Abstract
Efforts to restore firefly populations are ongoing; these populations have been declining due to the development of cities and industries. The first step in restoration is the development of a breeding system that can accommodate approximately 5000 hatched larvae of Luciola cruciate, an aquatic firefly species, until they reach the last instar larval stage. The bred larvae are released into a river in a restored forest in a chemical plant, where they can breed naturally. Firefly-viewing events are organized so that people can experience the pleasure of watching fireflies. Such events can be successful if the peak flight season of fireflies can be predicted accurately. There is scope to improve the methods used for flight season prediction, which have impaired prediction accuracy owing to the variability of regional characteristics. In this study, data on fireflies and meteorological data accumulated for the restored forest over 11 years were analyzed and the peak flight days of L. cruciate adults and climbing by L. cruciate larvae were discussed. The new regression equation-based method proposed in this study, which used the breeding temperature and minimum humidity, can estimate the peak flight date of adult fireflies better than the conventional method based on the effective cumulative temperature. As part of the ecology of fireflies, statistical analysis on climbing by larvae revealed that minimum air temperature and cumulative precipitation were significant predictors of climbing and that climbing occurred on a rainy night with a minimum air temperature of ≥ 10 °C.
Article Highlights
-
Data on firefly ecology for 11 years in a restored forest were utilized.
-
The proposed prediction formula can accurately predict the peak flight season of fireflies.
-
The trigger for landing firefly larvae was clarified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Fireflies in flight were a common aspect of rural landscapes in Japan [1], but such landscapes have been lost in many regions [2,3,4,5]. Ohba and Suzuki [6] reported that Genji fireflies (Luciola cruciate Motschulsky, 1854 (Coleoptera: Lampyridae)) were found in large numbers before 1988, but these numbers gradually decreased; however, investigations into the underlying reasons for this decline, along with activities to restore the lost firefly habitats and preserve the remaining fireflies, have started to emerge. In Japan, the word “fireflies” is used to refer to Genji fireflies (L. cruciate). Their light fascinates viewers, but the use of luminescence for communication is not fully understood [7]. L. cruciate is an organism that is ecologically important and is also the target of natural monuments [8]. L. cruciate is ranked as endangered species IB (high risk of extinction in the wild in the near future) in Tokyo and Gunma prefectures and as endangered species II (species with an increasing risk of extinction) in Saitama Prefecture. In Miyagi, Ibaraki, and Nagano prefectures, it is ranked as near-threatened (species that have a low risk of extinction currently but may become endangered depending on changes in habitat conditions) (http://jpnrdb.com/link.html). From the above, it can be concluded that L. cruciate is in danger of extinction in Japan. Restoration and conservation activities have been initiated to restore landscapes where the sight of wild fireflies becomes commonplace again. After their eggs hatch approximately at the end of June, L. cruciate larvae spend most of their lives in water. The larvae feed in the water and molt repeatedly to become mature larvae. They land in late March to early April and transform into pupae underground. They metamorphose from pupae to adults and fly from mid-May to the end of June. The authors of this study are trying to increase the number of adult fireflies by artificially assisting the aquatic larval stage. Since 2008, L. cruciate larvae have been bred and released into a river in a forest that was restored within the premises of a chemical plant. Furthermore, in 2020, a large-scale breeding method (for example, [9]) was established and can accommodate approximately 5000 hatched larvae. Through this method, several thousands of L. cruciate larvae have been successfully produced in a stable and easy manner. In the firefly flight season, firefly-viewing events, which are open to the public, have become annual events. In these events, climbing by larvae released to the river and the flight of adult fireflies are monitored. Previous studies on conservation activities, appreciation, and monitoring of fireflies related to our activity are outlined below.
