower EGT than CPO200 TiO2.
-
UBHC, CO, NOx, and smoke emissions are reduced as compared to diesel fuel respectively. The braking thermal efficiency values were slightly increased from ppm Al2O3 and TiO2 blended emulsified cashew nut shell pyro oil respectively. whereas the values were 31.6% and 29.8% for diesel and cashew nut shell pyro oil.
-
The smoke value and hydrocarbon emissions and CO emissions are reduced as compared to diesel fuel.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
As a result of their superior effectiveness and performance, Diesel Engines are typically used. However, it has a few downsides like excessive smoke, NOx production, and a propensity to deplete. Since it is a non-renewable resource, automotive fuel is becoming increasingly scarce. Therefore, scientists are looking towards non-polluting, alternative fuels that should be renewable. Bio oils are discovered forthcoming renewable in nature and to have qualities akin to those of conventional fuel. Fossil fuels, which are slowly running out, nonetheless account for a significant portion of the world's primary energy supply. Research has been focused on creating more sustainable and renewable energy systems in response to the increasing global energy demands and environmental concerns. In addition to being a source of energy, biomass also has the potential to produce a variety of high-quality goods and biochemicals, including composite materials, paints, adhesives, and other things. While the latter can be quickly modified, the former is a time- and money-consuming operation. Vegetable oils have characteristics that are comparable to those of conventional gasoline, but using them directly in an engine causes them to burn less efficiently and emit more smoke, carbon monoxide, and hydrocarbons. Vegetable oil has a high viscosity and density, which can cause nozzle sticking and gum buildup in fuel flow lines when used directly. Researchers have tried some of the approaches in an effort to decrease the density and viscosity of the fuel and use it more effectively. One such method is to pre-heat the oil using emulsified and trans-esterified oil. Trans-esterified vegetable oil reduces viscosity, which improves engine performance. The use of emulsified gasoline has a number of advantages, such as improved combustion and fewer contaminants. In this work, raw and emulsified vegetable oils that had been combined with Al2O3 and TiO2 nanoparticles as fuels were examined and their performance was contrasted with that of diesel. completely in pyro oil made from cashew nuts. Additionally, sonication was carried out at 150 and 200 ppm in a similar manner. After a few days of observation, the 150 ppm blended nanoparticles in the cashew nut shell pyro oil were allowed to settle, and 200 ppm was found to be the appropriate blend in this circumstance. Al2O3 and TiO2 were formed into four samples in two different sets for the research.
2 Literature review
In their analysis of the most recent trends and advancements in the use of cashew nut shells, as well as their extracts and isolates, as clean energy sources, James Mgaya et al. also covered numerous efforts made to use agro-waste in the energy industry [1]. Kumar V et al. concluded that bio-oils produced through fast pyrolysis are more practicable in terms of transportation, storage, and handling after doing a brief examination of the many improvements and current state of biomass pyrolysis technologies during the preceding thirty years [2]. According to A K Hossain & P A Davies, only one-fourth of the twenty-four confirmed tests using pyro fluid (both crude and refined) in diesel engines were successful in stabilizing engine performance [3]. Long ignition delays, corrosion of engine parts, coking of cylinder and piston liners, suddenly rises in-cylinder pressure, reduced BTE, prolonged combustion times, higher SFC and CO emissions, etc. According to Kumar S et al., using pure CPO with a CI engine caused it to operate poorly and to emit more NOx and less HC, CO, and smoke while also reducing these emissions [4]. A further point made by the authors was that it is feasible to replace bio-oil made from cashew nuts by up to 40% without sacrificing engine performance.
