Abstract
In this study we evaluate the extent of degradation of high-density polyethylene by bacterial isolates obtained from landfill. The microorganisms are isolated from plastic wastes deposited in the landfill for 2–3 years and 17 years. Experiments are conducted under laboratory conditions to degrade virgin high-density polyethylene used in the manufacture of packaging materials. Gravimetric and GC–MS analyses are performed to describe polyethylene decomposition. Of all the bacterial isolates tested, the degradation of polyethylene by Bacillus cereus is the highest, 1.78%, based on weight loss. On the other hand, degradation by Pseudomonas tuomurensis is 0.3%. Degradation products are detected, confirming the progressive degradation of the plastic. The hydrocarbons with single and double bonds are observed most frequently. Our study provides insight into the microbial biodegradation of polyethylene in the environment and contributes to the understanding of the biodegradation processes that may occur in landfills and their progress.
Article Highlights
-
Microorganisms isolated from the landfill are capable of high-density polyethylene degradation.
-
The biodegradation of high-density polyethylene is a slow process.
-
Out of degradation products the hydrocarbons with single and double bonds were observed most frequently.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
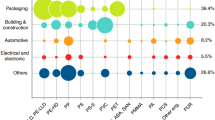
With their durability, weight, flexibility and availability, plastics have taken over the world. Without the ability to use plastics, life would be much more complicated nowadays. By the 1950s, plastic production was negligible. Polyethylene, for example, was discovered in 1933, and the very first synthesis of light density polyethylene was performed in 1935 by Reginald Gibson and Eric Fawcett of the United Kingdom [1]. In 1953 Karl Ziegler of the Kaiser Wilhelm Institute and Erhard Holzkamp invented High Density Polyethylene (HDPE). From there, two years later, in 1955, HDPE was produced as pipe [2]. Ziegler was awarded the 1963 Nobel Prize for Chemistry. Since the 1950s, demand for plastics has increased in all industries [3]. The European plastics industry has grown rapidly, production of plastics in Europe was about 58 million tonnes and the global production reached over 360 million tonnes in 2019 [4]. The largest end-use markets are packaging and construction represented in Fig. 1.
Distribution of global plastic production in 2019, by type; PP-Polypropylene; PE-Polyethylene; PVC-Polyvinyl chloride; PUR-Polyurethane; PET-Polyethylene terephthalate; other plastics: ABS-Acrylonitrile butadiene styrene; PBT-Polybutylene terephthalate; PC-Polycarbonate; PMMA-Polymethyl methacrylate; PTFE-Polytetrafluoroethylene; PS + EPS-PolyStyrene + Expanded PolyStyrene [3], modified
The amount of plastic entering the environment is alarming [3, 4]. Because of its physicochemical resistance and stability, plastics are a threat to all ecosystems. Decomposition complications are caused by additives that can be toxic, especially to marine fauna, where plastic particles spread easily due to their high molecular weight, hydrophobicity and, in some cases, the presence of stable functional groups that are difficult for oxidative processes to access [5]. The fact that various environments are affected also poses a threat to humans, who can ingest contaminants from plastic through their digestive systems [5]. Therefore, there is an interest in developing new effective methods to treat them. According to Geyer [3], about 6 300 million tonnes of plastic were produced in 2015. If the production of plastics remains as high as it is now and waste management is maintained, about 12 000 million tonnes of plastic waste could end up in landfills or in the environment by 2050 [3]. Geyer [3] notes that since 1950, only 9% of plastics used have been adequately recycled and nearly half either end up in landfills or accumulate in the natural environment. In 1960, municipal solid waste contained less than 1% of plastics. This amount increased in 50 years to more than 10% in middle- and high-income countries [3].
The limited reuse of plastic is the reason why new ways of dealing with plastic waste need to be found. One of these ways could be degradation. Degradation can be divided into abiotic (chemical, physical degradation) and biotic (biological degradation—biodegradation) depending on the factors involved [6]. Chemical degradation is based on the use of chemicals that can break a polymer chain and produce non-toxic substances [6]. Physical methods aim to break down and reduce particles through temperature, UV irradiation, crushing, combustion, etc., however, this often produces toxic products [6]. An advantage of this method is that no potentially toxic byproducts or products may be formed [7, 8]. This applies especially to biodegradable plastics, whose wide range of possible applications has been described [9, 10].
In biodegradation, microorganisms [6, 11] play an important role in the transformation and mineralization of various compounds including xenobiotics and other introduced substances due to their high adaptability. Some microorganisms are known to produce enzymes (depolymerase, hydrolase) that can be used for polymer degradation [12, 13]. Biodegradation can occur under both aerobic and anaerobic conditions and can be influenced by many factors, such as the density of the material, thickness, and chemical structure [14]. The higher the molecular weight, the more difficult it is to degrade plastic material [15]. It is also more difficult to degrade thick materials [6, 16]. Amorphous structures appear to be more easily degradable than structures with a regular shape [17]. Biodegradability is also influenced by the surface area of the polymer [18]. For example, pretreatment with UV light has been demonstrated to improve biodegradation [19].