With regard to firefly conservation activities, Kashio [10] has reported on public–private partnership activities, such as efforts toward the artificial breeding of L. cruciate and the cultivation of Semisulcospira libertina freshwater snails in Moriyama City, Shiga Prefecture. The author showed that “fireflies” and “clear rivers” were important keywords that united multiple generations in a common environmental conservation vision through environmental conservation activities. Kishi and Era [11] reported that after river conservation activities were initiated, the populations of S. libertina and Clithon retropictus, a freshwater and brackish water snail, as well as of L. cruciate and Luciola lateralis (Heike firefly), which are predator species of the two former species, increased dramatically from 2008 to 2016. With regard to viewing, the Yokosuka Environmental Policy Department [12] has reported on observation-related manners such as refraining from capturing fireflies and not using flashlights, mobile phone lights, and car headlights to avoid disturbing the community. Shinohara [13] reported on the creation of firefly maps to provide information about fireflies, improve tourism resources, call for strengthened cooperation with local communities through riparian zone conservation activities, and increase citizens’ awareness of water-related issues. Matsuoka et al. [14] investigated the association between the flight season of L. cruciate bred in and released to Kuwana City, Mie Prefecture, and meteorological and various phenological data to explore the possibility of predicting the flight season via a simple method. Using their method, the peak flight season can be predicted as approximately 55 days after the larvae have climbed onto land or Cerasus × yedoensis cherry blossoms have fallen. Kumode et al. [15] studied the relationship between adult emergence time and the larval climbing season and air temperature in Kyoto City and Santo-cho, Shiga Prefecture, to predict the emergence time of L. cruciate adults; they proposed the following equation to represent the relationship between the peak (or initial) date of larval climbing and the peak (or initial) date of luminous adults (both presented as the number of days from January 0):
Yuma [16] reviewed the behaviors and habitat of L. cruciate adults; a simple population estimation method based on continuous visual observations was introduced, followed by estimation of the survival rate based on changes in firefly population and average lifespan. In addition, some groups have observed and reported the number of flying L. cruciate adults around local rivers [17, 18].
L. cruciate, which is a large aquatic firefly species well known among Japanese people, was selected as the target in this study, and a project is underway to restore firefly populations. As the first step, the bred larvae are being released into a river since 2008 in order to preserve and increase the number of adults. During this activity, attempts have been made to further our ecological knowledge of fireflies through firefly observation. Firefly habitats were explored as the second step, and we are seeking to achieve the natural reproduction of fireflies as the final step. We are currently at the first step. Some previous studies have observed and evaluated the growth of L. cruciate from eggs into adults via observing hatching, larvae, larval climbing, and pupae in artificial breeding environments [19, 20]; however, the ecology of fireflies largely remains unclear as larvae spend most of their lives in water, in which it is difficult to make adequate observations, before they climb onto land and start flying as adults. Therefore, it is necessary to predict the peak flight season as early as possible in order to organize widely attended firefly-viewing events. The challenges related to firefly viewing are summarized below.
-
(1)
Prediction of the peak flight season is required to host a firefly-viewing event. Previous studies have proposed equations for prediction [14, 21]; however, they have room for improvements in prediction accuracy because regional climate differences such as temperature can affect firefly ecology.
-
(2)
With regard to climbing by larvae, Minami [19] stated that larvae start climbing all at once at approximately 7 pm on a night of steady rain when the water temperature matched the air temperature at approximately 14 °C. However, which weather condition triggers climbing remains unclear. In addition, it is not easy to make observations during rainy nights, and we want to simplify observation conditions where possible.
-
(3)
The factors affecting the number of flying adults remain unclear. Yuma [22] stated that rainfall in September and the second half of July had a major effect on population size reduction because frequent rainfall during this period washes away young larvae inhabiting rivers and reduces foraging behavior. However, this theory is not applicable to our case because the larvae were bred indoors.
To address the above issues, in this study, we have summarized the findings from the observations of L. cruciate ecology in the forest in which we released L. cruciate larvae over 11 years from 2011 to 2021.
2 Materials and methods
The overview of the forest, data collection period, data types, data collection methods, and data analysis methods are described.
2.1 Overview of the forest
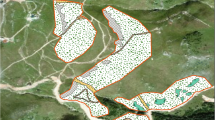
The forest area restored within the premises of the chemical plant where firefly larvae were released and bred naturally was reforested in 2007 as part of an effort to revitalize nature, farmlands, and woodlands and toward the conservation of creatures and ecosystems in the community. In an area of approximately 10,000 m2, there are aquatic plants in pond marshes, paddy fields, marshland, and natural forests of laurel trees found in a shrine. Therefore, various landscapes can be found, including ridges, valleys, flows, and ponds. A schematic of the forest is shown in Fig. 1.
The flowing spring water within the premises of the chemical plant is sent to Pond 1 in Fig. 1. Water flows counterclockwise in the following order before being discharged into the river: Pond 1 → Flow 1 → Pond 2 → Marshland/Paddy Field → Flow 2 → Pond 3 → (underground channel: not shown) → Flow 3. Flow 1,2 and 3 are 0.4–0.7 m wide and 0.1–0.3 m deep. As Genji fireflies inhabit clear streams, the larvae were released to Flows 1, 2, and 3.
2.2 Data collection period
Although we started to release firefly larvae in 2008, we decided not to use the data from 2008 to 2010 because of missing data and suboptimal accuracy in data collection. Therefore, the data collected over 11 years (from 2011 to 2021) were used in this study.
