Abstract
This work reports a simple and sensitive on-line solid-phase extraction system for the pre-concentration and determination of a trace amount of Co(II (ions by flame atomic absorption spectrometry (FAAS). This method is based on the on-line retention of cobalt ions with polyvinyl chloride modified by 3-(2-thiazolylazo)-2,6-diaminopyridine in a mini-column system. The retained Co(II) ions were eluted with 1.0 mol L−1 HCl, and direct aspiration to flame atomic absorption spectrometry (FAAS) for an accurate determination of it. The important experimental parameters such as the solution pH, length of column (adsorbent mass), sample flow rate, solvent eluent, type and concentration of the eluent, and co-existing ion effects were investigated in detail and optimized. Under the optimum conditions, the calibration graph was linear over the concentration range of 2–150 µg L−1 for pre-concentration of 10.0 mL of the sample solution. The interassay precession (n = 6) was in the range of 1.8–5.2% at the concentration levels of 10.0, 50.0, 100.0, and 140.0 µg L−1. The limit of detection and enhancement factor were 1.3 µg L−1 and 40, respectively. The method was applied for determination of trace levels of Co(II) ions in different real samples such as tap water, waste water, vegetable, fruit, and drug with satisfactory results.
Article Highlights
-
PVC-TADAP advantages are stability and high adsorption capacity.
-
Developed SPE method is on-line and environmentally friendly.
-
This method has been successfully applied for determination of trace levels of Co(II) in different real samples.
Similar content being viewed by others
1 Introduction
Heavy metals are potentially toxic elements. Thus, they can lead to serious ecological and health problems even at trace levels [1, 2]. Cobalt is one of these elements, which surplus in the concentration of it, can cause asthma, gastrointestinal tract irritation, rhinitis, prohibition of enzyme activities, allergic dermatitis vasodilation, and cardiomyopathy [3,4,5]. According to the World Health Organization (WHO), the maximum acceptable cobalt concentration in drinking water is 40 µg L−1 [3]. Consequently, determination of trace amounts of this element with a highly sensitive and accurate method is essential.
Up to now, different analytical methods have been reported for the quantitative determination of a metal in various matrices. Among the cited methods, flame atomic absorption spectrometry (FAAS) is a popular technique because its accuracy and precision is high and its cost is low [4, 6]. However, the sensitivity of this method limits its application [1, 6, 7]. To resolve this problem, different separation and pre-concentration techniques such as solidified floating organic drop microextraction (SFODME) [8], hollow fiber-liquid phase microextraction (HF-LPME) [9], dispersive liquid–liquid microextraction [10], and solid-phase extraction [4, 5, 11] have been developed to enrich the analyte concentration to an appropriate concentration. Due to the high pre-concentration factor, low cost, low solvent usage, and off-line and on-line operation chance, solid-phase extraction (SPE) is an excellent pre-concentration method [4, 11, 12] https://pubs.rsc.org/en/content/articlelanding/2020/ay/c9ay02762a]. The protocol applied in SPE is to use an adsorbent for adsorption of the analyte [13]. Thus, one of the main aspects of a SPE method is the nature of the adsorbent [11, 14]. Polymeric adsorbents have outstanding features such as cheapness, high porosity, and stability in acidic or basic media [12, 15], which make them suitable for the pre-concentration and determination of metals.
The Schiff base ligands form stable and selective complexes with transition metal ions [16, 17]. Therefore, by modification of the polymeric bed with the Schiff bases, one can create a new highly selective adsorbent for determination of a metal with a very low concentration.
In the present work, polyvinyl chloride (PVC) was modified with the 3-(2′-thiazolylazo)-2, 6-diamino pyridine (TADAP) Schiff base. TADAP reacts with heavy metal ions such as cobalt [18]. Considering this subject, the PVC-TADAP resin was used as an adsorbent in an on-line system (Fig. 2) for the pre-concentration and determination of cobalt in different samples. This is the first report for application of this polymeric adsorbent for the on-line pre-concentration of cobalt.
