Abstract
Continuous discharge of industrial and domestic inputs from various processes into the Lagos lagoon has significantly affected the quality of the aquatic environment, as a result of potentially toxic elements (PTEs) being released into the harbour during anthropogenic activities. This study involved monitoring the concentration and distribution of heavy metals in Lagos harbour during the dry and wet seasons. The PTEs can pose a serious ecological threat to the marine environment as well as human beings when the level of priority metals like cadmium, lead, and chromium is beyond World Health Organization (WHO) limits of 0.003, 0.05, and 0.1 mg/L, respectively. The shipping activities within the harbour play a significant role in the generation of these toxic metals. The diverse nature of these metals coexisting with their oxidation states in aquatic environments and their bioaccumulation influences the toxicity of PTEs towards the living organism. The quantification of these metals with highly selective and accurate instrumentation is imperative. Ion-selective exchangers and other functionalized composite nanomaterial are critical for harbour water remediation because of the high risk that could be associated with prolonged exposure to these toxic elements especially when the carcinogenic risk value is greater than 1 × 10−6 mg/kg/day.
Article highlights
-
Persistence and indiscriminate generation of industrial and domestic waste within and around the Lagos harbour led to increase the rate of potentially toxic metals in the harbour.
-
The legacy of historical inputs from the use of antifouling paints and shipping activities has generated a lot of concern due to the presence of some toxic elements.
-
Some results of carcinogenic and non-carcinogenic risk according to this paper exceeded the exposure daily intake (EDI), which could have a serious health implication on human being within and around the Lagos harbour, Nigeria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The intense development of anthropogenic activities in the last two decades has resulted in an increase in a load of pollution in the water bodies across the globe [1].
In lagoon systems, metal tends to accumulate in sediment in association with organic matter and it may be released into the water body due to changes in environmental conditions such as pH, dissolved oxygen, and temperature [2]. Mineral elements such as zinc, copper, manganese, iron, and calcium are classified as essential elements, and they play a lot of physiological functions in the body of living organisms. However, certain metals like lead, cadmium, chromium, arsenic, mercury, etc., do not play any essential role in the body and are highly toxic to all organisms even at low concentrations [3]. These metals are highly toxic, and because of that, they have been classified among priority metals by the European Union Commission (directives 2013/39/EU).
The marine environment has become contaminated due to a wide range of pollutants generated by various natural and anthropogenic activities, and this has prompted worldwide attention over decades [4, 5]. Processes like rapid industrial development, discharge from petrochemical industries, and waste discharged into the lagoon without proper treatment have contributed to the major sources of pollution. Also, the use of agrochemicals and fertilizers containing heavy metals from farmland settlements finds their way into many rivers [6]. During the transportation of these pollutants into larger water bodies like lagoons, there could be frequent changes due to dissolution, precipitation, and adsorption. Natural processes such as geological weathering of the earth’s crust and ocean tides could pose more stress on the lagoon water, thereby affecting the biodiversity of marine species as well as the ecosystem [7,8,9,10]. The contamination of the water body by heavy metals can be a potential threat to the environment, which can pose a risk to human health through the food chain because the water quality indices have an environmental and ecological impact on marine living organisms.
United Nations (UN) reports of the 2015 Millennium Development Goals (MDGs) showed that more than 40% of the global population was projected to be affected by water scarcity because of the accessibility to quality water resulting from the release of toxic pollutants into the lagoon system [11].
Human exposure to heavy metals has been linked to severe effects on human organs like kidney damage and various cancerous growths [6]. Hence, it is appropriate to have updates about the concentration of heavy metal present within and in areas of the harbour. Also, daily exposure to toxic chemicals could be established through major routes, which are, dermal, oral, and inhalation [12]. Health risk assessment is an important tool used for the evaluation of possible health effects caused by potentially toxic metals.
Studies have reported a lot of remediation processes for these diverse potentially toxic metal ions from wastewater by the use of innovative adsorbents, membrane separation, and photocatalytic-based treatment [13,14,15]. Different spectroscopic techniques like laser-induced breakdown spectroscopy (LIBS) and chromogenic analysis by the use of UV–visible spectroscopy have been employed in the quantitative evaluation of these diverse metal ions based on conjugated functionalized nanomaterials [16, 17]. Further research on the effective removal of toxic metal ions has also employed the use of ligand-based functionalized composite nanomaterials under acidic conditions [18,19,20].
Some of these potentially toxic metals which present as diverse metal ions in wastewater required sensitive and accurate analytical methods because of their selectivity as reported in the literature [14, 21, 22]
Lagos lagoon has been reported by several authors to be greatly polluted over the years, but the harbour [22], which is an important area of the lagoon system, has not been comprehensively addressed. It is against this background that necessitated this study.
Comprehensive monitoring of the Lagos harbour from November 2016 to July 2018 was conducted to assess the pollution status of the harbour in terms of contamination from anthropogenic pollution and its sources. The concentration of some priority metals was monitored in the lagoon water including some physico-chemical parameters. The carcinogenic and non-carcinogenic risks that could associate with these potentially toxic elements were also assessed based on exposure daily intake (EDI).
2 Experimental
2.1 Sampling and analytical procedure
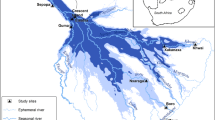
All the glass that was used was washed with non-ionic liquid detergent and rinsed with potable water and later with purified water. The glassware was soaked in 0.1% nitric acid overnight and rinsed with ultra-purified water before being used. Water samples were collected from six different locations with the aid of a stainless-steel grab sampler into air-tightened amber bottles on the field during sample collections. In situ parameters were carried out on the water samples. The samples were transported to the laboratory on an ice chest. This was then followed by the addition of 0.1% nitric acid to the water samples for preservation. Table 1 contains the summary of the sampling points and the activities taking place, while Fig. 1 shows the map of the study area which translated the sampling points into pictorial form.
