Abstract
In this research, Mg–3Zn–xSi (x = 0, 0.1, 0.3, and 0.6 wt%) biodegradable alloys were produced by simple stir casting method, their microstructure and phase changes were evaluated using X-ray diffraction (XRD), optical microscope (OM) and scanning electron microscope coupled with energy dispersive x-ray analysis (SEM–EDS). Potentiodynamic polarization was conducted to measure the alloys’ corrosion behavior in simulated body fluid (SBF). Tensile strength test and in-vitro biocompatibility evaluation regarding MTT cytotoxicity, ALP osseointegration assay and MG-63 cell growth pattern were conducted. Electrochemical investigations showed that Mg–3Zn alloys enclosing Si attained degradation rates suitable for structural support until bone healing, while the Mg–3Zn alloy without Si had a corrosion rate of 0.128 mm/year which is much lower than the required value. None of the inspected alloys exhibited a significant cytotoxic effect, meanwhile, Mg–Zn base alloy and the alloy with 0.3 wt% Si demonstrated the highest ALP level. The optimum cell growth pattern was demonstrated for Mg–Zn base alloy and the alloy with 0.1 wt% Si. Evidence of calcium phosphate precipitation was observed in the four investigated Mg–3Zn alloys. Therefore, based on the fore mentioned results, Mg–3Zn–xSi alloys were suggested as viable biodegradable materials due to their compatible degradation rates, proved cytocompatibility, high cell viability and excellent osseointegration potential.
Article Highlights
-
Increasing Si content from 0.1 to 0.6 wt% results in Mg2Si intermetallic phase coarsening which negatively affected the strength and the corrosion resistance of the alloy and accelerates its degradation rate.
-
Mg2Si intermetallic phase in Mg–3Zn–xSi alloy improves MG-63 cells’ viability and osseointegration
-
Mg–3Zn–xSi alloys are potential orthopedic biodegradable materials for orthopedic use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Each year, millions of people suffer from bone fractures brought on by aging, diseases and accidental injuries [1]. The majority of these fractures require surgical fixation with implants because they are typically too difficult to heal by external medical care.
Today, biodegradable implants are becoming more vital as a replacement for permanent implants used for temporary bone healing. A successful orthopedic implant should uniformly deteriorate in-situ to be gradually restored by the regenerating tissue till the osseous healing procedure is accomplished [2]. Therefore, to ensure the functionality of the metallic implant during the service period, its degradation rate should be maintained within a reasonable range [3]. Too slow deterioration will retard the formation of new bone while too fast deterioration cannot provide structural support for the defected site [4]. Moreover, a biomaterial should have sufficient strength and mechanical qualities comparable to that of bone to be able to provide structural support and avoid stress shielding [5].
Corrodible metals, as Mg, Fe, and Zn are attracting interest as orthopedic implantable biomaterials thanks to their biodegradability and biocompatibility. Although Fe and Fe alloys have excellent mechanical qualities, their deterioration rates are too sluggish to support bone healing. When compared to the rates at which natural bone grows, Zn and Zn alloys degrade at appropriate rates. However, the inadequate strength and ductility of pure Zn metals as well as the low fatigue strength and susceptibility to creep and natural aging of Zn and Zn alloys limit their applicability in bone restoration [6].
Mg is among the utmost prevalent cations in the human body, mainly found in bone [7], where it affects the architecture of RNA and DNA as well as the metabolism of enzymes. Mg alloys have elastic moduli and densities comparable to bone moduli and densities [8], but they are chemically very active and readily corrodes, resulting in the development of a permeable oxide layer on its surface [2]. Consequently, they deteriorate early in the physiological milieu of humans, causing a premature loss of strength before the bone heals.
There are several methodologies to slow down the rate of deterioration of Mg-based alloys, among these methods is alloying. Alloying Mg with other elements, including (Zn, Al, Re, Si, Ca, etc.) may control the grain size, microstructure, and phase distribution of the alloy, enhancing its ability to resist corrosion [9]. Moreover, elements that are found in the human body, such as Ca, Zn, Mn, and Si, in addition to diminutive levels of low toxicity rare earth metals may be endured to prevent the corrosion of magnesium alloys, thus are attractive alloying choices [10]. Calcium is one of the main components of human bone, and its release may enhance the process of bone repair [11,12,13,14]. Zinc is another fundamental element in the human body, which enhances the strength and corrosion resistance of the Mg alloy [15]. Song et al. [10] reported that a magnesium alloy enclosing Zn and traces of Ca, Mn, or Si could be a potential biodegradable alloy. Cai et al. [16] mentioned that adding of 1–5% Zn could enhance the corrosion resistance and strength of the Mg alloy.
Recently, silicon has already been recognized as an important element in the human body that helps in healing and the development of the immune system [17]. Furthermore, Si may be vital for bone and connective tissue growth and development. Attributable to the beneficial biological properties of Si, the use of Mg–Si alloys as biodegradable materials in orthopedic applications should be encouraged.
However, Si addition can lead to the creation of a secondary phase, e.g., \({\mathrm{Mg}}_{2}\mathrm{Si}\) [8, 18, 19]. The formation of micro-galvanic cells between \({\mathrm{Mg}}_{2}\mathrm{Si }\) and the surrounding matrix as a result of the greater potential of the intermetallic phase serves as the catalyst for localized corrosion and accelerates the Mg alloys’ corrosion in SBF [20].
The secondary phases’ size, shape, and allocation influence the corrosion performance of the \(\mathrm{MgSi}\) alloys thus they were examined. Amirnejad et al. [21] and Ben‐Hamu et al. [22] investigated how the \({\mathrm{Mg}}_{2}\mathrm{Si}\) morphology affected the corrosion behavior of the Mg alloy. They proposed that \({\mathrm{Mg}}_{2}\mathrm{Si}\) phases with a large Chinese script shape enhanced the corrosion progression, whereas the polygonal shape hindered it.
It was also reported that when \({\mathrm{Mg}}_{2}\mathrm{Si}\) had coarse shape, the corrosion of the Mg alloy increased [22], however, the inclusion of a third element can change the \({\mathrm{Mg}}_{2}\mathrm{Si}\) structure into a finer one. The negative effect that increases the rate of degradation is reduced in this finer morphology. According to Ben-Hamu et al. [22], the \({\mathrm{Mg}}_{2}\mathrm{Si}\) phase is refined by adding Zn to \(\mathrm{MgSi}\) alloys, increasing its corrosion resistance. Srinivasan et al. [23] stated that 0.5 wt% Si and 0.5 wt% Sb addition to the AZ91 Mg alloy enhanced its corrosion behavior, and that the presence of Sb modified the \({\mathrm{Mg}}_{2}\mathrm{Si}\) intermetallic Chinese script shape into a fine polygonal shape thus enhanced its corrosion performance.