2.3 Types of data and data collection methods
2.3.1 Firefly data
-
(1)
The number of larvae released
A total of 500 randomly selected larvae were weighed in advance; the mass was 0.29 g/larva. The number of larvae released was determined from their mass. The larvae, which were last instar larvae, were released uniformly into the river in the forest; the larvae were released in early March each year.
-
(2)
The number of climbing larvae
Since the day before the first day of climbing, three investigators walked on the road along the river and counted the visible light spots of larvae from 19:00 to 21:00 every night. One or two investigators participated in and were in charge of counting every night from before the initial day of climbing to the last day of observation to minimize counting errors due to person-to-person variability. The third investigator was in charge of recording and safety during night. The number of climbing larvae was the total counted while the investigators walked around the forest once. The count was repeated three times in total, and the largest of the three results was used to represent the number of climbing larvae of the day.
-
(3)
The number of flying adults
From before the initial day of flight, three investigators walked on the road along the river and visually counted light spots of adults from 19:30 to 21:00 every night. One or two of them participated in and were in charge of counting every night from before the initial day of flight to the last day of observation to minimize counting errors due to person-to-person variability. The remaining investigator was in charge of recording and safety during night. The number of flying adults was the total counted while the investigators walked around the forest once. The count was repeated three times in total, and the largest of the three results was used as the number of flying adults of the day.
2.3.2 Meteorological data (2011–2021)
We collected meteorological data from the month in which larvae were released to the month in which flying adults were found. Specifically, the following data (1 point/day) from March 1 to June 30 in Fuji City, Shizuoka Prefecture, extracted from the database of the Japan Meteorological Agency (https://www.data.jma.go.jp/obd/stats/etrn/index.php) were used: mean wind velocity, mean wind direction, maximum instantaneous wind velocity, maximum instantaneous wind direction, daily mean air temperature, daily maximum air temperature, daily minimum air temperature, daily mean relative humidity, daily maximum relative humidity, daily minimum relative humidity, effective humidity, station pressure, sea-level pressure, maximum 10-min rainfall, maximum hourly rainfall, cumulative daily rainfall, 0:00–9:00 weather, 9:00–15:00 weather, and 15:00–24:00 weather.
2.3.3 Meteorological and temperature data for the forest (from march 2021)
Water temperature gauges were placed at the bottom of the river. Ground temperature gauges were placed 5 cm below the ground surface (5 cm was chosen because mud cocoons made by the larvae are found 1–5 cm below the ground surface; the stability of installation was also considered) and 50 cm away from the riverbank at the locations indicated with red circles. The water and ground temperature gauges (R718B2; Netvox Technology Co., Ltd., Tainan City, Taiwan) were placed at four locations each. Red circles in Fig. 1 denote weather sensors (FSN-4062L-OD; Fujikura Ltd., Tokyo, Japan), which measured air temperature, humidity, atmospheric pressure, and luminance. The data were collected at a frequency of once per 6 min and sent via radio waves to an external cloud server for storage and retrieval; this system was built by us and has been used since March 2021.
2.4 Analysis of observation data
2.4.1 Statistical analysis
The observation-derived data were first visualized as figures and tables, which were used to give an overall picture of the firefly ecology in the area studied. The studies described below were then conducted. BellCurve for Excel ver. 2.21 (Social Survey Research Information Co., Ltd., Tokyo, Japan) was used for statistical analyses.
2.4.2 Study of peak flight season
For many poikilothermic animals, including insects, the time for development from larvae to adults and temperature are known to follow the rule of effective cumulative temperature, as expressed by the following equation [23].
K is the effective cumulative temperature (d °C); D is the number of days of development (d); t is the breeding temperature (°C); and t0 is developmental zero (°C). The number of days of development should be determined as a representative value (e.g., median, mean) after the observation of individuals separately to evaluate the suitability of data distribution; however, this was difficult because individuals could not be identified in the setting of this study. Thus, the number of days between the initial day of climbing by larvae and the peak day of flight by adults was used in this study. This period is referred to as the development period. The breeding temperature was the mean of the mean air temperatures of individual days (daily mean air temperatures) during the development period. The development rate and developmental zero are defined as the reciprocal of the number of days of development 1/D (1/d) and the temperature at which the development rate is 0, respectively. For the development of Genji fireflies from larval climbing to adult emergence, Furukawa [21] proposed a developmental zero point of 8.02 °C and an effective cumulative temperature of 408.4 d °C based on breeding observations. Furukawa also stated that an error of + 5–10 days was possible because the newly emerged adults remain in mud cocoons for a while.