2.2 Chemical and reagents
All reagents used were of analytical grades, and they were purchased from Merck in Germany, while Milli Q-water with conductivity less than 0.05 µs/cm was used for reagent preparation throughout the analysis.
2.3 Instrumentation
A Q-block automatic digester was used for the digestion of the water sample. An Agilent ICP-OES 4200 was used for the determination of heavy metals in the water samples. The Elmasonic P sonicator made from Germany was used in the course of sonication.
2.4 Quality assurance
To assure the accuracy of the data generated, a triplicate analysis of the samples was carried out. The blank samples were analysed at every ten determinations. A quality control standard was used to verify the accuracy of the machine.
2.5 Recovery study
Certified IAEA-SL-1 lake sediment was used for the recovery study of the method by weighing about 500 mg of the sediment and digested for total metal by aqua regia. The recovery values in the range of 85 to 110% were obtained for Cr, Cu, Ni, Pb, Al, Si, Fe, Cd, Sn with coefficients of variation less than 10%.
2.6 Statistical analysis of data
A series of statistical analyses have been applied by many researchers to analyse the results obtained from the analysis of metal based on the available software, such as SAS statistical package, Pearson’s correlation coefficient [3], and one-way analysis of variance (ANOVA) at P < 0.05. However, the results obtained during this study were analysed using statistical R-studio for the principal component analysis (PCAs) and one-way ANOVA [23] correlation of the physico-chemical analysis.
2.7 Sample preparation
Sample preparation was done in accordance with the method adopted by other researchers with modifications [2].
About 25 ml of the water samples was weighed into a 50 ml digester tube. 10 ml of concentrated nitric acid was added and placed inside an automatic digester for the digestion process.
The sample was heated and allowed to stay for about one hour inside the digester until the brown fume started to change to almost white. The digester machine was stopped, and the sample was allowed to cool and later made up to 25 ml mark with purified water. The sample was filtered through Whatman no 42 filter paper, and the clear filtrate was ready for analysis of heavy metals.
The standards of various concentrations for the metals of interest were prepared and run to establish linearity after which the samples were run. The amount of the metals present in the samples was calculated automatically by the machine.
3 Results and discussion
3.1 Lead
Lead has been classified by the United States Environmental Protection Agency [24] as potentially hazardous to aquatic organisms as well as human beings due to its neurological disorder and other toxic effects. In this study, the average concentration of lead varied from 2.55–5.85 mg/L in the dry season, while that of the wet season ranges from 2.40–4.46 mg/L as contained in Table 2, and the higher value was recorded in the dry season than in the wet season with the highest concentration detected in location E. This high concentration could be a result of effluent discharged containing metallic materials into the lagoon in addition to leaded fuel being used by boats and ferries on Lagos water [25, 26]. The concentration of lead detected in both seasons is above the WHO/USEPA limit (0.05 mg/L) for water. The higher value of lead detected during both seasons agreed with other researchers.
3.2 Cadmium
Cadmium has been recognized as one of the metals on the priority list by EU directives due to its toxic effect on marine organisms and human beings even at low concentrations and its possible bioaccumulation by aquatic organisms [27, 28]. The concentration of cadmium detected ranges from 0.03 – 0.15 mg/L with location C having the highest concentration. The concentrations of cadmium measured during this study were lower than what was reported on the Awash River in Ethiopia [4]. However, the presence of cadmium in harbour water could be a result of atmospheric deposition of non-ferrous metals in addition to domestic waste discharged into the harbour from different point sources and also industrial discharge that contained used batteries and dye from the Apapa industrial region down to the lagoon [28].
3.3 Chromium
The diverse occurrences of chromium ions in the marine environment play a significant role in the toxicity of the metal [29]. The chromium concentration in the lagoon water varied from N.D–0.9 mg/L at an average of 0.05 mg/L. The amount of chromium detected in almost all the locations was below the WHO limit (0.1 mg/L) except in location C which was much higher during the wet season (0.9 mg/L) than the regulatory specification, and studies have reported that a high concentration of chromium in the body can interfere with the iron uptake by haemoglobin [30]. The higher concentration of Cr in this location could be attributed to effluent-containing electroplating material [31].
3.4 Copper
Copper is the third most used metal in the world because of its economic and industrial importance [32]. It remains an important microelement needed in the growth of plants and animals as well as haemoglobin fortification in human beings. The variation of copper during the seasons showed that a higher concentration of copper was measured during the dry season than in the rainy season at an average of 5.97 mg/L. The higher values were recorded across the locations.
3.5 Iron
Iron occurs mainly as a ferric or ferrous state which is an essential trace metal with significant metabolic activities in the body system [24] and requires a certain amount of water. Iron is an essential heavy metal that plays a vital role in the growth and metabolism of a living organism. The deficiency of iron in the body can lead to diseases, while the excess of it in the aquatic environment could be dangerous to marine organisms as well as human beings [33, 34]. The concentration of iron measured during this study on Lagos harbour was higher in the dry season than wet season which ranged between 6.53 and 13.6 mg/L with location D having the highest concentration. The higher value recorded in this location could be a result of industrial inputs from Amuwo odofin/Apapa that discharged their partially treated or untreated waste into the lagoon in addition to metallic waste from shipping activities. The average concentration of Fe measured across all the locations is much higher than the WHO limit (0.3 mg/L). A recent study on Lagos lagoon had shown the presence of a higher amount of iron during the dry season [35]
3.6 Nickel
This is one of the non-essential elements which can form complexes in an aquatic environment with organic and inorganic materials which can absorb directly into clay particles or lagoon sediment [36]. The possibility of nickel co-precipitate with some anions and cations at pH greater than 5 as reported in the WHO peer review document can redistribute itself into the water phase [36,37,38]. Furthermore, the presence of nickel in Lagos lagoon water could be traceable to metallic scrap from the welding and battery industry situated around the harbour which discharges their waste into the lagoon.