2 Experimental
2.1 Instrumentation
A Shimadzu flame atomic absorption spectrophotometer (AA-670) was used for detecting and quantifying cobalt. The FASS instrument was equipped with a cobalt hollow cathode lamp (wavelength of 240.7 nm). The flow rates of acetylene and air were fixed at 2.2 and 8.0 L min−1, respectively. In the on-line column pre-concentration procedure, for pumping the sample solution, an electro-multichannel peristaltic pump (PP201V) was used. For selection of the sample or eluent flow through the column, a six-way rotary injection valve (model 5011) was applied. The used mini-column was fabricated from a polypropylene tube (2 mm i.d., 8 cm length). A Metrohm (model 744) pH-meter equipped with a combined glass electrode-calomel was used for the pH adjustments.
2.2 Reagents and solutions
All the reagents used including cobalt nitrate hexahydrate (Co(NO3)2·6H2O), nitric acid, hydrochloric acid, sodium nitrite, ethanol, sulfuric acid, citric acid, acetic acid, potassium hydrogen phthalate, potassium dihydrogen phosphate, dipotassium hydrogen phosphate, sodium hydroxide, PVC, sodium citrate, 2,6-diaminopyridine, and 2-aminothiazole were provided from the Merck company (Darmstadt, Germany). Deionized water was used for preparation of the aqueous solutions. The glassware and plastics were washed by 10% (v/v) HNO3 and deionized water, respectively, prior to use. A buffer solution (with pH 6.5) was obtained by mixing proper volumes of potassium dihydrogen phosphate (0.27 M) and potassium hydrogen phosphate (0.27 M). Hydrochloric acid (as the eluent solution) was prepared by dilution of its concentrated solution and standardized with sodium carbonate. The stock standard solution of cobalt(II) (1000 µg mL−1) was prepared from Co(NO3)2·6H2O (0.2464 g in a 50 mL volumetric flask). The working solutions were obtained daily by stepwise dilutions from this solution.
2.3 Preparation of polyvinyl chloride functionalized with TADAP (PVC-TADAP)
In previous our work, the ligand TADAP was synthesized according to the procedure reported by Chotima [18] and the synthesis of TADAP was confirmed by the FT-IR spectra and melting point [11]. Polyvinyl chloride functionalized with TADAP (PVC-TADAP) was prepared according to our previously reported procedure and the formation of covalent bond between PVC and TADAP was confirmed by FT-IR spectrum and the CHN analysis [11]. In this work, the scanning electron microscopy (SEM) images were also recorded for pure PVC (Fig. 1a) and PVC-TADAP (Fig. 1b). The SEM images for pure PVC (Fig. 2a) and PVC-TADAP (Fig. 2b) demonstrate that after immobilization of the ligand on the PVC bead, the surface of sorbent is rougher. This phenomenon that was reported by other researchers [19] confirms the loading of the ligand on the polymeric bead.
Schematic diagram of designed flow system (a) pre-concentration, (b) washing, and (c) elution. (P peristaltic pump, MC mini-column; FAAS flame atomic absorption spectroscopy; W waste.) [11]
2.4 Preparation of mini-column
The mini-column prepared as follows: about 265.0 mg of the synthesized PVC-TADAP sorbent was packed inside a laboratory-made column with 8.0 cm length. Then to prevent the loss of sorbent during the pre-concentration process, the two ends of the mini-column were blocked with a glass wool. The prepared mini-column was washed with HCl (concentration of 1.0 M) and then distilled water. It should be noted that re-packing of the column was not necessary because the packing material had an unlimited lifetime.