Zhang et al. [24] examined the refining influence of adding Ca, Zn, or Sr to \(\mathrm{MgSi}\) alloys on the \({\mathrm{Mg}}_{2}\mathrm{Si}\) phase, they mentioned that the refinement of \({\mathrm{Mg}}_{2}\mathrm{Si}\) can be accomplished by a little addition of Ca and Zn. Yang et al. [25] showed that Sr addition in the AZ61-0.7Si alloy could efficiently improve the \({\mathrm{Mg}}_{2}\mathrm{Si}\) Chinese script shape. Santos et al. [26] informed that the amount of Si influenced the speed of deterioration of the Mg–Si–Sr alloy. Si contents less than 0.15 wt% and Sr contents less than 0.25 wt% were advised to lower the degradation rate of this ternary system as compared to pure Mg.
Consequently, it should be promising that Mg–Zn alloys with added Si could be employed as potential corrodible bone implants. In order to develop biomaterials with characteristics similar to bone, it is vital to investigate their chemical compositions, mechanical qualities, biocompatibility and degradation rates. Hence, the present study aims to assess the influence of Si addition on the microstructure, mechanical qualities and corrosion behavior of biodegradable Mg–3Zn alloys for potential orthopedic applications. Zn was introduced to the Mg alloy to enhance its corrosion resistance and to refine the structure of Mg2Si secondary phases while Si was added for its good biological functions. Different prepared Mg–3Zn–xSi alloys should maintain their sufficient strength in order to withstand the functional forces subjected to them when implanted in bone until healing.
To the best of the authors’ knowledge, there is no published study investigating the effect of Si on the corrosion behavior, mechanical properties and in-vitro biocompatibility of Mg based alloys. Hence it was relevant in this work to study all these qualities to find an optimum concentration of Si to attain an Mg alloy having good tensile strength and biocompatibility while satisfying the requirements of bone healing rates, for efficient orthopedic implants.
2 Materials and methods
2.1 Alloys manufacturing
Several Mg alloys of Mg-3 wt% Zn chemical composition were prepared with the addition of 0, 0.1, 0.3, and 0.6 wt% Si by simple stir casting method. Commercially pure Mg (99.98 wt%), pure Zn (99.99%), and Mg-5 wt% Si master alloys were employed. Stir-casting is a flexible and affordable method for incorporating various types of reinforcements into the Mg matrix. By regulating the process parameters and reinforcing properties during stir-casting, uniform dispersion can be achieved [27, 28].
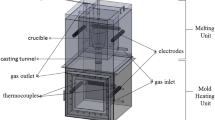
The investigated alloys were placed in graphite crucible and melted in electrical furnace under a protecting gas mixture of tetrafluoroethene (CF3CH2F, R134a, 1 volume%) and argon (Ar, Balance). Firstly, Mg ingots were heated at temperature above 650 °C; Zn and different compositions of Si were then added to achieve the desired alloys. To achieve homogenous mixes, the temperatures of the alloys melt were elevated above 760 °C and maintained 15 min while stirring. The slags were removed to obtain clean melting surfaces. Finally, the liquid was poured into preheated cylindrical cast iron metallic mold of 100 mm outer diameter, 40 mm inner diameter, and 250 mm height. X-ray fluorescence (XRF) analyzer (model Axios advanced-PANALYTCAL, Netherlands), was used to perform elemental analysis of the as-cast alloys as shown in Table 1.
2.2 Phase analysis and microstructure characterization
Investigated specimens obtained at 10 mm from the bottom of the cast rods were ground, polished then etched for 2–3 min. in an acidic solution of 30, 10, 120 and 40 ml of acetic acid, nitric acid, ethanol, and water respectively, Specimens were cleaned using anhydrous ethyl alcohol then dried. Different phases in the investigated specimens were detected utilizing thin film X-ray diffraction (XRD, model X’PERT PRO,) with a Cu-Kα radiation source. Their microstructure was assessed using optical microscope (OM) (Zeis model Axiotech, Germany) as well as a scanning electron microscope (SEM) (model JSM-5410, Japan) supplied with an energy-dispersive X-ray analysis (EDS).
2.3 Electrochemical behavior
Electrochemical measurements were performed employing (potentiostat/galvanostat, Autolab PGSTAT 302N, Netherlands, NOVA software) in SBF solution at 37 °C and pH 7.4. The SBF solution was prepared as reported by Kokubo et al. [29] and it consisted of (g/l) 8 NaCl, 0.14 CaCl2, 0.4 KCl, 0.35 NaHCO3, 1 C6 H12O6, 0.1 NaH2PO4, 0.1 MgCl2·6H2O, 0.06 Na2HPO4·2H2O and 0.06 MgSO4·7H2O [13, 30]. The employed cell was a three-electrode cell with an Ag/AgCl reference electrode, a platinum counter electrode, and working electrodes made of the alloys under investigation. The exposed circular area to the electrolyte solution was 0.785 cm2. Open circuit potentials (OCP) were recorded at a stabilization time of 30 min before beginning the potentiodynamic measurements. Electrochemical impedance spectroscopy (EIS) was achieved using perturbation amplitude of 5 mV and a frequency range of 10 mHz–100 kHz. Potentiodynamic polarization curves between − 2.0 and − 0.6 mV were recorded at a scan rate of 1 mV/s. The tests were carried out three times for each sample to confirm the reproducibility of results.
2.4 Mechanical properties evaluation
Tensile strength tests were made utilizing a universal Shimadzu (AG-X plus tabletop type) tensile machine. The tensile test was performed according to the ASTM E8/E8M standard, the test was conducted in triplets (n = 3), means and standard deviations were calculated. The actual test machine and the geometry of the tensile specimens are shown in Fig. 1. A loading rate of 0.1 mm/min was used for testing.
2.5 Assessing the MTT cytotoxicity of the prepared magnesium alloys
Medical research ethics committee, national research centre, Egypt/14311122022 approved this study in accordance with the provisions of the relevant Egyptian laws and with Helsinki Declaration, good medical and laboratory practice (GCP and GLP) guidelines, and World Health Organization (WHO) rules regarding the ethics of scientific research.
Mg alloy specimens were produced according to the ISO10993-12:2004 specification [31] using a micro cutter with high precision (Pico175) under an abundant water amount, producing 2 mm-diameter rod. Stainless steel specimens (316L) of similar size were utilized as a positive control, as specified by the ISO 10993-12:2004 [31]. Tested specimens were sterilized using 70% ethanol and UV light for 1 h.