3.7 Aluminium and silicon
The average concentration of aluminium ranges from 12.25–36.72 mg/L, while that of silicon ranges from 0.67–2.13 mg/L. The highest concentrations of these elements were recorded in location D. Location D is the extension of the harbour which serves as a reservoir for inputs from aluminium smelting industries located at Apapa. The possible discharge of partially treated waste into the harbour could be a contributing factor [39, 40] Also, there could be a possibility of natural deposition of these metals into the harbour which might have adhered to the lagoon sediment and eventually leached into the water body [41, 42].
3.8 Tin
The concentration of Sn ranges from 0.67–2.13 mg/L. Again, the highest concentration of Sn has been detected in location D. The detection of Sn across all the locations is an indication of the prevalence of the use of antifouling chemicals used in shipping activities [3, 43]. The metallic tin is not toxic, but when it is present in organic species, it becomes toxic, especially tri-organotin compounds, which are the basis of organotin compounds in aquatic environments [44].
3.9 Effect of pH and temperature on lagoon system
The pH of a medium is the level of acidity or alkalinity of the medium [45]. The pH of the lagoon is an important factor that affects the solubility and dispersibility of metals in the harbour.
The result of the physico-chemical analysis according to Fig. 2 showed that the pH of the Lagos harbour ranges from 6.2 – 6.60 during the wet season, while it was from 6.7–7.20 during the dry season. The highest pH value was recorded in location E. The pH of the harbour during this study is relatively neutral as this might not have a significant effect on marine organisms [46, 47]. The result of the pH values falls within the WHO standard for the level of acidity or alkalinity of the Lagoon water (6.5–8.5) [36].
The temperature is another important parameter which has a serious impact on the marine environment [48]. The mean temperature range during this study was from 24–30 °C. The results obtained according to Fig. 3 showed that the temperature recorded during the dry season is slightly higher than that obtained during the rainy season [49]. This could be a result of interaction between surface water and atmospheric temperature, which is usually high during the dry season, and this can lead to intense evaporation of the water [50]. Also, there could be possible mixing of surface water with underlying lagoon water, thereby leading to uniformity of the entire water column and consequently leading to higher temperatures during the dry season. Similar results have been reported in the works of the literature [50, 51]. These results were also in agreement with the range of temperature recorded by Oyeleke and his co-workers (27–30 °C) that worked on Lagos lagoon. However, the measured temperature was slightly higher than what was recorded by Adedayo and his co-researchers that worked on Lagos lagoon [52], but lower than the temperature recorded on Bonny island water in River State, Nigeria (32.2 °C ± 0.03) [16].
3.10 Effect of electrical conductivity, total dissolved solid (TDS), and salinity
The conductivity of water is a measure of its ability to carry electric current as a result of various ions in the water medium [45]. The effect of the conductivity of a medium is reflected in the salinity and the level of total dissolved solids of the medium.
The range of results obtained for conductivity, TDS, and salinity during the dry season is (1.95–72.52 × 103 µs/cm), (1.02–38.08 × 103 mg/L), and (15.21–75.1 mg/L), respectively. Location D has the highest value, while the results obtained during the wet season are (1.86–42.52 × 103 µs/cm), (0.93–24.62 × 103 mg/L), and (10.50–22.80 mg/L), respectively. The results obtained during the dry season are higher than that observed during the wet season as shown in Fig. 4; this showed that there is a significant difference at (P < 0.05) in seasonal variation and this could be a result of the increase in the levels of cations and anions due to possible evaporation in lagoon water which tends to increase the concentration of ions present in marine environments [50]. A similar result was observed by other researchers on marine water. Also, the higher values of electrical conductivities during the dry season could be a result of run-off of loaded potential toxic metals and other ions from chemical industries situated within the Apapa area of Lagos, which consequently led to higher total dissolved solids and salinity during the dry season as contained in Figs. 5 and 6, respectively [50].
3.10.1 Pearson’s correlation coefficient
The results of the Pearson’s correlation in Table 3 showed the interactions among the variables There is a strong positive correlation between pH and electrical conductivity EC, r = 0.96. Also, a significant correlation occurred between total dissolved solids (TDS and EC, r = 0.99) [41]. This strong correlation between TDS and EC follows the trend of regression analysis models for water quality parameters reported by other researchers [53]. The result of regression analysis for TDS and salinity showed a strong correlation between the two variables, r = 0.90, which is in agreement with what has been reported in the literature [54, 55]. However, a weak correlation between EC and TDS on river Benue in Makurdi, Nigeria, was reported by researchers (TDS, EC, r = 0.34) [56]. Furthermore, weak correlation occurred between the pH and DO, r = 0.48, while a negative correlation exists between DO and EC, r = −0.51. A similar pattern of correlations was observed by other researchers that worked on variations of physico-chemical parameters in the Lagoon [4].
3.10.2 Principal component analysis (PCA)
The statistical R-studio was used to perform PCA of the heavy metal of the data obtained during the study. The principal component analysis was utilized to identify the origin of heavy metals in water and was shown to be an efficient tool to define anthropogenic sources of heavy metals. The result of PCA for heavy metal contents in water is shown in Fig. 7. The first two component factors were extracted from the plot. As a consequence, heavy metals can be grouped into 2 components in which component 1 explained approximately 54.9% of all the data variation. Potential toxic metals such as Pb, Cd, Fe, Ni, and Cu with high coefficient values are highly correlated, and this showed that these metals would most likely originate from industrial and domestic discharges that contained these heavy metals [39]. The second component (PCA 2) accounts for about 20% variance and that Si, Al, Cr, Sn variation in the heavy metal concentration in water in location D, Si, Al and Cr are highly correlated and concentrated in that location, which suggests that these metals could originate from natural sources, while the Sn contaminant could be from ship repairing and other anthropogenic sources [57].