2.5 General procedure for quantitative determination of cobalt(II) ions
The designed flow system that was reported for the determination of cadmium was used [11]. In the designed flow system (Fig. 2) [11], the quantitative determination of cobalt(II) ions was made in a three-step process. (i): Pre-concentration step: by rotation of valve V and turning on pump 1, an aliquot of 10.0 mL of sample with a definite pH and flow rate was passed through the prepared mini-column, and the cobalt ions were quantitatively retained on the adsorbent (PVC-TADAP). (ii): Washing step: by switching valve V and turning on pump 2, distilled water was directed into the packed mini-column for 60 s to remove the impurities weakly adsorbed on the adsorbent. (iii): Elution step: by turning on pump 3, a stream of HCl (1.0 M) was conducted to the packed column at a flow rate of 3.3 mL min−1 to elute cobalt held by the PVC-TADAP bed, and then directed to the flame atomic adsorption spectrometer to display the analyte absorption signal.
3 Results
3.1 Optimization of variables
3.1.1 Effect of pH
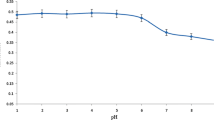
Among the chemical variables affecting the pre-concentration process, the solution pH has a critical role in the effective interaction of the analyte of interest and the adsorbent [4, 19, 20]. For this purpose, 10 mL of cobalt(II) solutions (50.0 μg L−1) buffered in the pH range of 4.0–8.5 were passed through the packed mini-column at a flow rate of 3.3 mL min−1. The up-taken cobalt(II) ions were eluted by loading HCl (1.0 mol L−1) to the mini-column and directly determined by FAAS. As it can be seen in Fig. 3, the maximum signal was obtained at pH = 6.5. At a lower pH, due to the protonation of electron-donor groups (nitrogen and sulfur) that exist in the TADAP ligand, and at a higher pH due to the formation of various species such as Co(OH)2, Co(OH)3−, etc. [21], the absorption signal decreases. Thus, based on the obtained results for all the subsequent experiments, the pH value was adjusted to 6.5.
The effect of buffer type on the analytical signal was also investigated using different buffer solutions (e.g. acetate, phosphate, and citrate) at pH 6.5, and the resultant data was shown in Fig. 4. The formation of cobalt(II)–citrate complexes is responsible for reduction of the signal at the citrate buffer solution [22]. As it can be seen in this figure, the analytical signal is identical in the phosphate and acetate solutions. However, due to the higher buffer capacity of the phosphate buffer solutions (with respect to acetate), the phosphate buffer solution was chosen.
3.1.2 Effects of type and concentration of eluent
The nature of the eluent solvent has a significant role in the desorption of the analyte. The protons of mineral acids can interact with the electron pairs in the ligand and break the metal-chelate bonds [11]. Therefore, different mineral acids such as hydrochloric acid, nitric acid, and sulfuric acid were investigated for the complete desorption of analyte from the polymeric bead. According to the experimental data, there is no difference between the selected acidic solvents. Nevertheless, since chloride ions have less interaction with the analyte, and due to a less corrosive effect of hydrochloric acid, this mineral acid was chosen as the desorption eluent. The effect of the eluent concentration was also investigated in the range of 0.1–2.0 mol L−1. The results obtained (Fig. 5) imply that by increasing the concentration of hydrochloric acid up to 0.8 M, the analytical signal increases, and then it is nearly constant. To ensure the complete elution of cobalt(II) ions, the concentration of 1.00 M HCl as the eluent was chosen.
3.1.3 Effects of sample flow rates
The amount of analyte adsorbed into the mini-column is dependent to the flow rate of the sample delivered through the column. This factor must be adjusted to get the highest amount of retained analyte on the sorbent in a considerably short time. For this purpose, 10 mL of the sample solution (50.0 µg L−1, pH 6.5) was passed through the mini-column with the flow rate in the range of 2.5–7.5 mL min−1. According to the results (Fig. 6a), it is clear that the analytical signal is independent from sample flow rate over the whole range examined, and that only a slight decrease in the analytical signal is observed at flow rates higher than 6.0 mL min−1. This means that the kinetic of the complex formation is fast, and that the contact time for complete sorption is sufficient. Due to the low repeatability of the analytical signal at a flow rate higher than 5 mL min−1, the flow rate of 5.0 mL min−1 was adopted as the best one.