Cell culture experiments were carried out in accordance with ISO 10993-5:2009 [32]. Human bone osteosarcoma cell line (MG-63, ATCC, USA) was utilized to evaluate the biocompatibility of the examined specimens. The medium used to culture the MG-63 cells enclosed Dulbecco’s Modified Eagle’s Medium, 10% fetal bovine serum, 2 mM l-glutamine in 250 mg/ml amphotericin B, 100 units/ml penicillin G sodium and streptomycin sulphate solution. Cells were grown to sub-confluence in humidified 5% CO2 air at 37 °C. For sub-culturing, monolayer cells were collected after trypsin/EDTA treatment. Cells were utilized when they achieved 75% confluence [33]. Cambrex BioScience provided all the cell culture materials (Denmark, Copenhagen), Sigma/Aldrich (USA) provide all the used chemicals.
The cytotoxicity of the examined specimens was assessed against MG-63 utilizing the MTT (3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide) cell viability assay [34]. In this test, 0.5 × 105 MG-63 cells/well were placed in duplicate in 48-well microplate and incubated for 24 h with the tested specimens at 37 °C in a humidified 5% CO2 atmosphere. Following the removal of the media, 80 µl MTT solution (5 mg/ml MTT in 0.9% NaCl) was added to each well and incubated for another 4 h. 180 µl acidified isopropanol was added to solubilize MTT crystals, before being photometrically inspected using microplate ELISA reader at 570 nm. Four specimen of each group was inspected (n = 4) and their mean was computed. Results were represented as the relative viability percentage compared with the untreated cells (cells with no added specimens/negative control), with cytotoxicity indicated by < 100% relative viability.
2.6 Osseointegration potential of the tested specimens
The osseointegration potential of the specimens was found by measuring the alkaline phosphatase (ALP) activity of the MG-63 cells [35], where 5 × 104 MG-63 cells/cm2 were plated and cultured for 24 h, in the presence of the tested specimens (n = 4). The supernatant from each well containing the cells in the vicinity of the specimens was collected to detect ALP activity using the alkaline phosphatase assay kit (fluorometric) (ab83371). Results were analyzed using the T-test to compare the ALP activities of each specimen versus the negative control (with no incubated specimens) and the positive control (stainless steel specimens).
2.7 Cell growth pattern and materials interaction
1 × 104 MG-63 cells were seeded on cell culture slides (SPL, South Korea) in the vicinity of the tested specimens. After 24 h, slides were washed using phosphate buffered saline then they were subjected to acridine orange/ethidium bromide (AO/EtBr) staining dye for 10 min. in dark before being examined under fluorescence microscope (Axio Imager Z2, Zeiss, Jena, Germany) at magnification ×20, with a fluorescent camera (AxioCam MRc3 S/N 4299, Carl Zeiss Microscopy GmbH, Jena, Germany). The fluorescence distribution of the AO/EtBr stain was quantified using ZEN 11 blue edition software for image analysis (Zeiss, Germany) in order to inspect the mode of cell death and to count the number of live, early apoptotic, late apoptotic and necrotic cells. The experiment was done on basis of 1000 cells [36].
2.8 SEM–EDS inspection of the optimum alloy specimen
Specimen that revealed the prominent ALP activity and cells attaching and growing capability was inspected after MG-63 cells incubation using SEM–EDS (model JSM-5410, Japan) to estimate their hydroxyapatite layer formation capability.
2.9 Statistical analysis
Data management and statistical analysis were performed using the Statistical Package for Social Sciences (SPSS) version 18. Numerical data were summarized using means and standard deviations. Data were compared between groups using one way analysis of variance (ANOVA) test. Significance level was set at P ≤ 0.05.
3 Results
3.1 Phase structure of investigated specimens
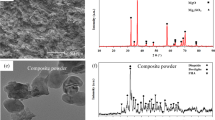
Figure 2 illustrates the XRD patterns of the Mg–Zn–xSi specimens. In addition to the prominent peaks related to the Mg phase, M\({\mathrm{gZn}}_{2}\) phases were also identified. Moreover, peaks at approximately 2θ = 40.177° corresponding to the diffraction peaks of \({\mathrm{Mg}}_{2}\mathrm{Si}\) were detected in specimens Mg-3Zn-0.3Si and Mg -3Zn-0.6Si with very low peak intensities. Since the contents of these secondary phases (\({\mathrm{MgZn}}_{2}\mathrm{ and} {\mathrm{Mg}}_{2}\mathrm{Si })\) were significantly lower than that of the base Mg metal, their peak intensities were very weak in comparison with the intensities of Mg peaks. It was also obvious that the increase in Si content increased the amount of \({\mathrm{Mg}}_{2}\mathrm{Si}\) secondary phases.
3.2 Microstructural characterization
Figure 3 shows optical micrographs of the Mg–3Zn alloys that were studied with and without the addition of Si. Figure 4 also shows SEM micrographs and elemental maps of the Mg–3Zn alloys that were studied with and without Si. The microstructure of all Mg–3Zn–xSi alloys (where x = 0, 0.1, 0.3, and 0.6 wt%) is mostly formed by dendritic primary α-Mg phases as the based matrix. Zinc, whose content is the same across all specimens, is either present as white Mg–Zn intermetallic phase in the eutectic region or dissolved in the matrix with concentrations higher in the center than at the grain edges as shown in Fig. 4a, c. Figure 5 shows an EDS point analysis of the Mg–Si intermetallic phase in the investigated alloy with a 0.6 Si addition.
3.3 Electrochemical behavior
Potentiodynamic polarization testing was conducted to measure the electrochemical behavior of Mg–Zn–xSi alloys in SBF solution at pH 7.4 and temperature 37 °C as displayed in the polarization curves (Fig. 6). The corresponding corrosion parameters: corrosion potential (\({E}_{Corr}\)), corrosion current density (\({I}_{Corr}\)), polarization resistance (Rp) and corrosion rate (Ri) are documented for all investigated specimens in Table 2.
The polarization resistance and the corrosion current densities are detected by the Stern–Geary equation: [37, 38]
βa and βc are the anodic and cathodic Tafel slopes respectively. The corrosion current density (\({i}_{Corr}\)) and rate (\({R}_{i}\)) are linked according to the subsequent Eq. (3): [39, 40]
where \({R}_{i}\) is given in mm/year, \({i}_{Corr}\) in µA/cm2, \(K=3.27 \times {10}^{-3} \mathrm{mm} \mathrm{g}/\mathrm{\mu A} \mathrm{cm} \mathrm{Year}\), \(EW\) is the equivalent weight and d is the density of the base Mg metal.