The impact of hexanol and pentanol addition to cashew nut biodiesel is further investigated. In both cases, a modest change in BTE and a significant decrease in BSFC were seen along with elevated NOx levels and lowered CO, HC, and HCO levels. G. Kasiraman et al.'s testing of blends of cashew nut shell oil with 10%, 20%, and 30% (by volume) camphor oil as fuels on a diesel engine was presented, along with long ignition delay, engine component damage, and other issues. They discovered that the "30% camphor oil blends with 70% cashew nut shell oil" performed nearly as well as diesel fuel, but with 3.6% more BTE, 5.8% higher NOx, and 7.4% higher [5]. V.K. Bupesh Raja & J Jaya Prabhakar examined the performance and emission characteristics of diesel engines using fuel blends of 20%, 40%, and 60% (by volume) cashew nut shell oil. When compared to diesel fuel, they discovered that the 20% blend had lower CO, HC, and CO2 emissions but created NOx levels that were somewhat higher [6]. Pushparaj et al., investigated the effects of Cashew nut shell biodiesel blends of 10%, 20%, and 30% by volume on the performance and emission evaluation in diesel engines and discovered that blend20 demonstrated improved power output over diesel and improved performance parameters, including up to 45% higher SFC along with the 37% reduced CO emissions and slightly increased Nox, etc. [7]. K Venkatesan tested different blends of "Cashew nut shell pyro oil blended with 20% Jathropa biodiesel-Diesel mixture" as fuels for diesel engines and found that "10% cashew nut shell pyro oil blended with 20% Jathropa-Diesel" closely resembled diesel properties with a slight increase in BTE and BSFC and a decrease in CO, HC, and Smoke emissions with diesel [8]. Venkatesan K et al., investigated how waste cooking oil combined with diesel fuel performed and what emissions it produced in a four-stroke DI diesel engine. CPO5WC5D90, CPO10WC5D85, and CPO15WC5D80 are pyro oil and diesel mixes that produced a performance that was comparable with a little decrease in BTE at all power settings [9]. A number of metal-based additives have been found to improve combustion quality when applied to biodiesels. When Cashew nut shell biodiesel with nano alumina particles was evaluated by Radhakrishnan et al., the results showed reductions in CO, HC, NOx, and smoke emissions of 10%, 8.8%, 12.4%, and 8.4%, respectively [10].
3 Materials and methodology
Cashew nut shells were gathered at a processing plant for cashew nuts in the Indian suburb of Neyveli. Shells were cleaned, exposed to the sun for 36 h to dry them out and remove any excess moisture, and then pulverized in a lab ball mill. and the samples were kept in a refrigerator for additional testing at 4–50 C. Figure 1 depicts photographic views of the cashew nuts, cashew nut shells, the ball mill that was used to grind the samples, and the pyrolysis setup used for the extraction of cashew shell pyrolysis oil.
4 Preparation of nanoparticle-blended emulsified cashew shell pyro oil
By adding 200 ppm Al2O3 and TiO2 and 80% Cashew Nut Shells oil, Cashew Pyro Oil was transformed into its emulsion (Fig. 2). Oil was placed in a beaker and swirled for 100 min at a rate of 1500 rpm. The fuel's viscosity is mostly determined by the amount of water and temperature in the emulsion. Emulsion's energy content is lower than that of raw oil, but because it enhances combustion by the micro explosion, it has no effect on BTE. The 200 ppm Al2O3 and TiO2 and also 150 ppm Al2O3 and TiO2 were then separately blended with EMO using an ultrasonicator. Table 1 shows the physicochemical characteristics of neat and mixed prepared fuel properties analysis. The engine was tested using the prepared fuel, and the Performance, and Emission were compared to those of Diesel fuel.