3.1.4 Effects of eluent flow rates
For optimization of the eluent flow rate, 10 mL of cobalt(II) solution (50 µg L−1) at pH 6.5 and a flow rate of 5.0 mL min−1 was passed through a packed mini-column. The flow rate of the eluent solvent was investigated in the range of 2.0–4.5 mL min−1. As it can be seen in Fig. 6b, the analytical signal was increased by increasing the flow rate of the eluent solution from 2.0 to 3.5 mL min−1. Upper it, due to the insufficient time for the complete elution of the retained cobalt, the signal was reduced. Thus the flow rate of 3.3 mL min−1 was chosen as the optimum one for the eluent.
3.1.5 Effect of column length (amount of adsorbent)
To study the effect of column length (amount of polymeric sorbent) on the retained analyte, mini-columns with different lengths (1.0–9.0 cm) were prepared, and their performance was investigated. The results obtained (Fig. 7) showed that the analytical signal improved with increase in the column length up to 6.0 cm. Behind it, the analytical signal was nearly constant. Therefore, a column of 8.0 cm length (265.0 mg of PVC-TADAP) was selected as optimum value for the complete adsorption of various concentrations of the cobalt(II) ions from 10 mL solutions.
3.1.6 Effect of sample volume
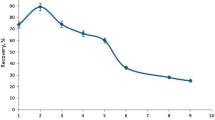
The sample volume is one of the important factors that affects the pre-concentration factor [4, 5]. To study the effect of loading time (sample volume) on the analytical signal, different volumes (from 5.0 to 50.0 mL) of cobalt solution with concentrations of 20, 50, and 100 µg L−1 were passed through the column, and quantitative determinations were made. The experimental data (Fig. 8) implied that at a higher sample volume (up to 30 mL), the absorption signal increased linearly, and then it was constant. However, for compatibility between sensitivity and reduction of loading time, 10 mL was selected as the best one. It should be noted that for a low concentration, a higher volume could be used.
3.1.7 Salt effect
To survey the influence of the ionic strength (salt effect), different salts such as potassium nitrate, potassium chloride, and sodium chloride with the concentration range of 0.00–0.20 mol L−1 were added to 10 mL of 50.0 µg L−1 cobalt(II) solutions. Then they were subjected to the pre-concentration process, and the associated signals were recorded. The results obtained (Fig. 9) demonstrated that the analytical signal reduces with increase in the salt concentration. This manner can be explained based on the competition between the cationic ions and cobalt(II) ions for adsorption on the adsorbent. To solve this problem, one can use the standard addition technique.
3.2 Analytical performance of SPE- on-line system
Under the optimum experimental conditions (pH value of 6.5, 10 mL of the sample with a flow rate of 5.0 mL min−1, and elution with HCl solution (1.0 M) at a flow rate of 3.3 mL min−1, and 265 mg of the adsorbent), various analytical parameters were computed. For a 10 mL sample volume of cobalt(II) ions, the calibration graph showed a linear manner over the concentration range of 2.0–150 µg L−1. The corresponding linear equation is A = 1.74CCo(II) + 0.005 (r = 0. 9998, n = 10), where A is the absorbance and CCo(II) is the cobalt(II) concentration in mg L−1.
The limit of detection (LOD) was calculated by LOD = 3Sb/m, where Sb is the standard deviation for the signal of the blank solution (a mixture of buffer and distilled water), and m is the slope of the calibration graph [10, 11]. By taking ten replicate measurements on the blank solution, a detection limit of 1.3 µg L−1 was obtained.
Evaluation of the precision of the proposed flow-FAAS technique was measured in terms of the relative standard deviations (RSDs) of six replicate measurements of cobalt(II) concentration containing 10.0, 50.0, 100.0, and 140.0 µg L−1. These values were 5.2%, 3.0%, 1.8%, and 1.8%, respectively, showing a good repeatability of the method.