Shifting of the electrochemical corrosion potentials (\({\mathrm{E}}_{\mathrm{Corr}}\)) to more negative values was observed after Si addition in Fig. 6. Values of \({\mathrm{E}}_{\mathrm{Corr}}\) decreased with increasing Si contents reaching the lowest value of (− 1.36 V) for the Mg–3Zn–0.6Si alloy which implied that Si addition accelerated the material degradation. It was also clear from Table 2 that the corrosion rates of the Mg alloys increased after the inclusion of different concentrations of Si. The fastest degradation rate was noticed after the inclusion of 0.6 Si in the Mg base alloy with a value of 0.391 mm/year corresponding to \({\mathrm{E}}_{\mathrm{Corr}}\) value of (− 1.36 V). This behavior is consistent with the observation of the corrosion potentials, which revealed a higher tendency for the material to corrode when more negative values of \({\mathrm{E}}_{\mathrm{Corr}}\) were attained.
Figure 7 presents the Nyquist plots using the electrochemical impedance spectroscopy (EIS) method for the Mg–Zn–xSi alloys immersed in SBF. As observed, all investigated samples had semicircle capacitance loops with different radii. The largest loop radius for Mg–Zn without Si addition suggests its high corrosion resistance. However, the smallest loop radius of Mg–Zn–0.6Si implies that it dissolves at the fastest rate in SBF. The outcomes were consistent with those of the polarization measurements.
Figure 8 shows a one-time constant simple model, which is the equivalent circuit model for the corrosion process. The circuit included the surface coating polarization resistance \({\mathrm{R}}_{\mathrm{p}}\), which is parallel to a constant phase element (CPE); both are in series with the solution resistance \({\mathrm{R}}_{\mathrm{s}}\). The constant phase element (CPE) is specified by its impedance value that is computed from the following equation:
where \(\alpha\) is surface heterogeneity exponent account, 0 ≤ \(\alpha\) ≤ 1, \(j\) is the imaginary number \(\left( {{\text{j}} = \left( { - 1} \right)^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 2}}\right.\kern-0pt} \!\lower0.7ex\hbox{$2$}}}} } \right),\;{\upomega } = 2{\pi f}\) is the angular frequency in \(\mathrm{rad}/\mathrm{s}\), \(f\) is the frequency in \(\mathrm{Hz}={\mathrm{S}}^{-1}\). The Electrochemical impedance analysis shown in Table 3 indicate that \({\mathrm{R}}_{\mathrm{p}}\) values decreased with the inclusion of Si. This reveals the lower resistance to charge transfer since corrosion rates are inversely related to \({\mathrm{R}}_{\mathrm{p}}\) and hence higher dissolution rates of Mg substrate.
3.4 SEM–EDS analysis of the corroded Mg–Zn–xSi alloys
SEM and EDS results of the Mg–Zn–xSi alloy surfaces after electrochemical tests in SBF are shown in Figs. 9 and 10. It was clear that the Mg based alloy without Si didn’t suffer from much corrosion since a small percentage of corrosion products appeared on the alloy surface. However, the other investigated alloys suffered from increased local corrosion with the addition of Si and were therefore covered with corrosion products on most of the surfaces.
EDS analysis in Fig. 10 demonstrated that the corrosion products were dominated by the basic components (Mg, Zn, Si), O, and Cl, P, and Ca elements originating from SBF. The presence of these ions suggests the formation of insoluble magnesium chloride, calcium–magnesium phosphates or calcium–zinc phosphates magnesium or zinc hydroxides [41, 42].
3.5 Mechanical properties evaluation
Figure 11 shows the tensile strength-elongation curves of the current Mg–3Zn–xSi alloys. Tensile strength and elongation are linearly correlated in all alloy curves with no statistical significant difference. It is observed that the alloy strength increased with 0.1 wt% Si from around 158 to 172 MPa. However, this value suddenly dropped again upon increasing Si content. Moreover, Si considerably reduces the total elongation when compared to Mg–Zn alloy.
3.6 In vitro cytotoxicity evaluation of tested specimens
Results shown in Fig. 12 revealed good cytocompatibility and high cell viability of all the tested specimens, with the highest percentage of relative viability for Mg–3Zn without Si addition, followed by Mg–3Zn–0.1Si. On the other hand, the increased percent of Mg2Si in the third and fourth groups containing 0.3 and 0.6 Si showed no significant difference between them but significantly lower percentage of relative viability compared to the first two specimens, however all tested specimens had significantly higher percentage of relative cell viability compared to the control at p < 0.001.
Effect of the four investigated Mg–3Zn alloys (sample 1: Mg–3Zn, sample 2: Mg–3Zn–0.1Si, sample 3: Mg–3Zn–0.3Si, and sample 4: Mg–3Zn–0.6Si) on MG-63 cells’ viability, estimated using the MTT assay. Results are presented as a percentage of untreated control cells [mean ± St. dev]. ***p < 0.001, ns not significant
3.7 Osseointegration potential of tested specimens
Results of osseointegration revealed significantly higher alkaline phosphatase activity (ALP) in all tested specimens compared to the positive (stainless steel specimens) and negative (cells with no added specimens) controls at p < 0.01 (Fig. 13). The highest ALP was for Mg–3Zn alloys without added Si (specimen 1), followed by those with added 0.3 and 0.6 Si (specimen 3 and 4), indicating their high osseointegration potential.
3.8 Cell growth pattern and materials interaction
As shown in Fig. 14, the inspected specimens revealed the bright green color of vital MG-63 cells attaching, invading, and growing onto the surfaces of all examined specimens with small amount of apoptotic cells presented in orange-yellow color. On calculating the amount of vital and apoptotic cells through analyzing the fluorescence distribution of AO/EtBr stain using Zen 11 Blue Edition Software, (Zeiss, Germany) it was revealed that Mg–3Zn (sample 1) and Mg–3Zn–0.1Si (sample 2) showed early apoptotic cells with less late and necrotic cells indicating their superior viability. On the other hand, Mg–3Zn–0.3Si (sample 3), and Mg–3Zn–0.6Si (sample 4) showed increased late apoptosis and some necrotic cells, indicating their lower viability. The experiment was done on basis of 1000 cells (Fig. 15).
Illustrative images showing the attachment of MG-63 cells to the experimental specimens, (1a, b) Mg–3Zn, (2a, b) Mg–3Zn–0.1Si, (3a, b) Mg–3Zn–0.3Si, and (4a, b) Mg–3Zn–0.6Si, stained using AO/EtBr stain and examined by fluorescence microscopy at a magnification ×20, scale bar = 50 μm (vital cells are bright green and apoptotic cells appear orange)
Interestingly, there were appositions of insoluble precipitates in all Mg–3Zn alloys, they appeared as green fluorescent fragments, these fragments didn’t appear in the stainless-steel positive control specimens (Fig. 16). After examining Mg–3Zn–0.3Si alloy as a representative specimen using SEM, it was revealed that a combination of spherical particles, which likely corresponded to the creation of a new bone-like apatite layer, was found. This observation was confirmed through EDS analysis as represented in Fig. 17. The EDS spectra demonstrated the presence of calcium and phosphorous ions on the sample surface, with an approximate 1.067 Ca/P ratio, indicating the apparent formation of dicalcium phosphate dihydrate (brushite) or dicalcium phosphate anhydrate (monetite). However, it is worth noting that the desirable Ca/P ratio for calcium phosphate formation is 1.67.