5 Experiment setup and procedure
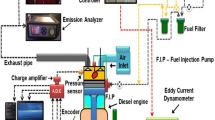
A schematic of the experimental test setup and the engine specifications are shown in Fig. 3 and Table 2, respectively. The tests were conducted using a KIRLOSKAR single-cylinder, four-stroke, naturally aspirated diesel engine that has an output power of 3.7 kW at 1500 rpm. The test rig was composed of a CI engine, a dynamometer, an exhaust gas analyzer, a smoke meter, a DAS (digital type) connected to a computer, and several sensors and measuring tools [11,12,13,14,15]. A CI engine shaft was connected to an eddy current dynamometer through a load sensor to provide the brake load. An rpm sensor of the non-contact type was affixed close to the flywheel to measure speed. The water within the calorimeter was circulated using a self-priming pump. The cooling water flow rate for the engine and dynamometer, which varied from 40 to 400 L per hour, was controlled by providing a separate rota metre [16,17,18]. To measure air flow rates, differential pressure transmitters were put in the air box next to an in-line air filter. A fuel metering system follows the in-line fuel tank and keeps track of gasoline usage rates [19,20,21,22]. A surge tank was employed upstream of the air intake manifold to ensure a consistent airflow by reducing the pulsations. A TDC Pulse pick-up and a piezo-electric pressure transducer were installed in the engine cylinder to read the crank angle and cylinder pressure, respectively. For offline analysis, the required data was obtained and put on a 16-bit NI USB-6210 high-speed DAS. K-Type thermocouples were used to measure the temperatures at various points throughout the engine. Initially, the engine was started, and a steady state level was allowed to establish. The gas analyzer and smoke metre were stabilized for a while before to the observations. The timing of the fuel injection was established at 270 before TDC with 200 bar of injection pressure [23,24,25]. Since CI engines are frequently set up to run on diesel fuel, diesel was initially fed to the engine in order to obtain the baseline data. after the addition of the next gasoline blend for testing, cleaning the fuel line, and gathering test-tank data. Each of the five blended materials tested—CPO, CPO200 Al2O3, CPO150 Al2O3, CPO200 TiO2, and CPO150 TiO2—was subjected to the same process.
The BSFC was calculated at a specified braking load using automated Excel computations. BTE was calculated at the specified load using inputs like fuel consumption, calorific value, and density in the Excel sheet. Exhaust gas temperatures were measured with a calorimeter that resembled a pipe. Emissions such as HC, NOx, CO, and smoke opacity were monitored with the aid of an exhaust gas analyzer for the purpose of recording. After the fuel line had been washed off, the subsequent fuel mixture was then fed into the tank. After scrubbing the gasoline line, testing the ensuing fuel mixture, and compiling test results in the tank. The procedure was repeated for each of the five tested combinations, and for accurate findings, the average values were taken into consideration.
6 Results and discussion
6.1 Performance parameter
The Figs. 4 illustrate, correspondingly, the Brake Thermal Efficiency (BTE), with load at various absorptions of Al2O3 and TiO2 nanoparticles. The concentration of nanoparticles was observed to boost the BTE. Cashew Pyro Oil with 200 ppm Al2O3 and TiO2 nanoparticles showed superior BTE to other blends. This could be a result of the gasoline-containing nanoparticles that better combustion. Because of their greater surface area-to-volume ratios, each nano-particle in the ignition served as a Heat source and improved combustion. Figure 4, the BTE with the load for a blended EMO of 200 ppm Al2O3 and TiO2.
It is clear from the graph that at full load, the nano-particle-combined CPO produced improved BTE than the nano-particle-with- Cashew Pyro Oil combination. Increased BTE was the result of the water droplets in this fuel enhancing the burning through spray fragmentation by the micro-explosion. The nanoparticle-blended CPO was less effective than the nanoparticle-blended Cashew Pyro Oil under partial load conditions. This is brought on by the fuel's inclusion of water, which lowers the heat inside the Combustion chamber. BTE value for 200 ppm Al2O3 blended CPO was superior to 200 ppm TiO2 blended CPO.
Al2O3’s better heat conductivity and oxygen availability compared to TiO2 may be the cause of this. This might be a result of Al2O3’s superior heat conductivity and oxygen availability compared to TiO2. The BTE of CPO including TiO2 nanoparticles and 200 ppm Al2O3 was determined to be 31.5% and 30.8%, respectively, while the corresponding values for diesel and CPO150 Al2O3 and CPO150 TiO2 were 30.5, 30.2% and 29.5%.