The enhancement factor (EF = \(\frac{{\mathrm{m}}_{1}}{{\mathrm{m}}_{2}}\), where m1 slope of the calibration curve with pre-concentration and m2 slope of calibration curve without pre-concentration (direct aspiration), was 40. The consumptive index (the consumed sample volume (mL) per unit of the enhancement factor (EF)) was also 0.25 mL [11]. The low value for this factor states the suitable efficiency of the proposed on-line-FASS method.
The recovery percentage (R%) was defined by the following equation:
where EF, VE, and VS are the enhancement factor, volume of the eluent, and volume of the sample solution, respectively. Since the time required for the complete elution of the adsorbed cobalt(II) ions with a flow rate of 3.3 mL min−1 was 4 s, the computed VE value was 220 µL, and consequently, the recovery percentage for 10 mL sample by the proposed on-line process was found to be 88.
The maximum capacity of the proposed sorbent was obtained in the batch technique. In this method, 100 mg of this adsorbent was added to 500 mL of 10 mg L−1 of the cobalt solution (buffered at pH 6.5) and stirred mechanically for 1 h. Then the amount of cobalt remained in the solution was measured by direct aspiration to FAAS. The difference between the initial and remaining amounts of cobalt was applied to obtain the maximum capacity. The proposed adsorbent had a maximum capacity of 22.5 mg g−1.
3.3 Effect of interference ions
In order to identify the possible interferences of different ions may be exist in the matrix of real samples in the measurement of cobalt, various amounts of diverse ions were added to the solutions containing 50.0 µg L−1 of cobalt and analyzed using the proposed on-line-FAAS method. A variation more than ± 5% in the absorbance was considered as a criterion to identify a foreign ion as an interference [10]. As it could be seen in Table 1, among a large number of ions surveyed, only Ag+, Ni2+, Zn2+, and Mn2+ had severe interferences. More investigation proved that the interference effect could be decreased to 200-fold using the masking agents such as dithizon (for Ag+), dimethylglyoxime (for Ni2+), Cl− (for Zn2+ and Mn2+), and F− (for Al3+).
3.4 Application of developed method to real samples
3.4.1 Analysis of tap water
The developed on-line-FAAS method was applied for the determination of cobalt(II) ions in tap water (Shahrood, Iran) by the standard addition technique. Since the concentration of cobalt in the sample was lower than the detection limit of the proposed method, the standard addition method was performed on the spiked solutions. The recovery values and calculated t values (Table 2) imply the accuracy of the proposed method.
3.4.2 Analysis of wastewater of a pharmaceutical company
After filtering, an aliquot of 40 mL of wastewater sample was transferred to a 50 mL volumetric flask containing 2.5 mL buffer and marked to volume with distilled water. Then a 10 mL aliquot of the prepared sample was subjected to pre-concentration and determination by the developed method by the standard addition method. The results obtained (Table 3) show that there were no statistical differences between the real and the experimental data.
3.4.3 Analysis of cobalt in vegetables and fruits
For the analysis of cobalt in vegetable and fruit by the developed method, to 1.5 g of the dried and powdered sample of white cabbage or cucumber, 10 mL HNO3 was added, and heated on a hot plate at 100 °C. After 1 h, 10 mL HNO3 and 4 mL H2O2 were again added and stirred slowly for 1 h to decrease the volume by half [23]. Then the resultant filtered solution was neutralized with NaOH (3 M), transferred to a 250 mL flask, and diluted to 250 mL with distilled water. 40 mL of the prepared solution was added to a 50 mL flask containing 2 mL phosphate buffer (pH 6.5), and diluted with distilled water. Then a 10.0 mL aliquot of the prepared solution was analyzed by the proposed FIA-FAAS technique for the quantitative determination of cobalt(II) ions. The results obtained (Table 4) approve the validity of the proposed method.