Illustrative images showing the insoluble precipitates in the vicinity of (1) Mg–3Zn–0.3Si specimens, compared to (2) the normal cells incubated with the stainless-steel positive control specimens with no precipitates, stained using AO/EtBr stain and examined by fluorescence microscopy (Axio Imager Z2, Zeiss, Jena, Germany) at a magnification ×20, scale bar = 50 μm
4 Discussion
The type of the alloying elements determines both the composition of the matrix and the minor existence of intermetallic phases. In this study, the microstructure of all Mg–3Zn–xSi alloys was mostly formed by dendritic primary α-Mg phases as the based matrix. Zinc was either present as white Mg–Zn intermetallic phase in the eutectic region or dissolved in the matrix with concentrations higher in the center than at the grain edges. As stated by a previous study [43], this intermetallic phase was Mg21Zn25 compound, although it was hard to precisely determine its composition owing to the small crystals that make up this phase. In Mg–3Zn–xSi alloys, a new intermetallic phase was formed with black Mg–Si intermetallic form, that was identified as Mg2Si phase from the XRD results and from its characteristic morphology as a mixture of needle and polyhedral shapes in the eutectic with Mg. With increasing the amount of Si addition, the amount of new Mg2Si intermetallic phase formed increased.
Corrosion mechanism of Mg–3Zn–xSi alloys in SBF was proposed as follows: When magnesium alloys were immersed in SBF, the corrosion process was an electrochemical reaction between Mg and water. The corrosion reactions involved the following anodic and cathodic reactions: [4, 8]
where the overall reaction was
Magnesium hydroxide which is the main corrosion product (Eq. 7) acts as a passive layer that protects the alloy from further corrosion, but its protection become weaker in SBF. This can be attributed to the breakdown of the oxide films revealing that its stability is affected by the presence of aggressive chloride ions in the SBF corrosive medium [44]. These chloride ions penetrate the \({\mathrm{Mg}(\mathrm{OH})}_{2}\) layer causing soluble magnesium chloride film formation using (Eq. 9) with subsequent substrate dissolution accelerating.
Zn can also act to give \(\mathrm{Zn}{(\mathrm{OH})}_{2}\) according to the next equation:
Potentiodynamic polarization testing shown in Fig. 6 was conducted to measure the electrochemical behavior of Mg–Zn–xSi alloys in SBF solution at pH 7.4 and temperature 37 °C. Corrosion current density \({i}_{Corr}\) and the Corrosion potential \({E}_{corr}\) data and the corresponding corrosion rates were obtained from the Tafel extrapolation method [45]. The results imply that the inclusion of Si accelerated the material degradation but to appropriate rates due to the shifting of (\({\mathrm{E}}_{\mathrm{Corr}}\)) to more negative values after Si addition. The fastest degradation rate was noticed after the inclusion of 0.6 Si in the Mg base alloy. However, it is important to note that a single set of electrochemical corrosion test is not sufficient to obtain reliable degradation rate [45].
The increase in corrosion rates could be attributed to the formation of \({\mathrm{Mg}}_{2}\mathrm{Si}\) and \({\mathrm{MgZn}}_{2}\) secondary phases as confirmed in the XRD results. Both \({\mathrm{Mg}}_{2}\mathrm{Si}\) and \({\mathrm{MgZn}}_{2}\) intermetallics seem to be nobler than the \(\propto\)-Mg matrix and therefore acted as cathodic sites which might have led to the establishment of the micro-galvanic corrosion [23] and the decrease in corrosion resistance of Mg ternary alloys. The pronounced rise in corrosion rate for the Mg–3Zn–0.6Si specimen was due to the increased percentages of these secondary phases which accelerated the deterioration of the alloy. This outcome was comparable to a study by Gil-Santos et al. [26] who examined the corrosion performance of multiple as-cast Mg–Si–Sr alloys in-vitro. They concluded that higher Si and Sr wt% led to higher degradation rates of the alloy and that decreasing the Si weight percentage below 0.15 wt% lessened the degradation rate than that of pure Mg.
Accordingly, controlling the degradation rates to be compatible with the bone healing rates assures their efficacy throughout their service period. Several literature stated that; for orthopedic applications, the corrosion rates of implants must fall in the range of 0.2–0.5 mm/year to match the bone healing process [4, 46,47,48,49]. The Mg–3Zn alloy without Si had a corrosion rate of 0.128 mm/year which is much lower than the reported value, however the inclusion of (0.1–0.6 wt%) Si had corrosion rates of 0.251 mm/year, 0.278 mm/year and 0.391 mm/year respectively, which seem to be suitable rates for structural support until bone healing occurs. This result implies that the addition of Si was favorable to reach the ideal rate of corrosion for orthopedic use.
The Nyquist plots obtained using the electrochemical impedance spectroscopy (EIS) method for the investigated alloys in SBF were presented in Fig. 7. The results revealed that the diameter of the capacitive loops dropped with increase in the Si content which may be attributed to the diffusion of corrosive ions (\({\mathrm{Cl}}^{-}\) ions) from SBF solution into the Mg oxide/Mg hydroxide corrosion products film which therefore reached the Mg matrix. This led to accelerated corrosion of the Mg alloy lowering its corrosion resistance. Moreover, micro galvanic corrosion between Mg substrate and the Mg2Si intermediate phases could have occurred, thereby decreasing the resistance of the alloy.
SEM and EDS results of the Mg–Zn–xSi alloy surfaces after electrochemical tests in SBF (Figs. 9, 10) revealed that the Mg based alloy without Si didn’t suffer from much corrosion while the investigated alloys with Si addition suffered from increased local corrosion. These observations comply with the electrochemical polarization tests since it was proven that the inclusion of Si caused an increase in the rate of degradation of Mg alloys. This rise in corrosion rates could be attributed to the formation of coarse structures of Mg2Si intermetallic phases and the micro galvanic corrosion that could be formed between these phases and the Mg matrix. According to a previous study [22], incorporating 0.5 wt% Si to the Mg–6Zn–0.5Mn alloy resulted in massive Chinese script Mg2Si structures, which reduced alloys’ corrosion resistance. EDS analysis demonstrated that the corrosion products were dominated by the basic components as well as P and Ca elements originating from SBF. This suggests the formation of insoluble calcium–magnesium phosphates, calcium–zinc phosphates, magnesium chloride, magnesium or zinc hydroxides.