Based on fuel efficiency, BSFC communicates engine performance. It calculates how much gasoline is used to produce a unit of brake power in a certain amount of time. For each of the tested fuels, the graphic shows how BSFC varies with BP. With increasing BP outputs, BSFC for all of the tested blends dropped, as seen in Fig. 5
At all BP outputs, CPO200 Al2O3 and CPO200 TiO2 used less fuel than the others. Due to their substantially lower calorific values, greater viscosities, and higher densities, BSFC CPO150 Al2O3 and CPO150 TiO2 may have an advantage over diesel. Shorter combustion times caused by the presence of EMO in the fuel mixtures cause pressure to rise before the anticipated point of combustion.
The temperature of exhaust gases reveals how much energy is dissipated as heat in the exhaust. For Al2O3 and TiO2 nanoparticle combined Cashew Pyro Oil, Fig. 6 shows the EGT fluctuations compared with various load situations. Cashew Pyro Oil combined with TiO2 and Al2O3 nanoparticles at 200 ppm had the lowest EGT of all the blends. This might be because the gasoline contains nanoparticles, which would have lowered the temperature of the exhaust stream. Reduced EGT was the result of improved combustion, where the majority of the heat was dissipated inside the combustion chamber.
Al2O3 and TiO2 nanoparticles combined with CPO are shown in Fig. 6 to exhibit differences in EGT with a load of 200 ppm. Because there is water in the fuel, the EGTs of 200 ppm Al2O3 and TiO2 blended CPO were found to be lower than those of 150 ppm Al2O3 and TiO2 blended Cashew Pyro Oil. The EGT was decreased by the latent heat of water absorption. Al2O3 and TiO2 nanoparticles both demonstrated superior EGT reduction than TiO2, which may be related to Al2O3's improved combustion.
7 Emission parameters
Air–fuel combinations, poorly formed combustion mixtures, shorter combustion times, and a deficiency of oxygen are thought to be the main contributors to CO emissions. All of the tested fuels' CO emission percentages vary with increasing BP output, as shown in Fig. 7. The graph shows that CO emissions increase with increasing Brake loads. It is clear from the Figure that since diesel is an oxygen-free fuel, its CO emissions are lower than those of a combination of 200 ppm Al2O3 and 200 ppm TiO2.
Because Al2O3 has a higher packed energy density than the others, TiO2 mixed CPO was found to have the lowest CO emissions of the evaluated fuels. At the highest BP condition, CO emissions for CPO150 Al2O3 and CPO150 TiO2, Diesel, 200 ppm TiO2 and 200 ppm Al2O3 were 16%, 14%, 10%, 8%, and 7.5%, respectively. The CPO150 Al2O3 and CPO150 TiO2 blends' high fuel content, low energy density, and low calorific values caused some carbon atoms to partially oxidize, which produced CO. In their studies, Temilola T. Olugasa et al. [23] found similar carbon monoxide emission outputs.
The Fig. 8 shows the amount of unburned hydrocarbon produced when TiO2 and Al2O3 nanoparticles are combined with Cashew shell pyro oil, respectively. In comparison to other blends, the HC emissions were lower when Cashew shell pyro oil with 200 ppm Al2O3 and TiO2 was used. The decrease in HC emissions may be attributed to the large amount of fuel used in combustion, which would have oxidized due to the presence of nanoparticles in the fuel. Inbanaathan Papla Venugopal et al. [24] found that HC emission was slightly reduced.
The Fig. 8 compares fluctuations of CPO with 150 ppm Al2O3 and TiO2 to all other evaluated fuels. Comparing the CPO with 200 ppm Al2O3 and TiO2 to other evaluated fuels, a decrease in HC emissions was observed. The combination of a micro-explosion and a high Cylinder may be blamed for this drop. Al2O3 nanoparticle-mixed CPO reduced the HC emissions more effectively than TiO2 blended CPO because Al2O3 burns more efficiently than TiO2. For 200 ppm Al2O3 and TiO2 nanoparticle-blended CPO, the HC emission values were found to be 82 ppm, 95 ppm, and 102 ppm, respectively, in comparison to diesel and 150 ppm Al2O3 and TiO2.