Several phases, including biodeterioration, depolymerization, assimilation, and mineralization form the biodegradation process of plastic waste [20]. During biodeterioration, plastic polymers are modified by microorganisms in cooperation with abiotic factors. The plastic surface is damaged by metabolites and extracellular enzymes released by the microorganisms. The polymers are then degraded by depolymerases to oligomers, dimers and monomers that are more easily processed by microorganisms [21]. These compounds easily pass through the cytoplasmic membranes and can be used as carbon and energy sources [6].
There is increasing information about the degradation of plastics, in the natural environment, which involves microorganisms. However, not much is known about the degradation of plastics stored in landfills over a long period of time. The objective of this study is to evaluate the extent of degradation of high-density polyethylene (HDPE) by bacterial isolates obtained from the landfill waste. These species were selected because they are considered to play an important role in decomposition. The bacterial species were isolated from 2 different landfill layers and their ability to degrade polyethylene particles, the most abundant type of plastic in the landfill, was evaluated using gravimetric method and GC–MS analysis.
In the next section, we discuss the materials and methods used. In Sect. 3, we present the results we obtained in the degradation of plastics under laboratory conditions, including the analyses of the culture medium to find typical products of plastics biodegradation. In Sect. 4, we discuss our results with those of other authors.
2 Materials and methods
2.1 Waste samples collection
Plastic samples were collected in November 2019 at the municipal landfill in Hradčany (Přerov district, Czech Republic). The sampling points are shown in Figure S1. A total of twelve samples were collected from two sampling points characterised by different age of waste (six samples each). The waste at sampling point No. 1 were 2–3 years old and the samples were collected from a depth of 2 m. The waste at sampling point No. 2 was 17 years old, these samples were collected from a depth of 4 m. The wastes were removed from the landfill body using an excavator with a jaw bucket. Approximately 0.5 m3 of the excavated material was moved to an area where samples were collected from the centre of the waste pile to avoid cross contamination with other wastes. The samples of plastics were transferred into sterile glass containers (bottles) using sterile instruments and transported to the laboratory. All samples were processed immediately. Samples from sampling point No. 1 were processed in the laboratory of the T. G. Masaryk Water Research Institute, public research institution, Brno branch (Brno, Czech Republic), and the remaining samples were processed in the Section of Microbiology, Faculty of Science, Masaryk University (Brno, Czech Republic).
2.2 Samples processing
The samples of plastic waste were cut into squares 2 × 2 cm, 1–3 mm thick, smooth edge with different density (food wrap, food packaging) in sterile condition in biological safety cabinets Herasafe KS (Thermo Fisher Scientific, Waltham, Massachusetts, USA). Subsequently g of the plastic waste was transferred into sterile Erlenmayer flasks. These flasks contained 120 ml of sterile physiological saline solution (0.85% NaCl solution). The mixture of medium and plastic pieces was shaken for 120 min. After 30 min of rest, 5 ml of the infusion was aseptically transferred with a pipette into a sterile Erlenmayer flask containing 100 ml of LB broth (10 g of tryptone, 5 g of yeast extract (Oxoid CZ, Brno, Czech Republic), 5 g of NaCl (PENTA s.r.o., Prague, Czech Republic) in 1 L of distilled water). The samples were then cultivated at 30 °C ± 0.5 °C for 72 h.
The suspensions were then cultivated on LB agar (13 g of Nutrient Broth and 20 g of agar (Oxoid CZ, Brno, Czech Republic) in 1 L of distilled water) and Actinomycete Isolation agar (HiMedia, Brno, Czech Republic) at 30 ± 0.5 °C for 48 h. Cross streaks of 26selected colonies were made. Cultivation was performed at 30 ± 0.5 °C for 16–24 h (LB agar) or 26 h (Actinomycete Isolation agar). Gram staining was performed to determine the number, shape, and configuration of the cell wall of all isolated cultures. Cultures were stored in a refrigerator at 5 ± 3 °C until further processing.
2.3 DNA isolation and sequencing
DNA was isolated from pure cultures. The DNeasy UltraClean Microbial Kit (QIAGEN, Hilden, Germany) was used to isolate DNA. The purity of DNA was checked using the NanoDrop 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA). For 16S rRNA amplification, the 515F-Y primer 5′-GTGYCAGCMGCCGCGGTAA-3' [22] and the 806R primer 5'-GGACTACHVGGGTWTCTAAT-3' [23] were used. DNA was amplified by PCR in final volume of 25 µl in a thermocycler Labcycler (SensoQuest GmbH, Göttingen, Germany). Each well contained 1 mM of dNTPs, 3 mM MgCl2, 1 mM of each primer, 1 × PCR buffer, and 1 U of Taq DNA Polymerase, 1 µl isolated DNA. Cycling conditions were 95 °C for 10 min, followed by 30 cycles of incubation at 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 120 s, with a final extension step at 72 °C for 2 min. PCR products were visualised by electrophoresis on 1.5% agarose gel.
Prior to sequencing, purification was performed using the MinElute PCR Purification Kit (QIAGEN, Hilden, Germany). The purity of the amplification products was checked using NanoDrop 2000 (Thermo Fisher Scientific, Waltham, Massachusetts, USA), Table 1.