3.10.3 Cluster analysis (CA) on potentially toxic elements in Lagos lagoon water
Cluster analysis was performed based on squared Euclidean distance and Ward’s linkage method in line with some researchers [2, 58]. From Fig. 8, two cluster plots with different geochemical characteristics can be identified. The first plot tetragonally grouped Cu, Pb, Cd, Si, Ni, Cr, and Sn. This pattern of grouping indicated that Cu, Cr, and Ni could originate from the same source point, which is likely to be from industrial inputs from neighbouring companies around the harbour [59]. Also, Pb and Sn could be as a result of boating activities, which is a usual practice around the Lagos harbour that uses leaded gasoline as a fuel source which might get absorbed onto the lagoon sediment and eventually remobilized into the water compartment during dredging and other physico-chemical processes [42]. The presence of Si in Lagoon water could be attributed to the geogenic nature of the element [60]. The second cluster that grouped Fe and Al showed that apart from anthropogenic activities that involved painting and welding within the harbour that generate these two metals [2, 25, 28], these metals could also be attributed to their geochemical natural abundance. [61,62,63]. The dendrogram in Fig. 9 also showed the hierarchical interaction among these metals.
3.10.4 Risk assessment
Human risk assessment comprises the determination of the type and extent of adverse effects on human health of prolonged exposure to toxic contaminants [64]. The health risk of potentially toxic elements is usually based on the estimation of potentially carcinogenic and non-carcinogenic analysis. The carcinogenic and non-carcinogenic health risk assessment based on oral and dermal exposure routes was calculated according to equations described by USEPA risk assessment methods [12, 65].
3.10.5 Non-carcinogenic risk equations
Oral route:
Dermal route:
3.10.6 Carcinogenic risk equations
Oral route:
Dermal route:
where IR, EF, ED, EV, BW, SA, AT, and t event are defined in Table 4 [76, 77].
HQo and HQD are hazard quotients (non-carcinogenic risk values) for oral and dermal ingestion, respectively, while Risko and RiskD represent the carcinogenic risk values for oral and dermal routes, respectively. CW is the mean concentration of potentially toxic metals measured in Lagos harbour water. ABSGiis the gastrointestinal absorption fraction, SFo is the oral slope factor, RFDo is the oral reference dose, and Kp is the permeability coefficient. Table 4 indicates the default parameters for the exposure route assessment of toxic contaminants, while Tables 5 and 6 show the default parameters for cancer toxicity data and non-cancer toxicity data of chemicals, respectively.
3.10.7 Non-carcinogenic risk
From the results in Table 7 of four potential toxic metals that were risk assessed, the non-carcinogenic risk values (hazard quotient) measured for Cd, Cr, and Pb were greater than 1 based on the estimated daily intake via oral exposure, this is an indication of possible health risk in both the children and adults that might engage in activities within and along the Lagos harbour [67, 68], and the literature has reported direct severe impact of these three potential toxic metals in vivo body system that could result in cancerous growth over some periods [66]. However, the non-carcinogenic risk assessment carried out by this study showed that dermal exposure to Cd and Cr in both adults and children might not pose a significant health risk because their HQ values were much less than 1 [69].
3.10.8 Carcinogenic risk
The results of the carcinogenic assessment are summarized in Table 7. The risk index is in the order Ni > Pb > Cr > Cd. The risk values through the oral medium from this study for Ni, Pb, and Cr were greater than the daily exposure limit (1 × 10−6 mg/kg/day) as recommended by USEPA 2010. The higher values for these metals are indications of an incremental potential cancer in both children and adults as reported by other researchers [66]. The literature has reported that Pb, Cr, and Ni could interact with selenium, which is an essential element in the body’s metabolism. This interaction can increase in vivo cancer growth which can consequently put both adults and children at risk. [66, 70]. The results also revealed that dermal exposure to Cr could cause an incremental cancer risk in both adults and children as can be seen by their cancer risk values of 3.17 × 10–5 and 1.08 × 10–4 mg/kg/day [64] Also, continuous exposure to lead (Pb) could have serious health implications in the blood of children and adults as reported in works of the literature [64].
Overall, the total non-carcinogenic risk values measured for Cr and Cd in this study were higher in children than adults; this is an indication that there could be more adverse and higher hazardous risk effects of these metals on children than in adults [66, 71]. Hence, children are more vulnerable to these toxic metals, most especially those children involved in fishing and other boating activities along Lagos harbour. The results obtained were in agreement with the study conducted by other researchers about the risk effects of Cd and Cr on adults and children [72]. The toxicity effects of the combined metals (Cr and Cd) on marine organisms have been reported to be severe in the literature as a result of the toxicological pattern of these metals on human being [29, 73].
3.10.9 Discussion of Table 8
The results of PTEs like Al, Fe, Ni, and Pb measured in Hooghly River by Ghosh and his co-workers according to Table 8 exceeded the permissible WHO limits. These higher concentrations, according to the authors, were attributed to rapid and unplanned urbanization coupled with industries in the neighbourhood of river Hooghly [76]. This trend reflected what Sticker and Lyon carried on a similar river. On comparing these results with the present study, it showed that the concentration of Pb is much greater than that of the present study.
Dharfer and his group of researchers measured six potential toxic metals in the mangrove lagoons of the red sea coastal cities, Saudi Arabia. The average concentrations of PTEs detected in this river were much greater than in the present study as contained in Table 8. The higher values for these measured toxic metals in this river were attributed to an increase in sewage water discharged into the lagoon [31]. Also, the river Jeddah, which is one of the biggest red sea coastal cities in Saudi Arabia, has historical inputs of over two decades of refuse from metallic waste which perhaps might have accumulated down the sediment and later remobilized into the water compartment.
Kortei and his co-researchers detected cadmium and lead in the Pra basin in Ghana as shown in Table 8. The results of these two PTEs measured were below the WHO limits. This indicated that the presence of these metals at this concentration might not pose a significant threat to the quality of the water. However, when the risk assessment of these metals was carried out based on the daily intake exposure (EDI), the total hazard quotient for Cd was greater than 1 in both adults and children and this means that there could be likely adverse effects of prolonged exposure to these toxic metals [72].