In this study, developmental zero t0 was determined from a linear regression line of the relationship between the development rate and breeding temperature for each year. Then, the effective cumulative temperature for each year was calculated using Eq. (1), and the mean value during the study period was used as the effective cumulative temperature in the forest Kave. After the number of days of development D was calculated from Kave, t0 and the actual breeding temperature t using Eq. (1), the predicted peak flight day was calculated for each year by adding the resulting D to the initial day of climbing and compared with the actual peak flight day.
Furthermore, a multiple regression analysis with the number of days of development as the objective variable and the temperature, humidity, and rainfall as explanatory variables was performed to derive an equation for estimating the number of days of development. The predicted peak flight day was calculated for each year by adding the number of days of development from the resulting estimation equation for the number of days of development to the initial day of climbing and compared with the actual peak flight day and predicted peak flight day from Kave, as described above.
2.4.3 Study on larval climbing by multivariate analysis
First, we compared the data on climbing by larvae and meteorological data to determine triggers for the climbing behavior. Next, we conducted discriminant analyses with the occurrence of climbing as the objective variable and meteorological data as explanatory variables.
2.4.4 Estimation of meteorological conditions presumed to affect the number of flights by multivariate analysis
A multivariate analysis with the number of fireflies flying as the objective variable and meteorological data as explanatory variables was conducted to find the meteorological conditions affecting the number of fireflies flying.
3 Results
3.1 Observation of fireflies
The firefly count for each year is shown in Fig. 2 to provide an overview of the firefly observation results. The number of larvae released is shown in Fig. 2a. While 2000–3000 larvae a year were released from 2011 to 2019, > 4000 larvae were released in 2020 and 2021 in order to increase the number of adults. The number of climbing larvae is shown in Fig. 2b. The count remained at 100–700. The rate of climbing, which was defined as the number of climbing larvae/number of released larvae × 100, is shown in Fig. 2c. The rate of climbing ranged from 3 to 17%; the period average was 10%. Possible reasons for the low rate of climbing of 10% include larval death or draining off from the river in the approximately 1-month period from release to climbing, errors in counts of climbing larvae, and an unexpectedly high number of larvae that climb after 9 pm; however, the exact cause could not be identified. Flight counts per night in May and June are shown in Fig. 3. It was considered possible to apply the normal distribution to the flight counts despite the differences from one year to another.
Table 1a shows the dates of climbing by firefly larvae and dates of flight by adults from 2011 to 2021, with information on cherry blossom (Cerasus × yedoensis) (adapted from the cherry blossom flowering record on Fuji City Iwamotoyama Park; http://www.pref.shizuoka.jp/kankyou/ka-080/sakura/stats.html) as a reference. The best viewing season was defined as the period in which (the peak daily flight number × 0.75) adults flew daily, and the first and last days of the best season were determined. Based on means, the date of climbing by larvae was found to be almost the same as the cherry blossom flowering date. Regarding the date of climbing by larvae, the relatively large standard deviation (σ) of 7.9 suggests a large variability. The initial flight date was May 14 (σ 2.9) and remained almost constant every year; thus, the initial date of flight was considered relatively easy to identify. These results indicate that to identify the initial flight date without fail, flight observation should be initiated immediately after the May Golden Week holidays in Japan. The calculation results for the number of days between important events are summarized in Table 1b. The peak day of adult flight was 65.8 days after the initial day of climbing by larvae. This was approximately 10 days longer than the approximate 55 day interval between the day of larval climbing/Cerasus × yedoensis blossom falling and the peak flight day, which was reported by Matsuoka et al. [14]. In addition, we used the flowering date, rather than when Cerasus × yedoensis flowers fell, for comparison in this study. The initial day of the best viewing season was approximately 14 days (σ 2.5) after the initial flight day, and the deviation was relatively small. Once the first flying adult is observed, firefly-viewing events can be scheduled to be held in 2 weeks, and preparations can be started. The results also showed that the duration of the best viewing season was approximately 6 days. Table 1c shows the breeding temperature and the rate of development for each year. The relationship between the two is shown in Fig. 4. The coefficient of determination was 0.82, indicating that the regression equation in the figure can be used to estimate the development rate from the breeding temperature. When the development rate = 0 was plugged into the regression equation, the developmental zero point was calculated as 8.65 °C. The effective cumulative temperature calculated for each year using Eq. (1) is shown in Table 1d. The mean effective cumulative temperature was 437.8 d °C. This is larger by approximately 30, which corresponds to approximately 2 days, compared with 408.4 d °C shown by Furukawa [21]. Equation (1) can be modified as shown below to predict the number of days of development in the forest studied.