DNA isolated from the bacterial cells was subjected to DNA sequencing. Sanger sequencing was performed at Eurofins Genomics GmbH, Germany, using cycle sequencing technology (dideoxy chain termination / cycle sequencing) on an ABI 3730XL sequencing machine. The purified DNA was prepared according to the company's requirements. 5 μl of DNA was mixed with 5 μl of primer (5 μM). The obtained aligned DNA sequences were compared using the widely known tool BLAST (blast.ncbi.nlm.nih.gov). The sequences of pure cultures isolated from landfill and sequences of the species from GenBank were compared to clarify their similarity in their genetic information. Searches were performed based on nucleotide sequence comparisons. The genomic sequences are available in GenBank, access No.: ON518098, ON515459, ON514628, ON514627, ON514623, ON514621, ON514615 and ON514594. The assignment of specific species to our isolated pure cultures was based on the highest identity from the GenBank database, Table 1.
2.4 Plastics degradation assay setup
An original apparatus (Fig. 2) was constructed for this experiment. The experiment was performed in sterile, clear 500 ml gas wash bottles according to Drechsel. Air from the central air distribution system flowed through the manifold into the bottles. Two syringe membrane filters, pore size 0.2 µm, diameter 33 mm (Thermo Fisher Scientific, Waltham, Massachusetts, USA) were attached to the tubing to protect the bottles from contamination from the environment. The air did not contain CO2, which was verified using GC Agilent 7890 Series (Agilent, Santa Clara, California, USA). The wash bottles according to Drechsel were dipped in a water bath (two-thirds) with the water temperature 30 ± 0.5 °C.
250 ml of sterile mineral salt medium according to Vimala and Mathew [24], was prepared. 50 ml of the bacterial culture was diluted in the mineral salt medium and 0.8–0.9 g of sterile virgin HDPE film was added. In our study 50 µm HDPE film with a size of 3 × 3 mm were used. Samples of HDPE were obtained from the local company Belt Plast Brno s.r.o., Czech Republic. Before starting the experiment, HDPE was irradiated with UV at a distance of 1 m with a germicidal lamp NBV 2 × 30 S (UNIMED Praha, s.r.o., Vestec, Czech Republic) with a radiation intensity of 3.6 W/m2 for 72 h. Liquid bacterial culture was prepared by inoculating LB broth with culture obtained from municipal landfill. The solution was incubated at 30 °C for 24 h. After incubation, the liquid bacterial culture was centrifuged (5,000 rpm) in sterile Falcon tubes for 10 min. Then the cells were washed with sterile Tris–HCl buffer (6.05 g Trizma® base, Sigma, USA, in 1 l of distilled water) and resuspended in mineral salt medium. Optical density was measured to check the turbidity of all samples. Fifty millilitres of this suspension were placed in Drechsel wash bottles. The experiment lasted for 30 days. During the experiment, the sterile medium sometimes had to be replenished in a laminar box to prevent contamination of the bottles. This was probably caused by medium´s evaporation. On the last day of the experiment, the residual media and HDPE samples were kept for further analysis by gas chromatography–mass spectrometry (GC–MS) to detect intermediates of biodegradation. In each experiment control flask containing only medium and HDPE was included.
2.5 Plastics dry weight determination
After the incubation period, the bottles were opened and the contents were filtered through a grade 41 cellulose filter paper with a diameter of 150 mm (Whatman, Little Chalfont, United Kingdom). After drying, HDPE particles were washed in 2% sodium dodecyl sulfate solution and then again in distilled water. Then, HDPE particles were left on a paper in a laboratory dryer ED-S 115 (BINDER GmbH, Tuttlingen, Germany) at 60 °C for 24 h and reweighted. The dry weight of the plastic before and after the incubation period was compared. An analytical balance APX-200 (Denver Instrument GmbH, Göttingen, Germany) with a readability of 0.1 mg was used. The dry weight loss was determined according to Eq. (1) and expressed as a percentage of the dry weight loss of the plastic:
where DW0-initial plastic sample dry weight (g). DW1-dry weight at the end of the incubation period (g).
2.6 GC–MS analysis of biodegradation intermediates
Samples were processed according to the method described by Park and Kim [25]. For analysis of organic matter developed around the surface of the HDPE during the test, 50 mg of PE and 10 ml of CHCl3 were mixed in a 60 ml glass vial. Each vial containing a sample was placed in an ultrasonic bath (37–42 kHz). The ultrasonic treatment lasted for two hours at 55 °C. The residue from HDPE was filtered through grade 41, 150 mm diameter cellulose filters (Whatman, Little Chalfont, United Kingdom) into an evaporation flask. The solution was then concentrated with nitrogen gas and heated to 40 °C. One millilitre of CHCl3 was added to the evaporation flask and the solution was quantitatively transferred into a new glass vial.