Mg-alloys tensile properties are generally strongly influenced by the size and distribution of the secondary intermetallic phases. Kumar et al. [50] showed that increasing Si in Mg–xSi alloys enhances the strength due to the formation of Mg2Si, however, beyond certain limits, the strength decreases again due to particle coarsening. In the current Mg–3Zn alloy, a similar trend was expected. Tensile strength dropped upon increasing Si content. Si considerably reduced the total elongation when compared to Mg–Zn alloy. By assessing the microstructure of these alloys, it was observed that increasing Si wt% from 0.1 to 0.6 resulted in Mg2Si coarsening which negatively affected the strength of the alloy. However, the tensile strength of the Mg–Zn–xSi alloys ranged between 152 and 178 MPa which is close to the average values of bone tensile strength [1, 5] and complies with the strength of Mg–Zn alloys reported in other previous studies [24, 51].
Testing Mg alloys in vitro for cell viability utilizes a range of cell cultures. According to the designed alloy application, the cell type is selected. For those relating to osteosynthesis, the chosen cell type would be human bone marrow mesenchymal stem cells [52], osteoblasts [53], or human bone osteosarcoma cells [54]. Human bone osteosarcoma cell line (Mg-63) was selected for this study.
Results of MTT cytotoxicity evaluation of tested alloys revealed good cytocompatibility and high cell viability, with the highest percentage of relative viability for Mg–3Zn without Si addition, followed by Mg–3Zn–0.1Si. These results may be related to the proven cytocompatibility of the MgZn2 intermetallic compound, which is dominant in these two alloys. Zn is an antioxidant and anti-inflammatory agent that enhances cell mediated immunity by reducing inflammatory cytokines and oxidative stress development [55]. Moreover, when Zn-containing Mg alloys were implanted in vivo, they elicited minimal inflammatory responses [56,57,58]. On the other hand, the increased percent of Mg2Si in the third and fourth groups showed a lower percentage of relative viability, yet, higher than the positive stainless-steel control. These findings support previous research that found that when Si was added to Mg–6Al–1Zn alloy it caused Mg2Si phase formation that in turn improved Saos-2 cells’ viability [21].
Results of osseointegration revealed significantly higher alkaline phosphatase activity (ALP) in all tested specimens. The highest ALP was for Mg–3Zn alloys without added Si, followed by those with added 0.3 and 0.6 Si, indicating their high osseointegration potential. A previous in vivo study suggested that Mg alloy implantation promoted new bone formation [59]. The high osseointegration potential of the base Mg–3Zn alloys specimen was an expected consequence of its high viability as revealed by the MTT cytotoxicity test, however, the high osseointegration potential of specimens inclosing 0.3 and 0.6 Si, may be due to the positive effects of the relatively higher concentrations of Si on the capability of cells to induce bone formation. Previous literature [60, 61] stated that silicon is an essential nutrient for the metabolism of connective tissue and bone in animal tests.
Additionally, all the inspected specimens revealed vital MG-63 cells attaching, invading, and growing onto the surfaces of all examined specimens, with appositions of insoluble precipitates, indicating the creation of a new apatite-like layer. These results go with the previously obtained corrosion results in this study, where the EDS analysis revealed the existence of Ca and P ions on the surface of the specimen after SBF incubation and was explained as the probability of insoluble calcium–magnesium phosphates or calcium–zinc phosphates formation.
5 Conclusion
In this study, Mg–3Zn–xSi (x = 0, 0.1, 0.3, and 0.6 wt%) biodegradable alloys were synthesized by a simple stir casting method. The impact of Si addition on the electrochemical behavior, mechanical properties, and biocompatibility of the Mg based alloys was explored. The results implied that the inclusion of Si had a negative effect on the corrosion rates of the Mg alloys. However, all the investigated alloys with added Si had degradation rates in the range of 0.2–0.5 mm/year which are suitable rates for the bone healing process, in contrast to the Mg–Zn alloy with no added Si which showed a low corrosion rate of 0.128 mm/year. The tensile strength of the Mg ternary alloy decreased with the addition of 0.3 and 0.6 Si and the highest strength was attained for the Mg–3Zn–0.1Si alloy. The cytotoxicity results revealed that all the tested samples had good cytocompatibilities and high cell viability with the highest percentage of relative viability for Mg–3Zn without Si addition. However, the inclusion of 0.3 and 0.6 Si showed high osseointegration potentials since Si promoted the capability of the cells to induce bone formation and there were prominent calcium phosphate precipitates on the Mg–3Zn–0.3Si alloy indicating the viability of the MG-63 cells in the vicinity of this alloy.
Finally, although all Mg–3Zn alloys with and without Si addition achieved high cell viability and osseointegration capabilities, the alloys with Si inclusion had degradation rates more acceptable for the bone healing process. Hence, Mg–3Zn–xSi alloys proposed in the present study are suggested as potential orthopedic biodegradable biomaterials due to their appropriate degradation rates, good mechanical properties and excellent biocompatibility.
Data availability
All data generated or analyzed in this study are included in this published article.