Okoro et al. 2016 measured PTEs in Cape Town harbour. The concentrations of those elements measured according to Table 8 were higher than the WHO limits. These higher values, according to the authors, were a result of rapid urbanization in a developing country like South Africa, which has led to the generation of a lot of storms and discharges into the harbour. Cape Town harbour is also one of the busiest harbours in terms of shipping activities which could be responsible for the higher value of the PTEs in the harbour [25].
Don Pedro and his group of researchers studied the distribution and occurrence of some potentially toxic elements (PTEs) in the Lagos lagoon. Significant amounts of these PTEs were measured according to Table 8. The high concentrations of these metals in the Lagos lagoon are attributed to the metal-laden industrial and domestic effluents that enter the lagoon via drainage channels [31], which have led to high pollution rates. Also, the non-degradability of these metals has enhanced the slow rate of desorption of these metals into the lagoon water compartment. Therefore, there could be a high risk associated with the prevalence of these PTEs in the Lagos lagoon. The results obtained by Don Pedro were much higher than in the present study. Furthermore, Adebola and her group of researchers carried out a comparative study about a decade after Don Pedro worked on Lagos lagoon, according to Table 8. The result of their analysis showed that most of the PTEs in Lagos lagoon have been reduced significantly (P < 0.05) compared to what was reported by Don Pedro. These improvements were attributed to enforcement by regulatory bodies like the Federal Environmental Protection Agency (FEPA) and Lagos State Environmental Protection Agency (LASEPA) who monitor and regulate the discharge of industrial effluents [75]
4 Conclusion and recommendation
This research work focused on the assessment and distribution of potentially toxic elements (PTEs) in the Lagos lagoon. This study has significant information about the quality of water collected from Lagos harbour. The major findings of this research showed that some PTEs like lead, cadmium, copper, iron, and nickel were above the WHO limits, and this could pose a great threat to the ecosystem. The discharge of industrial and domestic waste in addition to the use of historical inputs from antifouling activities has contributed greatly to the high concentration of potentially toxic elements in the harbour. Also, the correlation coefficient of the physico-chemical parameters showed the interdependence of these variables. The summary of the comparative analysis of this study with the few studies across the world in Table 8 reflected that PTEs contamination of lagoon waters is a global problem because most of the reported results were much higher than the regulatory limit.
Furthermore, the finding from the risk assessment showed that continuous exposure to some metals (Ni, Cd and Pb) by adults and children through various activities like boating, diving, and fishing could lead to an incremental potential cancer, damage to other organs like brain and lung, because the calculated risk indices of these metals were greater than (1 × 10–6 mg/kg/day) as recommended by USEPA 2010. The findings have reflected the polluted nature of Lagos harbour.
Limitations, the major challenges with this work, were that during the rainy season, the harbour used to be deeper and sampling with the use of grab sampler while on a speed boat was not convenient, in that case, the diver was employed which resulted in extra cost.
In conclusion, this research work has set a template and baseline for the monitoring of other harbours in Nigeria. This is very important because a lot of shipping and boating activities are still going on in Nigeria harbour which could generate toxic elements. This research work has provided evidence of the presence of organotin compounds and their associated metabolites in the harbour which predominantly resulted from anthropogenic activities. In addition, this research has provided novel quantitative data on nine potentially toxic elements in Lagos harbour which have not been previously studied by any authors on the Nigeria harbour system. Also, the risk assessment study has provided evidence of environmental contamination which could lead to marine extinction if not properly managed. This study will help in facilitating effective approaches for the management of lagoons in Nigeria to meet up with the environmental sustainability of the Millennium Development Goals (MDGs).
It is therefore recommended that there should be public enlightenment among the stakeholders. Also, the combination of close monitoring and improvement on legislative regulations within and around the Lagos lagoon is recommended. Further research should be tailored to the areas of eco-friendly remediation of the harbour by the use of highly selective and innovative composite nanomaterials, photocatalytic-based treatment for effective adsorption of diverse toxic metal ions from the Lagoon system to maintain environmental sustainability as well as safeguard the health of the populace.
References
Nijole RN, Galina GB, Galina L, Kestulis J, Algirda S, Vitalijus M, Ruta B (2018) Distribution of metals and extent of contamination in sediment from the south- eastern Baltic sea (Lithuanian Zone). Oceanologia 60:193–206
Basheeru KA, Okoro HK, Adekola FA, Abdus-Salam N (2021) Mobility and sequential extraction of potentially toxic elements in sediment of Lagos Lagoon. Chem Afr 4:411–427. https://doi.org/10.1007/s42250-020-00218-4
Okoro HK, Hadizat A (2016) Speciation and determination of priority metals in sediments of oyun river, Ilorin, Kwara Nigeria. Bull Chem Soc Ethiop 30(2):199–208