For the analysis of biodegradation products in the mineral salt medium, 20 ml of the medium was extracted in 10 ml of CHCl3 after the experiment. The samples were shaken using a shaker for 30 min. CHCl3 was then transferred to a new vial using a glass Pasteur pipette. Another 10 ml of CHCl3 was added into the sample and the mixture was shaken again for 30 min. The second portion of CHCl3 was transferred to the vial containing the first portion. This volume of CHCl3 was then dried with dehydrated Na2SO4 and filtered through cotton wool to an evaporation flask. Concentration was performed using nitrogen gas and heat as mentioned above [24]. The treated samples were analyzed using GC–MS.
The analysis was performed using Pegasus 4D gas chromatograph and mass spectroscopy instrument (LECO Corporation, USA). The LECO instrument used with the time of flight analyzer has very good quality of the measured spectra and sensitivity that corresponds to the SIM mode of the quadrupole. The software also includes high-quality data deconvolution. Compound identification was based on the value of spectra matched to the available library, as well as manual comparison of mass spectra and retention times between individual samples.
Ultrapure helium was used as carrier gas (flow: 1 ml/min) with an injection of 1 µl in splitless mode. Heating programme: 1 min at 50 °C, followed by a continuous increase of 10 °C/1 min to 340 °C. This temperature was maintained for 15 min. The injector temperature was 280 °C and the transfer line was heated to the same temperature. The range of molecular mass in the MS method was 50–600. The measurement rate was 10 spectra per 1 s, the detector voltage was set to 1800 V, and the temperature was set to 250 °C. The analysis was performed using the spectra library NIST MS Search 2.0.
Blank samples were also prepared to verify the method. Blank samples were prepared: one blank with solvent (CHCl3) and one blank with untreated HDPE. All blank samples were processed in the same way as the other samples. Substances present in the blank samples were considered irrelevant for our purposes and were not included.
2.7 Statistical analysis
The experimental data were used to calculate the basic statistical parameters (M-mean, SD-standard deviation, M ± SD). Plots were generated using the Origin2021b software package (OriginLab Corporation, Northampton, MA, USA).
3 Results
3.1 Bacterial species selection
In this study, plastic waste was sampled at the landfill. Thirty two species were isolated from both sampling points. According to the previously described ability to decompose plastics [26, 27, 30, 32] or other types of resistant material [28, 29], eight species (four species from each sampling point) were selected for the experiments and analysed for their ability to degrade HDPE (Table 2).
3.2 Plastics dry weight loss
At the end of the experiments, the weight loss of HDPE was determined. The highest weight loss was found in the samples containing Bacillus cereus, and the average dry weight loss was 1.78%. The second highest weight loss was found in samples containing Citrobacter koseri, with an average dry weight loss of about 1.31%. In contrast, the lowest dry weight loss was found in samples containing Pseudomonas tuomurensis with an average dry weight loss of 0.34%. In general, samples with species isolated from the 2–3 years old landfill layer (sampling point No. 1) had lower HDPE dry weight loss. The results are summarized in Table 3.
3.3 GC–MS analysis and biodegradation products
A nontarget screening procedure was performed to detect potential biodegradation products. Only compounds with a similarity of 800 or higher were considered. The maximum value 999 represents resemblance of analysed compounds to the substance in a spectra library (NIST MS Search 2.0.).
In total, 442 compounds were identified in extracts. In organic matter around the surface of the HDPE 260 of 442 compounds were identified. In the liquid medium 248 of 442 compounds were identified. However, based on manual control of mass spectra several compounds were excluded based on their probable origin (chlorinated compounds may be formed as a consequence of chloroform application, compounds with iodine and bromine functional groups or polyaromatic hydrocarbons were likely laboratory origin, complicated long structures might be misidentified due to instrument inaccuracy). Compounds that were present in control samples were not taken into consideration as well, since they probably do not represent products of biodegradation. Finally, 212 relevant compounds were identified in both matrices and divided into eleven groups according to their chemical structure (Fig. 3).
The hydrocarbons with single and double bonds were observed most frequently. On the other hand, numerous double bonded hydrocarbons were detected in the organic matter around the surface of HDPE. High amounts of carboxylic acid esters, alcohols and ketones with anhydrides were observed in both matrices. Esters of inorganic acids were detected mainly in media, 2.5 × less in the organic matter around the surface of HDPE. Among the less abundant compounds aldehydes and carboxylic acids were detected. Heterocyclic structures were represented in minority. Aromatic hydrocarbons, amides and amines were rarely detected. The number of chemical compounds observed in both matrices after HDPE degradation with pure bacterial cultures can be found in Fig. 4.
In the samples with Proteus vulgaris, Pseudomonas stutzeri and Jonesia denitrificans analyzed after degradation, the number of compounds from organic matter around the surface of HDPE was higher than that from the medium in which the species were cultivated. The sample with the Pseudomonas tuomurensis and the sample with the Jonesia denitrificans contained the highest number of compounds (both more than 100 compounds), in contrast, the lowest number of compounds (17 compounds) was detected in the sample containing Enterobacter hormaechei.
The overview of the groups of compounds found in the organic matter around the surface of HDPE and in the medium after degradation with all tested species is shown in Figs. 5 and 6. It is clear that the vast majority of double-bonded hydrocarbons were present in all samples from the organic matter around the surface of HDPE, with only the sample containing the Jonesia denitrificans comprising more alkanes. The second most common structural type in the samples consisted of single-bonded hydrocarbons, but none of these were found in the samples with the Enterobacter hormaechei. Aldehydes, ketones, alcohols, esters, etc. were evenly distributed among the different isolated cultures.