The number of days of development predicted from Eq. (2) was added to the initial date of climbing to predict the peak flight date. Differences between the predicted and actual peak flight dates (predicted date–actual date) are shown in Table 1e. The mean difference between the predicted and actual peak flight dates was 0.02 days and the standard deviation was 3.81. These results indicate that Eq. (2) can be used to predict peak flight days.
To improve the accuracy of peak flight date predictions, we performed a multiple regression analysis with the number of days of development as the objective variable and meteorological data as explanatory variables. In addition to the breeding temperature described above, we directed our attention to the temperature, humidity, and rainfall, which are likely to have effects on the development of organisms. We selected five meteorological data (maximum air temperature, minimum air temperature, maximum relative humidity, minimum relative humidity, and cumulative daily rainfall) and used a total of six meteorological data as explanatory variables. For the multiple regression analysis, the daily mean values of these meteorological data from March to May, a period in which larvae climb onto land and adults emerge, were used. Monthly mean values could not be used because of the problem of the number of valid cases and the number of explanatory variables. The data used for the statistical analysis are shown in Table 2a. The results of the multiple regression analysis are shown in Table 2b.
Explanatory variables were narrowed down to the breeding temperature t and minimum humidity hL with the forward–backward stepwise selection method. Although the minimum humidity was not significant (P = 0.1152), the coefficient of determination of the regression Eq. (3), which considers the breeding temperature and minimum humidity, was 0.8495, which was considered effective for estimating the number of days of development.
The peak flight date was predicted by adding the number of days of development predicted with Eq. (3) to the initial larval climbing date. The predicted peak flight dates were compared with the actual dates in Table 3. The mean difference between the predicted and actual peak flight dates was 0.00 days (standard deviation, 3.04). This result suggests a higher estimation accuracy using Eq. (3) than Eq. (2). The differences between the predicted dates with the two equations and the actual dates are shown in the form of a box-and-whisker plot in Fig. 5. Thus, Fig. 5 visualizes the higher prediction accuracy of Eq. (3) with shorter lengths of the box and whiskers.
Difference between the predicted and actual peak flight dates. Box-and-whisker plot for (predicted date) using Eq. (3) (actual date) on the left side; plot using Eq. (2) on the right side. The box indicates the range from the first quartile to the third quartile, and the horizontal line in the box denotes the median value. The upper end of the whisker from the top of the box and the lower end of the whisker from the bottom of the box indicate the maximum and minimum values, respectively. However, values higher than 1.5 times the length of the box from the top or bottom of the box were not included in the whiskers and are displayed as x points as outliers (there were no outliers in this case)
3.2 Season in which larvae climb onto land
The meteorological data for days with climbing larvae were observed (≥ 40 larvae/day) and those for days no climbing larvae were observed (0 larvae/day) in each year were compared (Table 4). With a focus on temperature and rainfall, which have been reported as relevant meteorological data in a previous study [8, 19], we evaluated the following five meteorological data: daily maximum air temperature, daily minimum air temperature, daily maximum relative humidity, daily minimum relative humidity, and cumulative daily rainfall. In 2017, there were no days with ≥ 40 climbing larvae/day observed. In Table 4, we randomly selected the same number of days without climbing as the number of days with climbing from 2 weeks before the first day of climbing. The data are shown in the form of a box-and-whisker plot in Fig. 6. Figure 6 suggests that the daily minimum air temperature, daily minimum relative humidity, and cumulative daily rainfall are possible significant predictors. As examples, Fig. 7a shows the rainfall and the number of climbing larvae in March–April 2021, and Fig. 7b shows the daily minimum air temperature and number of climbing larvae in March–April 2021. These data suggest that the rainfall, combined with an approximate minimum air temperature of ≥ 10 °C, triggers climbing behavior. In these figures, the larvae did not climb onto land on March 13, presumably not because it was rainy but because the minimum air temperature was too low. Larvae then climbed onto land on a day meeting the rainfall and daily minimum air temperature conditions. April 14 was the last climbing day; larvae did not climb onto land regardless of rainfall or air temperature after this date.
Climbing by larvae and meteorological data. a Rainfall and the number of larvae climbing in march–april 2021; b daily minimum air temperature and the number of larvae climbing in march–april 2021; and c changes over time in the number of larvae climbing and air, water, and ground temperatures in the vicinity of Flow 1 in Fig. 1
Since March 2021, the relationship between the number of climbing larvae and various measures of temperature could be investigated because the temperature was continuously measured with sensors. Figure 7c shows changes over time in the number of climbing larvae and air, water, and ground temperatures in the vicinity of Flow 1 in Fig. 1. After the water temperature becomes similar to the ground temperature, the air temperature starts to increase gradually in spring, and larvae climb onto land when the air temperature becomes equal to the water and ground temperatures on a rainy day. We observed that the air temperature was almost the same as the water and ground temperatures when larvae climbed onto land.