References
Gutiérrez Púa LDC, Rincón Montenegro JC, Fonseca Reyes AM et al (2023) Biomaterials for orthopedic applications and techniques to improve corrosion resistance and mechanical properties for magnesium alloy: a review. J Mater Sci 58:3879–3908. https://doi.org/10.1007/s10853-023-08237-5
Tan L, Yu X, Wan P, Yang K (2013) Biodegradable materials for bone repairs: a review. J Mater Sci Technol 29:503–513. https://doi.org/10.1016/j.jmst.2013.03.002
Yeung KWK, Wong KHM (2019) Biodegradable metallic materials for orthopaedic implantations: a review. Technol Health Care 20:345–362. https://doi.org/10.3233/thc-2012-0685
Shuai C, Li S, Peng S et al (2019) Biodegradable metallic bone implants. Mater Chem Front 3:544–562. https://doi.org/10.1039/c8qm00507a
Moghadasi K, Mohd Isa MS, Ariffin MA et al (2022) A review on biomedical implant materials and the effect of friction stir based techniques on their mechanical and tribological properties. J Mater Res Technol 17:1054–1121. https://doi.org/10.1016/j.jmrt.2022.01.050
Kabir H, Munir K, Wen C, Li Y (2021) Recent research and progress of biodegradable zinc alloys and composites for biomedical applications: biomechanical and biocorrosion perspectives. Bioact Mater 6:836–879. https://doi.org/10.1016/j.bioactmat.2020.09.013
Saris NEL, Mervaala E, Karppanen H et al (2000) Magnesium: an update on physiological, clinical and analytical aspects. Clin Chim Acta 294:1–26. https://doi.org/10.1016/S0009-8981(99)00258-2
Jamel MM, Jamel MM, Lopez HF (2022) Designing advanced biomedical biodegradable Mg alloys: a review. Metals (Basel) 12:85. https://doi.org/10.3390/met12010085
Cho DH, Nam JH, Lee BW et al (2016) Effect of Mn addition on grain refinement of biodegradable Mg–4Zn–0.5Ca alloy. J Alloys Compd 676:461–468. https://doi.org/10.1016/j.jallcom.2016.03.182
Song G (2007) Control of biodegradation of biocompatable magnesium alloys. Corros Sci 49:1696–1701. https://doi.org/10.1016/j.corsci.2007.01.001
ZhangaLia YQ, Guoa T, Lia S (2019) Effect of Y content on properties of extruded Zn–1.5Mg–xY alloys for medical applications. Mater Res. https://doi.org/10.1590/1980-5373-MR-2019-0004
Gu X, Wang F, Xie X et al (2018) In vitro and in vivo studies on as-extruded Mg–5.25wt%Zn–0.6wt%Ca alloy as biodegradable metal. Sci China Mater 61:619–628. https://doi.org/10.1007/s40843-017-9205-x
Abdel-Gawad SA, Shoeib MA (2019) Corrosion studies and microstructure of Mg–Zn–Ca alloys for biomedical applications. Surf Interfaces 14:108–116. https://doi.org/10.1016/j.surfin.2018.11.011
Jung YG, Yang W, Kim YJ et al (2021) Effect of Ca addition on the microstructure and mechanical properties of heat-treated Mg–6.0Zn–1.2Y–0.7Zr alloy. J Magn Alloys 9:1619–1631. https://doi.org/10.1016/j.jma.2021.01.010
Chandra G, Pandey A (2022) Preparation strategies for Mg-alloys for biodegradable orthopaedic implants and other biomedical applications: a review. IRBM 43:229–249. https://doi.org/10.1016/j.irbm.2020.06.003
Cai S, Lei T, Li N, Feng F (2012) Effects of Zn on microstructure, mechanical properties and corrosion behavior of Mg–Zn alloys. Mater Sci Eng C 32:2570–2577. https://doi.org/10.1016/j.msec.2012.07.042
Reffitt DM, Ogston N, Jugdaohsingh R et al (2003) Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 32:127–135. https://doi.org/10.1016/S8756-3282(02)00950-X
Jiang W, Yu W (2021) Corrosion behavior and osteogenic activity of a biodegradable orthopedic implant Mg–Si alloy with a gradient structure. Metals (Basel) 11:781. https://doi.org/10.3390/met11050781
Wang M, Xiao DH, Liu WS (2017) Effect of Si addition on microstructure and properties of magnesium alloys with high Al and Zn contents. Vacuum 141:144–151. https://doi.org/10.1016/j.vacuum.2017.04.005
Ben-Hamu G, Eliezer D, Shin KS (2007) The role of Si and Ca on new wrought Mg–Zn–Mn based alloy. Mater Sci Eng A 447:35–43. https://doi.org/10.1016/j.msea.2006.10.059
Amirnejad M, Rajabi M, Motavalli A (2018) Effect of addition of Si on microstructure, mechanical properties, bio-corrosion and cytotoxicity of Mg–6Al–1Zn alloy. Trans Nonferrous Met Soc China (Engl Ed) 28:1755–1762. https://doi.org/10.1016/S1003-6326(18)64819-8
Ben-Hamu G, Eliezer D, Shin KS (2008) The role of Mg2Si on the corrosion behavior of wrought Mg–Zn–Mn alloy. Intermetallics 16:860–867. https://doi.org/10.1016/j.intermet.2008.03.003
Srinivasan A, Ningshen S, Kamachi Mudali U et al (2007) Influence of Si and Sb additions on the corrosion behavior of AZ91 magnesium alloy. Intermetallics 15:1511–1517. https://doi.org/10.1016/j.intermet.2007.05.012
Zhang E, Yang L, Xu J, Chen H (2010) Microstructure, mechanical properties and bio-corrosion properties of Mg–Si(–Ca, Zn) alloy for biomedical application. Acta Biomater 6:1756–1762. https://doi.org/10.1016/j.actbio.2009.11.024
Yang M, Shen J (2009) Modification and refinement mechanism of Mg2Si phase in Sr-containing AZ61-0.7Si magmesium alloy. China Foundry 6:37–42
Gil-Santos A, Marco I, Moelans N et al (2017) Microstructure and degradation performance of biodegradable Mg–Si–Sr implant alloys. Mater Sci Eng C 71:25–34. https://doi.org/10.1016/j.msec.2016.09.056
Kasaeian-Naeini M, Sedighi M, Hashemi R, Delavar H (2023) Microstructure, mechanical properties and fracture toughness of ECAPed magnesium matrix composite reinforced with hydroxyapatite ceramic particulates for bioabsorbable implants. Ceram Int 49:17074–17090. https://doi.org/10.1016/j.ceramint.2023.02.069
Rahim SA, Mohan KS, Rabeeh VM et al (2023) Hot rolled Mg–Ca/nHA composite for biodegradable implant material—a novel approach. Mater Today Commun 35:106235. https://doi.org/10.1016/j.mtcomm.2023.106235
Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 27:2907–2915. https://doi.org/10.1016/j.biomaterials.2006.01.017
Francis AA, Abdel-Gawad SA, Shoeib MA (2021) Toward CNT-reinforced chitosan-based ceramic composite coatings on biodegradable magnesium for surgical implants. J Coat Technol Res 18:971–988. https://doi.org/10.1007/s11998-021-00468-y
ISO 10993-12:2012 (2012) Biological evaluation of medical devices—part 12: sample preparation and reference materials
ISO 10993-5 (2009) Tests for in vitro cytotoxicity. Biological evaluation of medical devices. Br Stand Inst 2021 Part 5:54
Ibrahim KM, Safwat EM, Ghayad IM, El-Hadad S (2023) In-vitro biocompatibility evaluation of cast Ni–Ti alloy produced by vacuum arc melting technique for biomedical and dental applications. Chem Pap 77:847–858. https://doi.org/10.1007/s11696-022-02523-3
Hansen MB, Nielsen SE, Berg K (1989) Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods 119:203–210. https://doi.org/10.1016/0022-1759(89)90397-9