3.4.4 Analysis of vitamin B12 ampoule samples
An aliquot of 1.0 mL of vitamin B12 (a tetrapyrrole complex containing cobalt ion [24]) ampoule was placed in a 100 mL beaker, and 10 mL mixture of concentrated HNO3 and H2SO4 (with ratio of 10:1 v/v) was added. The solution was heated, and then the semi-residue mass was neutralized with a diluted solution NaOH and diluted to 100 mL with distilled water [25]. Then 40 mL of the prepared solution was transferred to a 50 mL flask, buffered at pH 6.5, and diluted to volume with distilled water. A 10.0 mL aliquot of the prepared solution was subjected to FIA-FAAS for measurement of Co(II). The results in Table 5 imply the capability of the developed method in determination of cobalt ions in different matrices.
3.5 Comparison of proposed SPE-FAAS method with some other on-line reports
A comparison between the proposed on-line-FAAS method and some recent on-line SPE methods is demonstrated in Table 6. As it can be seen, the figures of merit of the developed protocol are similar or comparable with most of the reported methods. The proposed method exhibits a higher detection limit compared to the method of reference [30]. However, the method of reference [30] requires a new microcolumn to be prepared and replaced for each measurement, which is time-consuming and reduces reproducibility. In contrast, our proposed method employs a mini-column for all measurements, which is advantageous in terms of experimental convenience, reproducibility, and reduction of consuming materials.
4 Conclusion
A newly designed flow-FAAS method was used for the quantitative determination of trace amounts of cobalt(II) ions using the PVC-TADAP adsorbent. The prepared resin was an inexpensive and eco-friendly adsorbent because it could simply be recycled and used for different times. The major advantages of the proposed protocol are rapid kinetic in the adsorption and desorption process, simplicity, time-saving, and automation. This method showed reasonable results in the measurement of trace levels of cobalt in different samples (such as sample waters, waste water, vegetable, fruit, and drug) with complicated matrices. Comparing the figures of merit of the designed method with some reported research works indicate that although a number methods report better results they use a large volume of sample, and so require a more amount of sample. In the other word, the consumptive index of the proposed method is better or comparative with the reported mmethods.
References
Jamali MR, Boromandi A (2014) Preconcentration and determination of cadmium in water and food samples by in situ surfactant-based solid-phase extraction and flame atomic absorption spectrometry. J Braz Chem Soc 25:1078–1085. https://doi.org/10.5935/0103-5053.20140082
Soylak M, Erbas Z (2018) Vortex-assisted magnetic solid phase extraction of Cd (II), Cu (II) and Pb (II) on the Nitroso–R salt impregnated magnetic Ambersorb 563 for their separation, preconcentration and determination by FAAS. Int J Environ Anal Chem 98(9):799–810. https://doi.org/10.3906/kim-2009-26
Jing N, Tian M, Wang Y, Zhang Y (2019) Nitrogen-doped carbon dots synthesized from acrylic acid and ethylenediamine for simple and selective determination of cobalt ions in aqueous media. J Lumin 206:169–175. https://doi.org/10.1016/j.jlumin.2018.10.059
Soylak M, Alasaad M, Özalp Ö (2022) Fabrication and characterization of MgCo2O4 for solid phase extraction of Pb(II) from environmental samples and its detection with high-resolution continuum source flame atomic absorption spectrometry (HR-CS-FAAS). Microchem J 178:107329. https://doi.org/10.1016/j.microc.2022.107329
Shirani M, Salari F, Habibollahi S, Akbari A (2020) Needle hub in-syringe solid phase extraction based a novel functionalized biopolyamide for simultaneous green separation/preconcentration and determination of cobalt, nickel, and chromium (III) in food and environmental samples with micro sampling flame atomic absorption spectrometry. Microchem J 152:104340. https://doi.org/10.1016/j.microc.2019.104340