Furthermore, the results of discriminant analyses with the occurrence of climbing as the objective variable and the daily maximum air temperature, daily minimum air temperature, daily maximum relative humidity, daily minimum relative humidity, and cumulative daily rainfall as explanatory variables are shown in Table 5.
Among the five types of meteorological data analyzed, the daily minimum air temperature was significant. The cumulative daily rainfall fell slightly short of significant. Wilks’ lambda of the cumulative daily cumulative was close to 1, indicating that the rainfall difference between days with and without climbing by larvae was small. Positive discrimination coefficients for both the daily minimum air temperature and the cumulative daily rainfall indicate that the likelihood of climbing is higher when these are higher. The standardized discrimination coefficient for the daily minimum air temperature was 2.62 times as large as that for the cumulative daily rainfall; therefore, the former has a larger effect. From the discrimination coefficient, A > 0 and A ≤ 0 in Eq. (4) predict the occurrence and nonoccurrence of climbing, respectively.
In this equation, tL and r denote the daily minimum air temperature (°C) and cumulative daily rainfall (mm), respectively. The percentages of correct classifications for occurrence and nonoccurrence of climbing were 92.3% (24/26) and 92.3% (24/26), respectively.
3.3 Factors affecting the number of flying adults
The total number of flying adults is shown in Fig. 8. Here, the total number of flying adults is the total value of the maximum number of flying adults each night from the start date to the end date of the flying. The total number of flying adults was highest in 2014 (3,500) and low from 2017 to 2019 (< 1,000). In other years, the approximate count was 2000.
We then attempted to determine the underlying cause of the difference in the number of flights seen in Fig. 8. It is critical to know variables that have effects on the number of flights because the number of flights has a large impact on the public’s enjoyment of fireflies. The first factor considered was the association of the larger number of larvae released with a larger number of climbing larvae and, in turn, with a larger number of flying adults. However, x–y diagrams of these variables (not shown) indicated that coefficients of determination of the regression equations were < 0.2, indicating that the number of larvae released alone was not a favorable predictor.
Various multivariate analyses (multiple regression analysis, logistic regression analysis, discriminant analysis, and quantification method 1) with the total number or maximum number of flights as the objective variable and meteorological data such as air temperature and rainfall from March to May as explanatory variables were attempted, but no significant variables were found.
4 Discussions
Efforts to restore firefly populations have been initiated across Japan. As one of these efforts, we have bred and released the larvae of L. cruciate and hosted firefly-viewing events since 2008. Based on the information acquired through our activities, we studied the prediction of peak flight date and factors that affected the counts of climbing larvae and flying adults.
Regarding the peak flight date, we used the conventional method of effective cumulative temperature to derive Eq. (2) from the observation data of the forest studied. The number of days of development D calculated with Eq. (2) could be added to the initial date of climbing by larvae to predict the peak flight day. While Eq. (2) is a function only of the breeding temperature t, we developed a function of the breeding temperature, the minimum humidity, and a constant term as Eq. (3) in this study and demonstrated the usefulness of Eq. (3) in improving the prediction accuracy compared with the effective cumulative temperature equation. Even with Eq. (3), the predictions for some years had an error of ± 4 days, as shown in Table 3. Given the fact that the first and second halves of peak flight seasons shown in Table 1b were for 2.27 days and 3.36 days, respectively, a prediction with Eq. (3) can miss the best viewing season, and an even better prediction equation than Eq. (3) is desirable.
The larvae climbed onto land on rainy nights in mid-March. In the beginning, few climbing larvae are seen, but the climbing season does not reach a climax until the day the climbing larvae suddenly increase in number to 40–100. The data on the actual number of climbing larvae show that the number increased suddenly to ≥ 40 (< 30 larvae were observed rarely) after < 10 climbing larvae were observed several times; in this study, 40 was considered as the threshold for the beginning of climbing. Minami [19] stated that larvae climb onto land together at approximately 7 pm on a night of steady rain when the water temperature matches the air temperature at approximately 14 °C; our data confirm that this statement is an accurate description of the ecology. In this study, our statistical analyses showed that the climbing behavior was triggered when the air temperature increased and was the same as the water temperature at approximately ≥ 10 °C on a rainy day; consequently, we proposed discriminant Eq. (4).
The (number of climbing larvae)/(number of larvae released) rates were small and highly variable (3%–17%). The rates were low, presumably because the released larvae were drained off from the river or died before climbing, and there were errors in the counts of climbing larvae; however, contributions from these causes remain to be determined.