Nitric oxide fluctuations with load are shown in Fig. 8 for various concentrations of blended Al2O3 and TiO2 Cashew nut pyro oil, respectively. Comparing the NO emissions at 100 ppm Al2O3 and TiO2 mixed CPO to all other concentrations, there was a rise. The greater temperature inside the combustion chamber may be the cause of the rise in NO emissions. Figure 9 compares the fluctuations in NO with load for nanoparticles combined with Cashew shell pyro oil and base fuel with Al2O3 and TiO2 at 150 ppm. The 200 ppm Al2O3 and TiO2 mixed CPO released less NO than the other examined fuels. Qiuyan Fan et.al [25] and Venkatesan et.al [26] studies show that NO emission was gradually reduced.
This might be a result of water evaporating due to latent heat, which would have delayed the ignition period and decreased NO generation. Al2O3 blended gasoline had more NO emissions than TiO2 did when compared to the two oxides. This may be due to Al2O3's greater heat conductivity than TiO2’s. The NO values of CPO blended with TiO2 nanoparticles and Al2O3 at 200 ppm were determined to designate 390 ppm and 410 ppm, respectively, whereas, corresponding values for Diesel, CPO150 Al2O3, CPO150 TiO2 were 450 ppm, 460 ppm, and 489 ppm.
For different amounts of Al2O3 and TiO2 mixed CPO, respectively, Fig. 10 illustrates how smoke opacity varies with load. In comparison to all other concentrations, the 200 ppm Al2O3 and TiO2 mixed CPO produced better results for smoke. Nanoparticles utilized in gasoline may be to blame for the reduced amount of smoke. Smoke was significantly decreased by the use of nanoparticles in the fuel. As a heat source, the nanoparticles enable CPO to swiftly evaporate by heating it to a sufficient level. Figure 10 compares the fluctuations in smoke with load for CPO that is blended with 100 ppm Al2O3 and TiO2 against 150 ppm of nanoparticles. Due to the added effect of the OH micro explosion and free radicals together with the fast heat transfer of the fuel, the 150 ppm Al2O3 and TiO2 nanoparticle-blended CPO produced significantly fewer smoke emissions than other evaluated fuels. Balasubramanian et al. [27] show that the combustion characteristics and cumulative heat release rate increase. Al2O3 blended fuel reduced smoke more effectively than TiO2 than either TiO2 or Al2O3 alone. Due to Al2O3's higher heat conductivity than TiO2, this may have occurred. In contrast to diesel and CPO oil, which had respective smoke values of 40% and 84%, 200 ppm Al2O3 and TiO2 nanoparticle-blended CPO was found to have 32% and 34.4%.
8 Conclusions
The features of CI engine performance and emissions were examined when 200 ppm Al2O3 and 150 ppm TiO2 nanoparticles were added to various CPO-diesel combinations. Nanoparticles were shown to be well miscible in combinations of 200 ppm Al2O3 and 150 ppm TiO2, and over the course of a week, no phase separation took place. It has been established that incorporating nanoparticles into gasoline can somewhat enhance engine performance. Performance findings of CPO200 Al2O3 at rated power output demonstrated 2.31% larger BTE, 6% lower BSFC, and 3.0% lower EGTs than CPO200 TiO2 at the same power output. In that order, emissions of UBHC, CO, NOx, and smoke decreased by 3.2%, 15%, 8%, and 5.27%, respectively. Lower smoke emissions were observed for the tested fuels at all BP outputs as compared to diesel. The efficiency of diesel engines was shown to be better than that of CPO200 Al2O3 and CPO200 TiO2. Despite being the best among the studied materials, CPO200 Al2O3’s promise as a dependable alternative fuel is constrained by the modest increases discovered.