Pourreza N, Zolgharnein J, Kiasat AR, Dastyar T (2010) Silica gel–polyethylene glycol as a new adsorbent for solid phase extraction of cobalt and nickel and determination by flame atomic absorption spectrometry. Talanta 81(3):773–777. https://doi.org/10.1016/j.talanta.2010.01.010
Deveci S, Çetinkaya E, Dönmez KB, Orman S, Doğu M (2018) Development of preconcentration process of iron by using graphene adsorbent and experimental design methodology. Microchem J 143:272–279. https://doi.org/10.1016/j.microc.2018.08.006
Viñas P, Campillo N, Andruch V (2015) Recent achievements in solidified floating organic drop microextraction. TrAC Trends Anal Chem 68:48–77. https://doi.org/10.1016/j.trac.2015.02.005
Sharifi V, Abbasi A, Nosrati A (2016) Application of hollow fiber liquid phase microextraction and dispersive liquid–liquid microextraction techniques in analytical toxicology. J Food Drug Anal 24(2):264–276. https://doi.org/10.1016/j.jfda.2015.10.004
Bagherian G, Arab Chamjangali M, Shariati Evari H, Ashrafi M (2019) Determination of copper (II) by flame atomic absorption spectrometry after its perconcentration by a highly selective and environmentally friendly dispersive liquid–liquid microextraction technique. J Anal Sci Technol 10(1):1–11. https://doi.org/10.1186/s40543-019-0164-6
Bagherian G, Pourbahramian A, Bahramian B, Arab Chamjangali M, Ashrafi M (2021) Evaluation of polyvinyl chloride functionalized with 3-(2-Thiazolylazo)-2, 6-diaminopyridineas a new chelating resin for on-line pre-concentration and determination of traces of cadmium in real samples by flame atomic absorption spectrometry. Anal Bioanal Chem Res 8(1):39–53. https://doi.org/10.22036/abcr.2020.204689.1409
Tuzen M, Sahiner S, Hazer B (2016) Solid phase extraction of lead, cadmium and zinc on biodegradable polyhydroxybutyrate diethanol amine (PHB-DEA) polymer and their determination in water and food samples. Food Chem 210:115–120. https://doi.org/10.1016/j.foodchem.2016.04.079
Fahimirad B, Rajabi M, Elhampour A (2019) A rapid and simple extraction of anti-depressant drugs by effervescent salt-assisted dispersive magnetic micro solid-phase extraction method using new adsorbent Fe3O4@ SiO2@ N3. Anal Chim Acta 1047:275–284. https://doi.org/10.1016/j.aca.2018.10.028
Camel V (2003) Solid phase extration of trace elements. Spectrochim Acta Part B 58:1177–1233. https://doi.org/10.1016/S0584-8547(03)00072-7
Kantipuly C, Katragadda S, Chow A, Gesser HD (1990) Chelating polymers and related supports for separation and preconcentration of trace metals. Talanta 37(5):491–517. https://doi.org/10.1016/0039-9140(90)80075-Q
Marahel F, Ghaedi M, Shokrollahi A, Montazerozohori M, Davoodi S (2009) Sodium dodecyl sulfate coated poly (vinyl) chloride: An alternative support for solid phase extraction of some transition and heavy metals. Chemosphere 74(4):583–589. https://doi.org/10.1016/j.chemosphere.2008.09.034
Gupta KC, Sutar AK (2008) Catalytic activities of Schiff base transition metal complexes. Coord Chem Rev 252:1420–1450. https://doi.org/10.1016/j.ccr.2007.09.005
Chotima R (2008) Synthesis, characterization and application of complex between 3-(2-Thiazolylazo)-2, 6-diaminopyridine and Ruthenium(III) (Doctoral dissertation, Kasetsart University)
Chamjangali MA, Bagherian G, Mokhlesian A, Bahramian B (2011) Synthesis and application of chloromethylated polystyrene modified with 1-phenyl-1, 2-propanedione-2-oxime thiosemicarbazone (PPDOT) as a new sorbent for the on-line preconcentration and determination of copper in water, soil, and food samples by FAAS. J Hazard Mater 192(3):1641–1649. https://doi.org/10.1016/j.jhazmat.2011.06.093