An annual increase in the number of flights can be a sign of reproduction among the released fireflies; however, Fig. 8 did not reveal this sign. This suggests that the forest is not equipped with the environmental conditions for fireflies to grow naturally. The mean and standard deviation of the total number of flying adults shown in Fig. 8 were 1836 and 878.3, respectively. The number was as high as 3600 in 2014, but as low as 554, 415, and 808 in 2017, 2018, and 2019, respectively. The t-test at the 1% significance level indicated that in all cases, the null hypothesis was rejected and the differences were significant. In other words, the total number of flights in 2014 was significantly higher than that in the average year, and the total counts in 2017–2019 were significantly smaller. No variables representing meteorological conditions had significant effects on the number of flights. These results suggest that representative values, such as mean values, of the meteorological data are not favorable variables to represent the peculiar conditions or factors other than meteorological data involved. We also investigated forest vegetation conservation work, such as trimming, and changes in water quality, but we could not identify the specific variables involved.
The time of appearance of fireflies is affected by regional characteristics, particularly the climate. The method used in this study is considered to be applicable to other environments. Furthermore, it is speculated that it can be applied to other species, but verification is required.
To summarize, the following issues related to this study remain to be addressed.
-
(1)
Identification of factors that affect the number of flying adults
No variables that affected the number of flying adults could be identified from among climate conditions and forest conservation activities. This was interpreted to mean that regarding climate and forest environmental elements (vegetation, water quality, and soil), we could not identify a favorable breeding environment for fireflies. We must review the unanalyzed data again and include future observations to identify the variables that affect the number of flying adults. Furthermore, to increase the number of flights, we need to change the forest environmental elements, such as the vegetation and river flow, to those that are considered to be more favorable for the development of fireflies and repeat the development and testing of a hypothesis.
-
(2)
Establishment of a method for counting adults
We counted living organisms emitting light as adult fireflies through visual observation from 7:30 pm to 9:00 pm every night. The observation time frame was established based on the activation of fireflies and reduction of burden on observers. Regarding the former, our assumptions included the following: fireflies are active for 1 or 2 h after sunset, i.e., they are most active from 7:30 to approximately 8:00 and do not fly or emit light much other than during this period [8]; and fireflies are commonly active in flight and emit light frequently three times a night, i.e., shortly before 9:00 pm, around 12:00 am, and 3:00 am, among which they fly most actively just before 9:00 pm [19]. Errors in counting adults are attributable to the following: adults that do not emit light between from 7:30 pm and 9:00 pm; adults that emit light too weakly or in the shade of leaves and are thus practically invisible; and differences in experience among observers. To solve these problems, the development of digital transformation (DX)-based tracking technologies, such as construction of a system that uses the line transect method and a night-vision camera to count firefly blinks overnight, is essential. It would be even better if the system could distinguish between males and females and find the male-to-female ratio based on blinking patterns.
-
(3)
Improvement in accuracy of climbing larvae tracking
It is difficult to observe larvae that emit weak light between vegetation and rocks and climb onto land during a rainy night. As described in (2), the development of DX-based tracking technologies, such as climbing trajectory-based counting of climbing larvae, is required. Comparisons with visually observed results are necessary to verify the reliability of an automated tracking system.
-
(4)
Rates of climbing and emergence in released larvae
Data on (2) and (3) above will help to more accurately determine the percentages of the released larvae that successfully climb onto land and fly into the air as adults after emergence. Knowing the rate-limiting steps will help increase the number of adults, and quantifying the improvements resulting from the restoration efforts are expected to contribute substantially to the path toward restoration and conservation of firefly habitats.
-
(5)
Improvement of prediction formula accuracy
We will continue to collect forest data and improve the prediction formula derived in this study.