Eliku T, Leta S (2018) Spatial and seasonal variation on physico –chemical parameters and heavy metals in Awash River Ethiopia. Appl Water Sci 8:177. https://doi.org/10.1007/s13201-018-0803-e
Adekunle AS (2012) Removal of heavy metals from industrial effluents by water hyacinth (Eichornia crassipes). J Environ Chem Toxicol 4:11. https://doi.org/10.5897/JECE12.037
Adebanjo JA, Adedeji WO (2019) Studies on heavy metals contents of Osun River at the pre-urban settlement and across Osogbo City, Nigeria. J Taibah Univ Sci 13(1):318–323. https://doi.org/10.1080/16583655.2019.1567899
Olmedo P, Pla A, Hernández AF, Barbier F, Ayouni L, Gil F (2013) Determination of toxic elements (mer-cury, cadmium, lead, tin and arsenic) in fish and shellfish samples. Risk assessment for the consumers. Environ Int 59:63–72
Islam SM, Ahmed M, Raknuzzaman M, Al Mamun MH (2015) Heavy metal pollution in surface water and sediment: a preliminary assessment of an urban river in a developing country. Ecol Indic 48:282–291
Martin J, Arana C, Ramos-Miras J (2015) Impact of 70 years urban growth associated with heavy metal pollution. Environ Pollut 196:156–163
Genc TO, Yilmaz F (2018) Heavy metals content in water, sediment, and fish (Mugil cephalus) from Koycegiz lagoon system in Turkey: approaches for assessing environ-mental and health risk. J Agric Sci Technol 20(1):71–82
UN: United Nation Millennium Development Goals MDGs report (2015) Environmental sustainability. MDGs Number 7:53
USEPA: United State Environmental Protection Agency (2013) Drinking water contaminants, national primary drinking water regulations available at. http:// water.epa.gov/drink/contaminants/index.cfm# Primary
Refaat FA, Mostafa MA, Hosam MS (2019) Selective and sensitive determination of Cadmium (II) ions in various samples using a novel modified carbon paste electrode. J Anal Sci Technol. https://doi.org/10.1186/s40543-019-0166-4
Aminul IMD, Rabiu AMD, Michael JA (2019) A reveiew on Nickel (II) adsorption in single and binary component system and future path. J Environ Chem Eng 7:103305. https://doi.org/10.1016/J.ece.2019.103305
Aminul IMD, Michael JA, David WM, Biplob KB, Rabiul AMD (2020) A Mechanistic approach of Chromium (VI) adsorption onto manganese oxides and boehmite. J Environ Chem Eng 8:103515. https://doi.org/10.1016/j.ece.2019.103515
Rezk RA, Abdel-Salam Z, Abdel-Ganiy NA, Abdel-Kareem M, Abdel-Harith M (2022) LIBS and PXRF validation for the removal of Pb by bio-CaCo3 nano particles from contaminated water. SN Appl Sci 4:151. https://doi.org/10.1007/s42452-022-05014
Rabiul AMD (2016) Solid phase sensitive Palladium (II) ions detection and recovery using ligand based efficient conjugate nano materials. Chem Eng J 300:264–272. https://doi.org/10.1016//j.ecj.2016.04.071
Khadiza TMD, ShadSalam MD, Nazmul Hazan AMD, Munjur HMD, Rabiul A (2021) Utilizing an alternative composite material for effective Copper (II) capturing from waste water. J Mol Liq 336:116325. https://doi.org/10.1016/j.molliq.2021.116325
Rabiul AMd, Munjur HMd, Jibran I, Aminul IMd, Aminul I, Shahjala K, Abdullah MA, Mohammed MR (2019) Ligand based sustainable composite material for sensitive Nickel (II) capturing in aqueous media. J Environ Chem Eng 8:103591. https://doi.org/10.1016/j.ece2019.103591
Rabiul AMD (2019) A facile composite material for enhanced Cadmium (II) ion capturing from waste water. J Environ Chem Eng. https://doi.org/10.1016/i.jece2019.103378
Mayara dos Santos C, Reginaldo SF, Ana CRL, Clesia CN, Wander GB, Josue CCS (2021) Evaluation of potentially toxic elements in mundau Lagoon (Maceio, Al-Brazil): a systematic environmental monitoring water and food quality. J Brazil Chem Soc 32(9):1–11
Abiodun OA, Oyeleke PO (2016) Analysis and seasonal distributions of some heavy metals in the sediment of Lagos lagoon using environmental pollution indices. Phys Sci Int J 10(2):1–11
Christopher F, David R, Anne-marie E, Guiomar G (2017) Gas chromatographic approach to evaluate the efficacy of organism degrading microbes. Int J Bioremed Biodegrad 5(1):18–26
Clara C, Antonella N, Laura S (2020) Iron dis orders revisited in hepcidin era. Hematol J 105(2):260
Okoro HK, Fatoki OS, Adekola FA, Ximba BJ, Synman RG (2014) Fractionation, mobility and multivariate statistical evaluation of metals in marine sediments of cape town Harbor South Africa. Chem Speciat Bioavailab 26(3):126–138
Kpee F, Edori OS (2014) Trace metals content in shore crabs (cardisoma guanhumi) from coastal area of Port Harcourt city, river state Nigeria. Achiev Appl Sci Res 6(6):16–21
Ezeji EU, Anyanwu CO, Ukwandu NCD (2019) Evaluation of heavy metals and physicochemical parameters of oil- contaminated soil and water samples from bonny, South- South Nigeria. Arch Curr Res Int 19(2):1–11. https://doi.org/10.9734/ACR/2019/v19i230152
Elhussien ME, Adwok BA (2018) Determination of heavy metals in fish and water of White Nile during watery diarrhea outbreak from June to July, 2017, Gezira Aba-Sudan. Sci J Anal Chem 6(1):1–6
Zbigniew H, Dorota K (2021) Selective removal of heavy metal ions from waters and waste waters using ion exchange method. Ion Exch Technol 8:195
Halina S (2019) The combined effects of Cr (III) supplementation and Iron deficiency on the copper and zinc status in Wista Rats. Biol Trace Elem Res 190:414–424. https://doi.org/10.1007/s12011-018-1568-7
Dhafer AA, Samir GA, Refaat AA, Jorg R, Sabry MS (2021) Assessment of water contamination by potentially toxic elements in mangrove of the Red Sea Saudi Arabia. Environ Geochem Health. https://doi.org/10.1007/s10653-021-00956-5