The distribution of chemical compounds in the cultivation mediums varied poorly compared to samples of the organic matter around the surface of HDPE. The most numerous types of compounds in the medium samples were single-bonded hydrocarbons (maximum in the sample with the Jonesia denitrificans), except for the sample with the Pseudomonas tuomurensis with more of double-bonded hydrocarbons (in total 20). Alkenes were the second most abundant structures found in the medium samples. A higher number of all types of compounds were found in the samples of organic matter around the surface of HDPE, but the distribution of compounds appeared to be similar.
In addition, among the detected compounds, dodecanol, heneicosane, and benzoic acid were observed as previously described specific biodegradation products of HDPE. Their occurrence is shown in Table 4.
4 Discussion
Our study focused on the comparison of the ability to degrade HDPE film by species isolated from different layers of the landfill. The results showed that higher weight loss was observed in the samples with species from the sampling point in the older part of the landfill. This could mean that the microorganisms from this sampling point have a higher ability to degrade HDPE and thus can adapt to the landfill environment as they age. The average weight loss of HDPE in the samples with species from the older part was about 1.4%, compared to 0.5% in the samples with species from the younger part. The dry weight loss determined in our study was lower than in most similar studies in recent years [24, 33,34,35]. The range of dry weight loss was described from 2–3% [24] to almost 20% [33] in similar experiments. This difference could have been caused by a different type of polyethylene and slightly different experimental conditions. For example, Jeon et al. [35] used a feeding solution containing glucose and yeast extract every 48 h to maintain microbial activity. Experiment by Kyaw et al. [33] was carried out at 37 °C for up to 120 days, while the biodegradation in the study of Dey et al. [34] was observed after aerobic incubation at 30 °C for 100 days. All above mentioned experiments were done with low-density polyethylene (LDPE). High weight loss was described by Sangeetha et al. [36] even for HDPE biodegradation by bacteria isolated from the plastic waste of coastal regions of India after 30 days incubation at 30 °C. Extremely high weight loss of HDPE strips (up to 60%) after 120 days of incubation of thermophilic bacterial consortia at 55 °C was detected by Skariyachan et al. [37]. Reported results for bacterial consortia were better when compared to the degradation of plastics with pure isolates.
Chemically sensitive polymers have better biodegradability than virgin polymers [38]. Therefore, many additives and pretreatment processes are used to affect their thermal sensitivity and enhanced UV absorption so that the polymers can be more easily broken down into smaller pieces available for degradation by microorganisms [38]. In our study, UV pretreatment of HDPE particles was used. It has been described that UV pretreatment can influence the performance of the experiment as UV light can act as an initiator of oxidation which promotes biodegradation. The study by Vimala and Mathew [24] suggests that UV pretreatment along with the addition of biosurfactants improves the rate of biodegradation. In the study by Kyaw et al. [33], anaerobic incubation was performed in mineral media at 37 °C for 40 days. To improve bacterial attack on the particles of polyethylene, an experiment with the Bacillus amyloliquefaciens was conducted for 2 months at high temperature, with pretreatment enhanced by gamma irradiation. However, the weight loss analysis did not reach more than 3.2% [33].
Other different methods to monitor the degradation rate have been reported, e.g. Fourier transform infrared spectroscopy (FTIR) to detect functional groups on the surface, atomic force microscopy (AFM) to describe mechanical properties, scanning electron microscopy (SEM) to observe surface topography [17, 39]. Molecular mass can also be determined using gel permeation chromatography [40]. The gravimetric method used in this study to determine dry weight loss is very simple and rapid. However, it is highly dependent on the shape and size of the polyethylene particles. The experiment of Skariyachan et al. [37] using a thermophilic consortium derived from cow dung showed that the biodegradation rate of strips was much higher compared to pellets. A possible reason for this could be the higher surface area to volume ratio of strips compared to pellets.
The variability of results in the degradation of polyethylene may also be related to the bacterial species used in the experiments. There is a wide range of bacterial species associated with the biodegradation of plastics. To name a few, genera of Comamonas, Pseudomonas, Rhodococcus, Staphylococcus, Streptomyces, Bacillus, Acinetobacter, Micrococcus have been experimentally shown to degrade polyethylene [36]. Fungi have also been confirmed as polyethylene degrading genera, for example, Aspergillus, Cladosporium, Fusarium, Penicillium or Phanerochaete [41]. The species from our study have also been previously described as plastic degraders. Similar species were used in LDPE degradation experiments by Veethahavya et al. [31]. Biodegradation by bacterial genera Comamonas, Delftia and Stenotrophomonas was observed by Peixoto et al. [30].
The key factor of the experiment is also the chosen cultivation medium, which must not be too nutritious, but nutritious enough to keep the microorganisms in a viable state. Ideally, it should not contain organic carbon so that the microorganisms can use synthetic materials as their sole carbon source. However, a medium without organic carbon proved to be insufficient, as the viability of the microorganisms decreased within a few days of the start of the experiment [42].