Anthemidis AN, Ioannou KIG (2006) Evaluation of polychlorotrifluoroethylene as sorbent material for online solid phase extraction systems: determination of copper and lead by flame atomic absorption spectrometry in water samples. Anal Chim Acta 575(1):126–132. https://doi.org/10.1016/j.aca.2006.05.072
Manousi N, Kabir A, Furton KG, Zachariadis GA, Anthemidis A (2021) Automated solid phase extraction of Cd(II), Co(II), Cu(II), and Pb(II) coupled with flame atomic absorption spectrometry utilizing a new sol-gel functionalized silica sorbent. Separations 8(7):100. https://doi.org/10.3390/separations8070100
Nikulshin PA, Mozhaeva AV, Pimerzin AA, Victor V (2012) CoMo/Al2O3 catalysts prepared on the basis of Co2Mo10–heteropolyacid and cobalt citrate: effect of Co/Mo ratio. J Fuel 100:24–33. https://doi.org/10.1016/j.fuel.2011.11.028
Ismail F, Anjum MR, Mamom AN, Kazi TG (2011) Trace metal contents of vegetables and fruits of Hyderabad ratail market. Pak J Nutr 10(4):365–372. https://doi.org/10.3923/pjn.2011.365.372
Bartosiak M, Jankowski K, Giersz J (2018) Determination of cobalt species in nutritional supplements using ICP-OES after microwave-assisted extraction and solid-phase extraction. J Pharm Biomed Anal 155:135–140. https://doi.org/10.1016/j.jpba.2018.03.058
Baghban N, Mohammad A, Dadfarnia S, Jafari A (2009) Flame atomic absorption spectrometric determination of trace amounts of cobalt after cloud point extraction as 2-[(2- Mercaptophenylimino) methyl]phenol complex. J Braz Chem Soc 20:832–838. https://doi.org/10.1590/S0103-50532009000500005
Amin AS (2014) Study on the solid phase extraction and spectrophotometric determination of cobalt with 5-(2-benzothiazolylazo)-8-hydroxyquinolene. Arab J Chem 7(5):715–721. https://doi.org/10.1016/j.arabjc.2010.12.008
Chamjangali MA, Sharif-Razavian L, Bahramian B, Bagherian G (2010) Synthesis and application of a functionalized polystyrene resin for on-line pre-concentration and determination of cobalt(II) in water samples by flow injection/FAAS. J Braz Chem Soc 21:525–532. https://doi.org/10.1590/S0103-50532010000300019
Mohammadi SZ, Hamidian H, Karimzadeh L, Moeinadini Z (2016) Tween 80 coated alumina: an alternative support for solid phase extraction of copper, nickel, cobalt and cadmium prior to flame atomic absorption spectrometric determination. Arab J Chem 9:S1290–S1296. https://doi.org/10.1016/j.arabjc.2012.02.002
Duran A, Tuzen M, Soylak M (2009) Pre-concentration of some trace elements via using multi-walled carbon nanotubes as solid phase extraction adsorbent. J Hazard Mater 169(1–3):466–471. https://doi.org/10.1016/j.jhazmat.2009.03.119
Haji Shabani AM, Dadfarnia S, Dehghan K (2003) On-line preconcentration and determination of cobalt by chelating microcolumns and flow injection atomic spectrometry. Talanta 59:719–725. https://doi.org/10.1016/S0039-9140(02)00609-4
Acknowledgements
The authors are thankful to the Shahrood University of Technology Research Council for the financial support of this work.
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
Dr. Ghadamali Bagherian: Supervision, Conceptualization, Methodology, Writing- Reviewing and Editing, Tahere Zarnoushe Farahani: Data curation, Investigation Mansour Arab Chamjangali: Data curation Motahare Ashrafi: Writing- Original draft preparation, Validation.:
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
All data generated or analysed during this study are included in this published article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zarnoushe Farahani, T., Bagherian, G., Arab Chamjangali, M. et al. On-line determination of the trace amount of cobalt(II) in real samples by flame atomic absorption spectrometry method after pre-concentration by modified polyvinyl chloride. SN Appl. Sci. 5, 183 (2023). https://doi.org/10.1007/s42452-023-05402-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05402-y