Mustafa SK, Alsharif MA (2018) Copper (Cu) on essential redox-active transition metal in living system- a review article. Am J Anal Chem 9(1):15–26. https://doi.org/10.4236/ajac.2018.91002
Angelova MG, Marinova TVP, Pogorielov MV, Loboda AN, Kolarova VNN, Bozhinova AN (2014) Trace elements status (Iron, Zinc, Copper, Chromium, Cobalt, and Nickel) in Iron-deficiency Anaemia of children under 3years. Anaemia. https://doi.org/10.1155/2014/718089
Ali H, Khan E, Ilahi I (2019) Environmental chemistry and ecotoxicology of hazardous heavy metals: environmental persistence, toxicity, and bioaccumulation. J Chem 2019:6730305. https://doi.org/10.1155/2019/6730305
Soneye ASO, Abiodun OA, Ayeni AO (2018) Spatial analysis of heavy metals concentration around the Lagos lagoon. Nigeria Savanna J 24(2):106–115
Singh H, Pandey R, Sudhir KS, Shukla DN (2017) Assessment of potentially toxic elements contamination in the sediment of river Ghaghara, a major tributary of the river Ganga in Northern Indian. Appl Water Sci 7:4133–4149. https://doi.org/10.1007/sl.3201.017.0672.y
World Health Organization (2021) Draft document of WHO guidelines for Nickel in drinking- water. WHO/HEP/ECH/WSH/2021.6 accessed on. http://apps.w.h.o.int/bookorders
Genchi G, Carocci A, Lauria G, Sinicropi MS, Catalano A (2020) Nickel: human health and environmental toxicology. Int J Environ Res Public Health 17(3):679. https://doi.org/10.3390/ijerph17030679
Richa B, Anshu-Gupta JK (2017) Evaluation of heavy metal contamination using environs metric and indexing approach for river Yamuna, Delhi stretch, India. Water Sci 31:52–66
Mojeed AA, Abiodun OA, Martins AA, Omobola OO (2020) Heavy metals in waste water and sewage sludge from selected municipal treatment plants in eastern cape province. South Afr Water 12:2746. https://doi.org/10.3390/w12102746
Keller C, Mccrath SP, Dunham SJ (2020) Trace metal leaching through a soil- grassland system after sewage sludge application. J Environ Qual 31:1550–1560
Li Q, Zhun JL, Chen B (2014) Toxic metal contamination and distribution in soils and plants of a typical metallurgical industrial area in south west of china. Environ Earth Sci 72:2101–2109. https://doi.org/10.1007//s12665-014-3118-8
Basheeru KA, Okoro HK, Adekola FA, Abdus-Salam N (2020) Speciation & quantification of organotin compounds in Lagos harbor, Nigeria. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1849647
Hoch M (2001) Organotin compounds in the environment. Appl Geochem 7–8:719–743
Opadokun IO, Falaye AE, Ajani EK (2015) Seasonal variation in physiochemical parameters of Lekki Lagoon and the conservation of its ecosystem. J Geosci Environ Prot 3:11–17. https://doi.org/10.4236/sep.2015.39002
Hodabalo DS, Kamilou OS, Gnon T, Badassan TE, Husseini A, Akoete YN, Akouvi MDA, Kissao G (2018) Seasonal & spatial variability of physico-chemical parameters of semi enclosed lagoon system: the complex of Lake Zowla-Aneho Lagoon. Int J Res Environ Stud 5:56–69
Popoola LT, Yusuff AS, Aderibigbe TA (2019) Assessment of natural ground water physico- chemical properties in major industrial and residential locations of Lagos metropolises. Appl Water Sci 9:191. https://doi.org/10.1007/S1320-019-1073-9
Oyeleke PO, Popoola SO, Abiodun OO (2019) Assessment of physico-chemical parameters of Lagos lagoon, south western Nigeria. Acad J Chem 4(3):9–11
Babalola OA, Agbebi FO (2013) Physico-chemical characteristics and water quality assesment from Kuramo Lagoon, Lagos. J Mar Sci Res Dev 3:3. https://doi.org/10.4172/2155-9910.s1.002
Ayah M, Grybros M, Tampo L, Bawa LM, Bril H, Boundjou G (2015) Quality and protection of the waters of a tropical coastal hydro system: case of lagoon system of Lome. Togo Eur Sci J 11(15):95–119
Traone A, Soro G, Kouadio EK, Bamba BS, Oga MS, Soro N, Emi B (2012) Evaluation of the physical, chemical and bacteriological parameters of the waters of a tropical lagoon during a period of low water, The aghien lagoon, (Ivory Coast). Int J Biol Chem Sci 6(6):7048–7058
Ouro-Sama K, Solitoke HD, Tanouayi G, Badassan TE, Ahoudi H, Nyametso AY, Gnandi K (2018) Spatio- temporal variation of the physicochemical parameters of waters from the Hydrosystem Lake, Topo, lagoon of aneno (South East of Togo). J Sci Eng Res 5(6):164–178
Mihir P, Nihir RS, Pankay KR, Malaba RB (2015) Electrical conductivity of Lake water as environmental monitoring- a case study of Rudrasagar lake (IOSR). J Environ Sci Toxicol Food Technol 9(3):66–71. https://doi.org/10.9790/2402-09316671
Bakhtair Jemily NH, Ahmad Saad FN, Mat Amin AR, Othman MF, Mohd’ Yusoff MZ, (2019) Relationship between electrical conductivity and total dissolved solids as water quality parameter in Teluk Lipat by using regression analysis. In: Abubakar M, Mohamad Sidiq M, Ochsner A (eds) Progress in engineering technology advanced structured materials. Springer, Cham
Saleem A, Dandigi M, Kumev KV (2012) Correlation- regression model for physic-chemical quality of ground water in the south Indian city of Gulbarga. Afr J Environ Sci Technol 9:353–364
Akaahan TJA, Olabanji FM, Azua ET (2017) Study on regression analysis between electrical conductivity and total dissolved solids as environmental variables in lower river Benue, Markurdi, Nigeria. Afr J Water Conserv Sustain 5(5):221–226
Vasiliu D, Busce A, Lupascu N, Ispas B, Gheablau C, Stanescu I (2020) Assessment of the metal pollution in surface sediment of coastal Tasaul Lake (Romania)”. Environ Monit Assess 192:749
Chiara F, Livia VA, Monica M, Gilmo V(2013) Speciation of potentially toxic elements at water-sediment interface eqa – environmental quality/Qualité de l’environnement/qualità ambientale 10: 51–64
Wuana RA, Okieimen FE (2011) Heavy metals in contaminated soils: a review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. https://doi.org/10.5402/2011/402647