Microorganisms attempting to decompose polyethylene particles produce enzymes and coenzymes responsible for the formation of polar carbonyl and hydroxyl bonds that readily destabilize the structure of polyethylene [43]. In agreement with this study, there is a strong possibility that specific compounds with these properties can be formed by microorganisms [44]. Among the detected compounds, hydrocarbons with single and double bonds were the most abundant. Single-bonded hydrocarbons predominated in the media; in contrast, numerous double-bonded hydrocarbons were observed in the organic matter around the surface of HDPE. Large amounts of carboxylic acid esters, alcohols and ketones were detected in both types of material. Esters of inorganic acids were mainly detected in the medium. Aldehydes, carboxylic acids, heterocyclic and aromatic structures, amides and amines were low. Similar polyethylene degradation products were identified by Hakkarainen and Albertsson [44]. The degradation of polyethylene leads to the production of various by-products, which depend on the nature and conditions of the degradation process. For example, Shahnawaz et al. [45] showed the presence of 1-trimethylsilylmethanol, 1,2,3-trimethylbenzene, ethyl-3,5-dimethylbenzene, hexadecanoic acid, 1,4-dimethyl-2-ethylbenzene or 1,2,3,4-tetramethylbenzene. Roy et al. [46] observed oxygenated compounds and unoxidized low molecular weight hydrocarbons after cultivation with a consortium of Bacillus pumilus, Bacillus halodenitrificans and Bacillus cereus with polyethylene particles. Kyaw et al. [33] described the presence of alkanes, fatty acids, substances with ester groups after biodegradation by the Pseudomonas aeruginosa, Pseudomonas putida and Pseudomonas syringae. Other degradation products were identified by Pramila et al. [47] and Mahalakshmi et al. [48]. Among the specific degradation products, heneicosane, benzoic acid and 1-dodecanol were identified in our study [25, 32].
5 Conclusion
Plastics are one of the major contributors to environmental pollution. This is the reason why researchers are working to develop methods to break down plastics in an environmentally friendly degradation. Many microbial species capable of degrading plastics have already been identified.
In our study, eight species isolated from landfill waste were tested for degradation of virgin HDPE. The results of gravimetric and GC–MS analysis showed slow degradation. This could indicate that there are unsuitable conditions for the environmental bacterial species that affect the process of biodegradation. Based on our results, inert plastic material embedded in the landfill body seems to be preserved instead of allowing effective microbial biodegradation.
Further research on the biodegradation of plastic waste deposited deep in landfills should be performed in order to better understand the biodegradation processes by environmental bacterial species at such sites.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Andrady AL, Neal MA (2009) Applications and Societal Benefits of Plastics. Philos Transact R Soc B Biol Sci 364:1977–1984. https://doi.org/10.1098/rstb.2008.0304
Goddard J (2011) A brief history of the development and growth of the corrugated polyethylene pipe industry in North America. J ASTM Int 8(6):102693. https://doi.org/10.1520/JAI102693
Geyer R, Jambeck JR, Law KL (2017) Production, use and fate of all plastics ever made. Sci Adv. https://doi.org/10.1126/sciadv.1700782
Plastics Europe. Plastics—the Facts 2020 In: https://plasticseurope.org/ [online]. [Brussels]: Plastics Europe, November 22, 2021. Available at: https://plasticseurope.org/knowledge-hub/plastics-the-facts-2020/. Accessed 30 August 2022
Li Z, Wei R, Gao M, Ren Y, Yu B, Nie K, Xu H, Liu L (2020) Biodegradation of low-density polyethylene by microbulbifer hydrolyticus IRE-31. J Environ Manage 263:110402. https://doi.org/10.1016/j.jenvman.2020.110402
Moharir RV, Kumar S (2019) Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: a comprehensive review. J Clean Prod 208:65–76. https://doi.org/10.1016/j.jclepro.2018.10.059
Mishra UN, Das S, Kandali R (2020) Bioremediation of synthetic polymers: present and future prospects of plastic biodegradation. Int J Curr Microbiol App Sci 9:1234–1247. https://doi.org/10.20546/ijcmas.2020.912.152
Science Advice for Policy by European Academies (SAPEA) Biodegradability of Plastics in the Open Environment; Science Advice for Policy by European Academies (SAPEA): DE, 2020; ISBN 978-3-9820301-8-0
Narancic T, Cerrone F, Beagan N, O’Connor KE (2020) Recent advances in bioplastics: application and biodegradation. Polymers 12:920. https://doi.org/10.3390/polym12040920
Moshood TD, Nawanir G, Mahmud F, Mohamad F, Ahmad MH, AbdulGhani A (2022) Sustainability of biodegradable plastics: new problem or solution to solve the global plastic pollution? Current Research Green Sustain Chem 5:100273. https://doi.org/10.1016/j.crgsc.2022.100273
Khan S, Nadir S, Shah ZU, Shah AA, Karunarathna SC, Xu J, Khan A, Munir S, Hasan F (2017) Biodegradation of polyester polyurethane by Aspergillus tubingensis. Environ Pollut 225:469–480. https://doi.org/10.1016/j.envpol.2017.03.012
Shah AA, Hasan F, Hameed A, Ahmed S (2008) biological degradation of plastics: a comprehensive review. Biotechnol Adv 26:246–265. https://doi.org/10.1016/j.biotechadv.2007.12.005
Kale SK, Deshmukh AG, Dudhare MS, Patil VB (2015) Microbial degradation of plastic: a review. J biochemical Technol 6(2):952–961
Gu J-D, Ford TE, Mitchell R (2011) Microbiological Corrosion of Metallic Materials In: Uhlig’s Corrosion Handbook; John Wiley & Sons, Ltd, pp. 549–557 ISBN 978-0-470-87286-4.