Data availability
All data generated or analyzed during this study are included in this published article.
References
Mgaya J, Shombe GB, Masikane SC, Mlowe S, Mubofu EB, Revaprasadu N (2019) Cashew nutshell: a potential bio-resource for the production of bio-sourced chemicals, materials and fuels. Green Energy 21:1186–1201
Kumar V, Nanda M (2016) Environmental effects biomass pyrolysis-current status and future directions, energy sources. Part A Recover Util Environ Eff 38:2914–2921. https://doi.org/10.1080/15567036.2015.1098751
Hossain AK, Davies PA (2013) Pyrolysis liquids and gases as alternative fuels in internal combustion engines—a review. Renew Sustain Energy Rev 21:165–189. https://doi.org/10.1016/j.rser.2012.12.031
Kumar S, Dinesha P, Rosen MA (2018) Cashew nut shell liquid as a fuel for compression ignition engines: a comprehensive review. Energy Fuels 32:7237–7244. https://doi.org/10.1021/acs.energyfuels.8b00579
Kasiraman G, Nagalingam B, Balakrishnan M (2012) Performance, emission and combustion improvements in a direct injection diesel engine using cashew nut shell oil as fuel with camphor oil blending. Energy 47:116–124. https://doi.org/10.1016/j.energy.2012.09.022
Bupesh Raja VK, JayaPrabakar J (2017) Performance and emission characteristics of cashew nut shell oil on the CI engine. Int J Ambient Energy 1:1. https://doi.org/10.1080/01430750.2017.1421584
Pushparaj T, Ramabalan S, Arul Mozhi Selvan V (2015) Performance evaluation and exhaust emission of a diesel engine fueled with CNSL biodiesel. Energy Sources Part A Recover Util Environ Eff 37:2013–2019. https://doi.org/10.1080/15567036.2011.643343
Venkatesan K (2016) Experimental investigation on performance of cashew nut shell pyrolysed oil blend with bio diesel and diesel. Exp Therm Fluid Sci 14:2918–2926
Kuppusamy V, Sankerlal S (2018) Performance and emission characteristics of cashew nutshell pyrolyzed oil – waste cooking oil with diesel fuel in a four stoke di diesel engine. Int J Mech Prod Eng Res Dev 8(1):181–188
Radhakrishnan S, Munuswamy DB, Devarajan Y, Arunkumar T, Mahalingam A (2018) Effect of nanoparticle on emission and performance characteristics of a diesel engine fueled with cashew nut shell biodiesel, Energy Sources. Part A Recover Util Environ Eff 40:2485–2493. https://doi.org/10.1080/15567036.2018.1502848
Kuberan J, Venkatesan K (2020) Effect of injection pressure on a diesel engine using pyrolysis Bio-oil from spirulina algae. Int J Mech Prod Eng Res Dev 10(1):731–740
Venkatesan K, Rahul RJ (2018) Diesel engine performance and emission analysis using mosambi peel pyro oil with nano additive particles. Int J Mech Prod Eng Res Dev 8(6):311–316
Venkatesan K, Rahul RJ (2019) A comprehensive assessment on performance and emissions of ci engine fuelled with diesel and mosambi peel pyro oil blended with methanol. Int J Mech Prod Eng Res Dev 9:100–110
Venkatesan K, Sathyaraj S (2019) Evaluation of ci engine performance and mission fuelled by diesel-mosambi peel pyro oil blended with copper oxide nanoparticles. Int J Mech Prod Eng Res Dev 9(3):1–12
Venkatesan K, Kiran KB, Prema Kumar PS (2019) Evaluating performance and emissions of CI engine run by blends of mosambi peel methyl ester and diesel fuel. Int J Mech Prod Eng Res Dev 9(3):593–600
Venkatesan K, Kuberan J (2020) A comprehensive assessment on performance and emission characteristics of diesel engine by using mosambi peel pyro oil as fuel. Int J Mech Prod Eng Res Dev 10(3):873–884