Ding Y, Tao B, Ma R et al (2023) Surface modification of titanium implant for repairing/improving microenvironment of bone injury and promoting osseointegration. J Mater Sci Technol 143:1–11. https://doi.org/10.1016/j.jmst.2022.09.044
Talebi A, Salehi M, Khaleghian A, Kubicki M (2023) Evaluation of anticancer activities, apoptosis, molecular docking, and antioxidant studies of new Ni(II), VO(IV), Cu(II) and Co(III) Schiff base complexes. Inorg Chim Acta 546:121296
Abdel-Gawad SA, Osman WM, Fekry AM (2019) Characterization and corrosion behavior of anodized aluminum alloys for military industries applications in artificial seawater. Surf Interfaces 14:314–323. https://doi.org/10.1016/j.surfin.2018.08.001
Abdel-Gawad SA, Sadik MA, Shoeib MA (2019) Preparation and properties of a novel nano Ni–B–Sn by electroless deposition on 7075-T6 aluminum alloy for aerospace application. J Alloys Compd 785:1284–1292. https://doi.org/10.1016/j.jallcom.2019.01.245
Hammam RE, Abdel-Gawad SA, Moussa ME et al (2023) Study of microstructure and corrosion behavior of cast Zn–Al–Mg alloys. Int J Met. https://doi.org/10.1007/s40962-022-00944-0
Shoeib MA, Abdel-Gawad SA (2023) High performance nano hydroxyapatite coating on zinc for biomedical applications. J Mater Sci 58:740–756. https://doi.org/10.1007/s10853-022-08034-6
Vahedi S, Aghdam RM, Sohi MH, Rezayan AH (2023) Characteristics of electrospun chitosan/carbon nanotube coatings deposited on AZ31 magnesium alloy. J Mater Sci Mater Med 34:8. https://doi.org/10.1007/s10856-022-06703-1
Akshay KS, Rabeeh VPM, Rahim SA et al (2022) Electrophoretic deposition of alginate/bioglass composite coating on Mg–Ca alloy for degradable metallic implant applications. Surf Coat Technol 448:128914. https://doi.org/10.1016/j.surfcoat.2022.128914
Černý R, Renaudin G (2002) The intermetallic compound Mg21Zn25. Acta Crystallogr Sect C Cryst Struct Commun 58:i154–i155. https://doi.org/10.1107/S0108270102018103
Leleu S, Rives B, Bour J et al (2018) On the stability of the oxides film formed on a magnesium alloy containing rare-earth elements. Electrochim Acta 290:586–594. https://doi.org/10.1016/j.electacta.2018.08.093
Shi Z, Liu M, Atrens A (2010) Measurement of the corrosion rate of magnesium alloys using Tafel extrapolation. Corros Sci 52:579–588. https://doi.org/10.1016/j.corsci.2009.10.016
Krüger D, Zeller-Plumhoff B, Wiese B et al (2021) Assessing the microstructure and in vitro degradation behavior of Mg–xGd screw implants using µCT. J Magn Alloys 9:2207–2222. https://doi.org/10.1016/j.jma.2021.07.029
Fischerauer SF, Kraus T, Wu X et al (2013) In vivo degradation performance of micro-arc-oxidized magnesium implants: a micro-CT study in rats. Acta Biomater 9:5411–5420. https://doi.org/10.1016/J.ACTBIO.2012.09.017
Chen J, Tan L, Yang K (2016) Recent advances on the development of biodegradable magnesium alloys: a review. Mater Technol 31:681–688. https://doi.org/10.1080/10667857.2016.1212587
Kong L, Heydari Z, Lami GH et al (2023) A comprehensive review of the current research status of biodegradable zinc alloys and composites for biomedical applications. Materials (Basel) 16:4797. https://doi.org/10.3390/ma16134797
Ajith Kumar KK, Srinivasan A, Pillai UTS et al (2022) Microstructure and mechanical property correlation of Mg–Si alloys. Silicon 14:9499–9515
Nanda IP, Hassim MH, Idris MH et al (2019) Mechanical and degradation properties of zinc adopted magnesium alloys for biomedical application. IOP Conf Ser Mater Sci Eng 602:12094
Chen YT, Hung FY, Lin YL, Lin CY (2020) Biodegradation ZK50 magnesium alloy compression screws: mechanical properties, biodegradable characteristics and implant test. J Orthop Sci 25:1107–1115. https://doi.org/10.1016/j.jos.2020.01.018
Gu X, Zheng Y, Cheng Y et al (2009) In vitro corrosion and biocompatibility of binary magnesium alloys. Biomaterials 30:484–498
Kubásek J, Vojtěch D, Lipov J, Ruml T (2013) Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg-X (X = Sn, Ga, In) alloys. Mater Sci Eng C 33:2421–2432. https://doi.org/10.1016/j.msec.2013.02.005
Prasad AS (2014) Zinc: an antioxidant and anti-inflammatory agent: role of zinc in degenerative disorders of aging. J Trace Elem Med Biol 28:364–371. https://doi.org/10.1016/j.jtemb.2014.07.019
Witte F, Ulrich H, Rudert M, Willbold E (2007) Biodegradable magnesium scaffolds: part I: appropriate inflammatory response. J Biomed Mater Res A 81:748–756. https://doi.org/10.1002/jbm.a.31170
Willbold E, Kaya AA, Kaya RA et al (2011) Corrosion of magnesium alloy AZ31 screws is dependent on the implantation site. Mater Sci Eng B 176:1835–1840. https://doi.org/10.1016/j.mseb.2011.02.010
Kraus T, Fischerauer SF, Hänzi AC et al (2012) Magnesium alloys for temporary implants in osteosynthesis: in vivo studies of their degradation and interaction with bone. Acta Biomater 8:1230–1238. https://doi.org/10.1016/j.actbio.2011.11.008
Witte F, Kaese V, Haferkamp H et al (2005) In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials 26:3557–3563. https://doi.org/10.1016/j.biomaterials.2004.09.049
Nielsen FH (2000) Importance of making dietary recommendations for elements designated as nutritionally beneficial, pharmacologically beneficial, or conditionally essential. J Trace Elem Exp Med 13:113–129. https://doi.org/10.1002/(SICI)1520-670X(2000)13:1%3c113::AID-JTRA13%3e3.0.CO;2-D
Hassan SF, Islam MT, Saheb N, Baig MMA (2022) Magnesium for implants: a review on the effect of alloying elements on biocompatibility and properties. Materials (Basel) 15:5669. https://doi.org/10.3390/ma15165669
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
MEM and SE-H prepared samples, performed microstructure characterization, and evaluated mechanical properties. REH, SAA-G and MS performed corrosion tests investigation and analysis. EMS performed biocompatibility investigation and analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Ethical approval
Medical research ethics committee, national research centre, Egypt/14311122022 approved this study in accordance with the provisions of the relevant Egyptian laws and with Helsinki Declaration, good medical and laboratory practice (GCP and GLP) guidelines, and World Health Organization (WHO) rules regarding the ethics of scientific research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Safwat, E.M., Hammam, R.E., Moussa, M.E. et al. Corrosion behavior, mechanical properties, and in-vitro biocompatibility of cast Mg–3Zn–xSi biodegradable alloys for bone regeneration. SN Appl. Sci. 5, 251 (2023). https://doi.org/10.1007/s42452-023-05477-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05477-7