Palmisano AC, Pettigrew CA (1992) Biodegradability of Plastics. Bioscience 42:680–685. https://doi.org/10.2307/1312174
Otake Y, Kobayashi T, Asabe H, Murakami N, Ono K (1995) Biodegradation of low-density polyethylene, polystyrene, polyvinyl chloride and urea formaldehyde resin buried under soil for over 32 years. J Appl Polym Sci 56:1789–1796. https://doi.org/10.1002/app.1995.070561309
Restrepo-Flórez J-M, Bassi A, Thompson MR (2014) Microbial degradation and deterioration of polyethylene—A Review. Int Biodeterior Biodegrad 88:83–90. https://doi.org/10.1016/j.ibiod.2013.12.014
Donlan RM (2002) Biofilms: microbial life on surfaces. Emerg Infect Dis 8:881–890. https://doi.org/10.3201/eid0809.020063
Pospíšil J, Nešpůrek S (1997) Highlights in chemistry and physics of polymer stabilization. Macromol symp 115(1):143–163
Asiandu AP, Wahyudi A, Sari SW (2020) A review: plastics waste biodegradation using plastics-degrading bacteria. J Environ Treat Tech 9:148–157. https://doi.org/10.47277/JETT/9(1)157
Domb AJ, Kost J, Wiseman DM (1997) Handbook of biodegradable polymers; Harwood academic publishers: Amsterdam, ISBN 978-90-5702-153-4
Parada AE, Needham DM, Fuhrman JA (2016) Every base matters: assessing small subunit rrna primers for marine microbiomes with mock communities, time series and global field samples: primers for marine microbiome studies. Environ Microbiol 18:1403–1414. https://doi.org/10.1111/1462-2920.13023
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI et al (2010) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. https://doi.org/10.1038/nmeth.f.303
Vimala PP, Mathew L (2016) Biodegradation of polyethylene using bacillus subtilis. Procedia Technol 24:232–239. https://doi.org/10.1016/j.protcy.2016.05.031
Park SY, Kim CG (2019) Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 222:527–533. https://doi.org/10.1016/j.chemosphere.2019.01.159
Auta HS, Emenike CU, Fauziah SH (2017) Screening of bacillus strains isolated from Mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ Pollut 231:1552–1559. https://doi.org/10.1016/j.envpol.2017.09.043
Ren L, Men L, Zhang Z, Guan F, Tian J, Wang B, Wang J, Zhang Y, Zhang W (2019) Biodegradation of Polyethylene by Enterobacter Sp. D1 from the Guts of Wax Moth Galleria Mellonella. Int J Environ Research Public Health 16(11):1941. https://doi.org/10.3390/ijerph16111941
Saratale GD, Saratale RG, Chang JS, Govindwar SP (2011) Fixed-Bed decolorization of reactive blue 172 by Proteus Vulgaris NCIM-2027 immobilized on Luffa Cylindrica Sponge. Int Biodeterior Biodegradation 65:494–503. https://doi.org/10.1016/j.ibiod.2011.01.012
Wang L, Gao Y-Z, Zhao H, Xu Y, Zhou N-Y (2019) Biodegradation of 2-Bromonitrobenzene by Pseudomonas Stutzeri ZWLR2-1. Int Biodeterior Biodegradation 138:87–91. https://doi.org/10.1016/j.ibiod.2018.12.008
Peixoto J, Silva LP, Krüger RH (2017) Brazilian Cerrado Soil Reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J Hazard Mater 324:634–644. https://doi.org/10.1016/j.jhazmat.2016.11.037
Veethahavya KS, Rajath BS, Noobia S, Kumar BM (2016) Biodegradation of low density polyethylene in aqueous media. Procedia Environ Sci 35:709–713. https://doi.org/10.1016/j.proenv.2016.07.072
Farzi A, Dehnad A, Shirzad N, Norouzifard F (2017) Biodegradation of high density polyethylene using Streptomyces species. J Coast Life Med 5:474–479. https://doi.org/10.12980/jclm.5.2017J7-94
Kyaw BM, Champakalakshmi R, Sakharkar MK, Lim CS, Sakharkar KR (2012) Biodegradation of low density polythene (LDPE) by Pseudomonas species. Indian J Microbiol 52:411–419. https://doi.org/10.1007/s12088-012-0250-6
Dey AS, Bose H, Mohapatra B, Sar P (2020) Biodegradation of unpretreated low-density polyethylene (LDPE) by Stenotrophomonas Sp. and Achromobacter Sp., isolated from waste dumpsite and drilling fluid. Front Microbiol 11:3095. https://doi.org/10.3389/fmicb.2020.603210
Jeon J-M, Park S-J, Choi T-R, Park J-H, Yang Y-H, Yoon J-J (2021) Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 Isolated from soil grove. Polym Degrad Stab 191:109662. https://doi.org/10.1016/j.polymdegradstab.2021.109662