Venkatesan K, Rithanya S (2020) Experimental investigation on dual-fuel engine performance and emission characteristics using diesel and mosambi peel pyro oil with oxygen concentration. Int J Mech Prod Eng Res Dev 10(3):341–356
Venkatesan K (2021) Investigation on the performance and emissions profile of CI engine using cashew nut shell pyro oil-toluene-diesel blends. SN Appl Sci. https://doi.org/10.1007/s42452-021-04580-x
Venkatesan K, Dhanasekaran P, Harish V (2022) Effect of trapezoidal fuzzy decisions on cashew shell pyro oil blend in compression ignition engine. J East China Univ Sci Technol 65(2):573–584
Venkatesan K, Kubaran J, Harish V (2022) Experimental investigation on a CI diesel engine fuelled with Mosambi peel bio-oil blended toluene emulsions. Spec Ugdym Spec Educ 1(43):7831–7842
Venkatesan K (2022) Comparative performance and emissions of CI engine fuelled with diesel and blends of mosambi peel pyro oil, methanol and nano Rh2o3 with diesel. Int J Veh Struct Syst 14(7):925–930. https://doi.org/10.4273/ijvss.14.7.19
Venkatesan K, Kiran Kumar B, Harish V (2022) Combustion and Emissions performance of direct use of mosambi peel pyro oil diesel blend in a diesel engine by methanol enrichment. Aust J Mech Eng 45:2146706. https://doi.org/10.1080/14484846.2022.2146706
Olugasa TT, Adekanbi ML, Fasogbon SK (2022) Performance and emission characteristics of compression ignition engine running on a blend of cashew nut shell liquid and biodiesel produced from orange peel. Eur J Sustain Dev Res 6(4):0197
Venugopal IP, Balasubramanian D, Rajarajan A (2021) Potential improvement in conventional diesel combustion mode on a common rail direct injection diesel engine with PODE/WCO blend as a high reactive fuel to achieve effective Soot-NOx trade-off. J Clean Prod 327:129495
Fan Q, Abbas J, Zhong Y, Pawar PS, Adam NA, Alarif GB (2023) Role of organizational and environmental factors in firm green innovation and sustainable development: moderating role of knowledge absorptive capacity. J Clean Prod 411:137262
Perumal Venkatesan E, Balasubramanian D, Samuel OD, Kaisan MU, Murugesan P (2021) Effect of hybrid nanoparticle on DI diesel engine performance, combustion, and emission studies. Novel Intern Combust Engine Technol Perform Improv Emiss Reduct 10:235–263. https://doi.org/10.1007/978-981-16-1582-5_10
Balasubramanian D, Inbanaathan PV, Gugulothu SK, Noga M (2021) Characterization of single-cylinder di diesel engine fueled with waste cooking oil biofuel/diesel blends. In: Balasubramanian D (ed) Alternative fuels and advanced combustion techniques as sustainable solutions for internal combustion engines. Springer, Singapore, pp 173–196
Barik D, Bora BJ, Sharma P, Medhi BJ, Balasubramanian D, Krupakaran RL, Varuvel EG (2023) Exploration of the dual fuel combustion mode on a direct injection diesel engine powered with hydrogen as gaseous fuel in port injection and diesel-diethyl ether blend as liquid fuel. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2023.06.083
Author information
Authors and Affiliations
Contributions
V,H Wrote the main manuscript and analysis result. K,K prepare tables and figures All authors reviewed the manuscript
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuppusamy, V., Venkatesan, H., Jayaraman, K. et al. Effective utilization of cashew nut shell pyro oil blended with optimum amount of Al2O3 and TiO2 nanoparticles in DI engine. SN Appl. Sci. 5, 269 (2023). https://doi.org/10.1007/s42452-023-05486-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05486-6