Giri S, Singh AK (2014) Assesment of surface water quality using heavy metal pollution ndex in Subamarekha River, india. Water Qual Expo Health 5(4):173–182
Ghosh S, Ram SS, Bakshi M, Chakraborty A, Sudarshan M, Chaudhuri P (2016) Vertical and horizontal variation of elemental contamination in sediments of hooghly estuary. India Mar Pollut Bull 109(1):539–549
Shaila S, Zakir HM, Shikazono N (2010) Fractionation profile and mobility pattern of trace metals in sediments of Naomi River, Tokyo, Japan. J Soil Sci Environ Manag 1(11):001–014
Ghosh S, Majunder S, Roychowdhury T (2019) Assessment of the effect of urban pollution on surface water-ground water system of Adi-Ganga, a historical outlet of river Ganga. Chemosphere 237:124507
Mohammadi AA, Zarei A, Majidi S, Ghaderpoury A, Hashempour Y, Saghi MH, Alinejad MY, Hoseingholizadeh N, Ghaderpoori M (2019) Carcinogenic and non-carcinogenic health risk assessment of heavy metals in drinking water of Khorramabad Iran. Methodsx 6:1642–1651. https://doi.org/10.1016/j.mex2019.07.017
USEPA: United State Environmental Protection Agency (2004) Risk assessment guidance for superfund. Human health evaluation manual, Part E supplement guidance for dermal risk assessment, 1, Final EPA/540/99/005
Adewuyi GO, Etchie AT, Etchie TO (2014) Health risk assessment of exposure to metals in a nigerian water supply, human and ecological risk assessment. Int J 20(1):29–44
Mohammadyan M, Moosazadeh M, Borji A, Khanjani N, Rahimi-Moghadam S (2019) Investigation of occupational exposure to lead and its relation with blood lead levels in electrical soldiers. Environ Monit Assess 191:126. https://doi.org/10.1007/s1066-1066019-7258-x
Ezemonye LI, Adebayo PO, Enuneku AA, Tongo I, Ogbomida E (2019) Potential health risk consequences of heavy metal concentrations in surface water, shrimp (Macrobrachium macrobrachion) and fish (Brycinus longipinnis) from Benin river. Nigeria Toxicol Rep 6:1–9. https://doi.org/10.1016/j.toxrep.2018.11.010
Koki IB, Bayero AM, Umar A, Yusuf S (2015) Health risk assessment of heavy metals in water, air, soil and fish. Afr J Pure Appl Chem 9(11):204–210. https://doi.org/10.5897/AJPAC2015.0654
Salihu N, Yan M, Baband A (2019) Heavy metals, concentration and human health risk assessment in grand water and table water sold in tundun murtals area, nassarawa local area, kano state, Nigeria. J Appl Sci Environ Manag 23(8):1445–1448
Obibri S, Essumans DK, Armah FA (2001) Cancer and non-cancer risk assessment from exposure to arsenic, copper and cadmium in borehole, tap and surface water in the Obu Asi municipality Ghana. Hum Ecol Risk Assess 16(3):651–665
Oguguah NM, Ikegwu OJ (2017) Concentration and human health implications of trace metals in fish of economic importance in Lagos lagoon Nigeria. J Health Pollut 7:13
Kortei NK, Heymann ME, Essuman EK, Kpodo FM, Lokpo APT, SY, Boadi NO, Amonor MA, (2020) Health risk assessment and levels of toxic metals on fishes (oreochronis noliticus and Claria anguillaris) from Ankobrah and Pra basins: impact of Illegal minning activities on food safety. Toxicol Rep 7:360–369. https://doi.org/10.1016/j.toxrep.2020.011
Adebola BAK, Kayode SA, Akeem OA (2017) Intergrated assessment of heavy metal pollution status and potential ecological risk in Lagos lagoon South West Nigeria. Hum Ecol Risk Assess Taylor Francis Int J. https://doi.org/10.1080/10807039.2017.1384694
Don Pedro KN, Oyewo EO, Otitoluju AA (2004) Trend of heavy metal concentration in Lagos lagoon ecosystem, Nigeria. West Afr J Appl Ecol 5:103–114
Ghosh S, Bakshi M, Mahanty S, Gaine T, Bhattacharyya S, Biswas JK, Chaudhuri P (2021) Spatiotemporal distribution of potentially toxic elements in the lower gangetic delta and their implications for non-carcinogenic health risk management. Geosci Lett 8:19. https://doi.org/10.1186/s40562-021-00189-5
Acknowledgements
I thank the management and staff of Nigeria Naval Dockyard for granting us permission and logistics for the collection of samples. Appreciation also goes to Prof. Wellington Oyibo (The Chairman DK Olukoya Central Research and Reference Laboratories, University of Lagos, Akoka, Lagos. The corresponding author, Dr Kazeem Basheeru, and other co-authors thank the University of Ilorin, Ilorin, Nigeria, and the University of Lagos, Akoka, Nigeria, for making available their respective library database and laboratory facilities. Dr Kazeem Basheeru also thanks the management of the University of Lagos for the support given him to undertake his Doctoral research study.
Funding
No funding was provided for this research.
Author information
Authors and Affiliations
Contributions
Prof. F.A Adekola, the main supervisor, initiated the research project and guidelines, while the Co-supervisor (Prof. Abdus-Salam) consolidated on the technical advice provided by the main supervisor. Dr Kazeem Basheeru prepared the manuscript which was reviewed by the supervisors and Dr Okoro.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no competing financial or personal interest that is directly or indirectly related to the work submitted to this journal for publication.
Ethical approval
This article did not involve the use of animal study or human beings; therefore, it does not contravene any national or international laws.
Open Access
This article is licensed under open access.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Basheeru, K.A., Adekola, F.A., Abdus-Salam, N. et al. Spatio-temporal monitoring of potentially toxic elements in Lagos harbour water and its health risk implications. SN Appl. Sci. 4, 298 (2022). https://doi.org/10.1007/s42452-022-05186-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05186-7