5 Conclusions
In this study, we analyzed the data on fireflies (such as the number of larvae released, number of larvae climbing onto land, and number of flying adults) and meteorological conditions that were accumulated for > 10 years in a restored forest in the premises of a chemical plant with reference to vegetation in the surrounding region and discussed the ecology of L. cruciate. We proposed a new method for predicting the peak flight date of adult fireflies using a regression equation composed of the breeding temperature, the minimum humidity, and a constant term and demonstrated that our method was superior to the conventional effective cumulative temperature-based method in terms of accuracy of prediction. We also found that the minimum air temperature and cumulative rainfall were significant predictors of climbing by larvae and showed that the larvae climbed onto land on a rainy night with a minimum air temperature of ≥ 10 °C. No specific meteorological conditions affected the number of flying adults; hence, other factors may be the major determinants.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Lewis S (2016) Silent sparks. Princeton University Press, New Jersey
Fujiso T, Kawaguchi Y, Takegawa Y, Yabuhara Y, Yamasshiro A (2017) Analysis of density determinate environmental factors of Semisulcospira libertina, and their correlation to observed number of Genji firefly (Luciola cruciate) in Kawata river, Misato, Yoshinogawa city, in Tokushima Prefecture. J Jpn Soc Civil Eng Ser G Environ Res 73:II_373-II_377. https://doi.org/10.2208/jscejer.73.II_373
Kohama T, Hayashi M (2012) The research of relationship between habitat and right water quality of Luciola cruciata in Choshi city. Chiba Inst Sci 5:29–34
Ohba N (1988) Genji Botaru. Bunichi Publishing, Tokyo
Yuma M (1995) With the aim of making new rivers. Biotic communities in nearby waterfront. In relation to paddy field farming. J Environ Conservat Eng 24:695–700
Ohba N, Suzuki H (2019) Flashing behavior and mitochondrial DNA analyses of the firefly, Luciola cruciata in the institute for nature study. Rept Inst Nat Stu 50:1–12
Kawano K (2011) An observation note on mating behavior of Luciola cruciata in natural habitat. New Entomol 60:46–49
Yokohama City Pollution Research Institute, Hotaru no Kai Y (1986) Creating the firefly breath environment, technical manual draft. Yokohama City Pollut Res Inst Mater 1986:71
Moriya S (2004) Creating a village where fireflies fly. Comprehensive learning through fireflies. Try X Publishing, Hiroshima
Kashio T (2018) Effects of an environmental local event on a local society a case from Moriyama, Shiga prefecture, Japan. Shiga Univ Res Cent Sustain Environ Annu Res Rep 15:33–41
Kishi Y, Era H (2017) Restoration of flesh water snails and fire flies at the Koajiro valley. Hiyoshi Rev Nat Sci Keio Uni 61:53–62
Yokosuka Environmental policy department (2019) Approaching fireflies in the miura peninsula. Parks Open Space 80:29
Shinohara S (2014) Ueda city’s “firefly map” project to “visualize” the waterside. J Sewerage Mon 37:18–22
Matsuoka M, Kawazoe A, Takemura K (2020) Prediction of flight season of Luciola cruciata. Bull Fac Educ Mie Univ 71:43–49
Kumode M, Tanaka K, Yuma M (1999) Estimation of best season of Genji firefly. Ecol Civil Eng 2:205–210. https://doi.org/10.3825/ece.2.205
Yuma M (1987) Genji firefly adult population in artificial waterways. Seibutu Kagaku Iden 41:48–52
Mayahara T (2010) Firefly monitoring in the area around Nihon university college of bioresource sciences. Nihon Univ Coll Mus Bioresour Sci 19:151–155
Nagai K, Kawashima I, Oizumi F, Horiuchi Y, Yagishita Y, Murayama S (2014) The report of occurrence individuals number of Luciola cruciata Motschulsky (Coleoptera: Lampyridae) at Ikuta-Ryokuchi Park in 2014. Bull Kaw Munic Sci Mus Youth 25:9–11
Minami K (1983) A study of fireflies. Scientist Publishing, Tokyo (U.S. Army translation no 1–9415 (1966). I.D. no. 2204017766, 0371)
Ohba N (1993) Firefly breeding and observation. Heart Publishing, Tokyo
Furukawa Y (2021) Firefly encyclopedia. http://www.tokyo-hotaru.com/jiten/hotaru.html. Accessed 25 Feb 2023
Yuma M (2007) Effect of rainfall on the long-term population dynamics of the aquatic firefly Luciola cruciata. Entomol Sci 10:237–244. https://doi.org/10.1111/j.1479-8298.2007.00219.x
Sekiguchi S, Yamamoto M (2014) Diapause and thermal constant of firefly (Luciola lateralis). Ann Environ Sci Shinshu Univ 36:109–113
Acknowledgements
We thank current and former staff of the Fuji office of Asahi Kasei Corporation for working overtime every night to observe fireflies, even during holidays and bad weather. We thank everyone at Mochizuki Teien Co., Ltd., for their support in firefly observation. We also thank Mr. Takeishi of Corporate Production Technology of Asahi Kasei Corporation for consultation about the creation of the data collection system for air temperature, water temperature, and other parameters. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
KS made contributions to the conception or design of the work. TY collected firefly data. JK agreed to be accountable for all the aspects of the work. All authors critically contributed to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or nonfinancial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sano, K., Yokoyama, T. & Kato, J. Ecology and behavioral patterns of Luciola cruciate in a restored forest in a chemical plant. SN Appl. Sci. 5, 193 (2023). https://doi.org/10.1007/s42452-023-05400-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-023-05400-0