Sangeetha Devi R, Ramya R, Kannan K, Robert Antony A, Rajesh Kannan V (2019) Investigation of biodegradation potentials of high density polyethylene degrading marine bacteria isolated from the coastal regions of Tamil Nadu. India Mar Pollut Bulletin 138:549–560. https://doi.org/10.1016/j.marpolbul.2018.12.001
Skariyachan S, Setlur AS, Naik SY, Naik AA, Usharani M, Vasist KS (2017) Enhanced biodegradation of low and high-density polyethylene by novel bacterial consortia formulated from plastic-contaminated cow dung under thermophilic conditions. Environ Sci Pollut Res 24:8443–8457. https://doi.org/10.1007/s11356-017-8537-0
Kumar S, Panda AK, Singh RK (2011) A review on tertiary recycling of high-density polyethylene to fuel. Resour Conserv Recycl 55:893–910. https://doi.org/10.1016/j.resconrec.2011.05.005
Gajendiran A, Krishnamoorthy S, Abraham J (2016) Microbial degradation of low-density polyethylene (LDPE) by Aspergillus Clavatus strain JASK1 isolated from landfill soil. 3 Biotech 6(1):52. https://doi.org/10.1007/s13205-016-0394-x
Abrusci C, Pablos JL, Corrales T, López-Marín J, Marín I, Catalina F (2011) Biodegradation of photo-degraded mulching films based on polyethylenes and stearates of calcium and iron as pro-oxidant additives. Int Biodeterior Biodegrad 65:451–459. https://doi.org/10.1016/j.ibiod.2010.10.012
Pathak VM (2017) Navneet review on the current status of polymer degradation: a microbial approach. Bioresour Bioprocess 4:15. https://doi.org/10.1186/s40643-017-0145-9
Ojha N, Pradhan N, Singh S, Barla A, Shrivastava A, Khatua P, Rai V, Bose S (2017) Evaluation of HDPE and LDPE degradation by fungus. Implemented Stat Optim Sci Rep 7:39515. https://doi.org/10.1038/srep39515
Novotný Č, Malachová K, Adamus G, Kwiecień M, Lotti N, Soccio M, Verney V, Fava F (2018) Deterioration of irradiation/high-temperature pretreated, linear low-density polyethylene (LLDPE) by bacillus Amyloliquefaciens. Int Biodeterior Biodegrad 132:259–267. https://doi.org/10.1016/j.ibiod.2018.04.014
Hakkarainen M, Albertsson A-C (2004) Environmental Degradation of Polyethylene In: Albertsson A-C (ed) Long Term Properties of Polyolefins Advances in Polymer Science; Springer Berlin Heidelberg: Berlin, Heidelberg, 2004; Vol. 169, pp. 177–200 ISBN 978-3-540-40769-0
Shahnawaz M, Sangale MK, Ade AB (2016) Bacteria-based polythene degradation products: GC-MS analysis and toxicity testing. Environ Sci Pollut Res 23:10733–10741. https://doi.org/10.1007/s11356-016-6246-8
Roy PK, Titus S, Surekha P, Tulsi E, Deshmukh C, Rajagopal C (2008) Degradation of abiotically aged LDPE films containing pro-oxidant by bacterial consortium. Polym Degrad Stab 93:1917–1922. https://doi.org/10.1016/j.polymdegradstab.2008.07.016
Pramila R, Ramesh K (2011) Biodegradation of low density polyethylene (LDPE) by fungi isolated from marine water—a SEM analysis. Afr J Microbiol Res 5:5013. https://doi.org/10.5897/AJMR11.670
Mahalakshmi V, Siddiq A, Andrew SN (2012) Analysis of polyethylene degrading potentials of microorganisms isolated from compost soil. Int J Pharm Biol Arch 3:1190–1196
Acknowledgements
The authors thank BELT PLAST, s.r.o. for providing samples of virgin polyethylene and SUEZ CZ a.s. for assistance in collecting plastic samples from the landfill. This study was financially supported from Institutional funds for the development of the research organization TGM WRI, p.r.i., within the framework of the internal grant No. 3600.52.26/2020 and 3600.54.04/2021.
Funding
This study was financially supported from Institutional funds for the development of the research organization TGM WRI, p.r.i., within the framework of the internal grant No. 3600.52.26/2020 and 3600.54.04/2021.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by KS, TV, MV, RK, IK and LM. The first draft of the manuscript was written by RK and IK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kopecká, R., Kubínová, I., Sovová, K. et al. Microbial degradation of virgin polyethylene by bacteria isolated from a landfill site. SN Appl. Sci. 4, 302 (2022). https://doi.org/10.1007/s42452-022-05182-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05182-x