Abstract
The harmless disposal and recycling treatment technology of livestock manure has received increasing attention in recent years. In this study, Fe2(SO4)3 was added during anaerobic digestion (AD) of pig manure (PM) to investigate the effects of different doses of Fe2(SO4)3 on biogas yield and heavy metal passivation. The results showed that the highest biogas yield was observed after adding a moderate dose of Fe2(SO4)3 (3%, based on the total solids), while the elevated result was inhibited as the Fe2(SO4)3 dosage increased. The analysis of solid digestate (solid matter remaining after AD) revealed that AD effectively passivated Cu, Zn, and As, which can be further improved with the addition of Fe2(SO4)3. However, the passivated Cd performance during this process was negligible. Furthermore, seed germination index (GI) trial results indicated that Fe2(SO4)3-assisted AD reduced the toxicity of end products to plants. To summarize, AD assisted by the addition of an appropriate amount of Fe2(SO4)3 is feasible to treat PM, and the addition of Fe2(SO4)3 at 3% was the most economic and environmental-friendly. This work could provide useful methods for the control of heavy metal pollution in the soil.
Article highlights
-
Adding 3% dose of Fe2(SO4)3 could increase methane yield by 66.76%.
-
Fe2(SO4)3-assisted AD passivated HMs and reduced their bioavailability.
-
The 3% Fe2(SO4)3-assisted AD significantly reduced the toxicity of end products to plants.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
In recent decades, with the rapid development of modern animal husbandry, the production of livestock manure has increased at an amazing speed worldwide. For example, China, as a relatively large agricultural country, produces 3.8 billion tons of livestock manure every year [1]. Livestock manure is often considered as an organic fertilizer that can improve soil fertility and boost food productivity. Most livestock manure is applied to farmland without proper pretreatment [2]. However, livestock manure in many regions of China contains heavy metals (HMs), such as Cu, Zn, Cd, and As, due to the addition of HMs to feed [3, 4]. The long-term application of manure containing HMs will reduce soil productivity and threaten food security [3]. The large amount of HMs remaining in livestock manure has become one of the main factors limiting the development of its recycling [5]. Therefore, it is critically urgent to develop an effective technology to stabilize HMs in livestock manure to reduce the potential risks of its application in the soil.
Anaerobic digestion (AD) is an effective and low-cost technique for decomposing organic matter through microbial metabolism under anaerobic conditions with the generation of biogas and biomass fertilizer [6], and such technology has been widely used to treat livestock manure. At present, pig manure (PM) is one of the major livestock manures, and its amount was reached 411 million tons per year in China [7]. The release of a huge amount of PM could cause many environmental problems, e.g. water contamination, unpleasant odors, uncontrolled heavy metals release and so on [1]. Hence this study used PM as the raw material and try to optimize the conditions of AD means to reduce the possible risks that introduced by PM.
The efficiency of AD depends on the concentration and activity of microorganisms, which are influenced by many factors, such as trace elements [8]. At present, trace metal elements have been reported to improve the capacity of AD [9]. Compared to other trace elements, iron is an ideal and versatile choice for enhancing methanogenic activities at appropriate dosages [10]. This is because iron participates in various cellular activities of anaerobic organisms and promotes the degradation of volatile fatty acids (VFAs) to methane [11]. A previous study found that FeCl2 (10 mg L−1) could increase cumulative biogas yield by 18.1% and prolong the peak period of biogas yield [12]. It has also been demonstrated that Fe3O4 nanoparticles (20 ~ 100 mg L−1) can effectively promote methanogenesis [13]. Although most studies reported that trace iron-based materials could increase biogas yield, Amen et al. [14] reported the inhibitory effect of adding nanoscale zero-valent iron (50 ~ 100 mg L−1) to AD. Yang et al. [15] also reported the inhibition of methane yield by nanoscale zero-valent iron at all doses (56 ~ 1680 mg L−1) studied. It is evident that the enhancement or inhibition effects of iron-based additives on biogas yield are controversial, thus the dose-dependent effect of iron-based additives on AD should be further revealed. In addition, biomass fertilizer needs to be returned to farmland, so heavy metal passivation should also be taken into account. Previous studies have indicated that AD is an important way to achieve heavy metal passivation in livestock manure. For instance, Liu et al. [16] showed that the addition of Fe3O4/fly ash in the process of AD had good passivation ability for Cu and Zn; Suanon et al. [17] found that nanoscale zero-valent iron also played a role to a certain extent in passivating various HMs (Cd, Co, Cu, Zn, Ni, and Cr). In summary, the addition of iron-based materials to the AD process is of research interest, and whether and how iron-based addition levels affect biogas yield and heavy metal passivation deserve to be explored in detail.
Fe2(SO4)3 is hypothesized to improve the AD process and passivate HMs due to the following reasons: Fe2(SO4)3 is easily dissolved in the neutral liquid phase environment of AD, which can facilitate the release of ionic iron into the liquid digestate (residual liquid after AD). In the meantime, it has a more pronounced stimulating effect on anaerobic microorganisms. Additionally, the proper amount of SO42− can stimulate the activity of sulfate-reducing bacteria, thus promoting the conversion of propionic acid to methane [18]. Moreover, iron can interact with HMs to form stable chemical forms [17], and sulfides which are reduced by SO42− can react with heavy metal ions, and precipitate and passivate HMs [19].
The objectives of this work were to (1) investigate the effects of Fe2(SO4)3 on biogas yield during AD; (2) analyse the changes in the total amount and morphological distribution of Cu, Zn, Cd, and As in solid digestate (solid matter remaining after AD) with or without Fe2(SO4)3 addition and assess the bioavailability of HMs in solid digestate; and (3) evaluate the toxicity of solid digestate and liquid digestate to plants.
2 Materials and methods
2.1 Experimental materials
The raw material used in the experiment was naturally air-dried PM, which was taken from a pig farm in Lujiang County, Anhui Province, China. The manure was thoroughly mixed and stored at a refrigerator at − 20 °C for further use. The inoculum was fresh anaerobic granular sludge taken from Lvhe Environmental Protection Company (Bengbu City, Anhui Province, China). Fe2(SO4)3 of analytical grade was purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). The physicochemical properties of PM and inoculum are shown in Table 1.
2.2 Experimental design
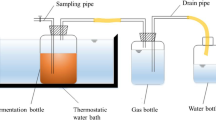
In this work, the roles of Fe2(SO4)3 were to improve the methane yield and metal passivation efficiency. The addition of Fe2(SO4)3 was selected on the basis of iron-based materials dosages which applied to AD according to previous studies [11, 15,16,17]. Therefore, a total of four batch experiments were set: control group (CK) and three treatment groups that corresponding to 0.5% (0.5%-Fe), 3% (3%-Fe), and 9% (9%-Fe) Fe2(SO4)3 addition contents (based on TS). The anaerobic fermenter was a custom made glass vessel with a volume of 4000 mL. A total of 118 g of PM and 500 mL of granular sludge (domesticated in the straw digestion solution in our laboratory for 15 days and filtered through gauze) were added to each fermenter, followed by 2500 mL of water (PM: inoculum = 21: 1 (VS: VS basis)). After feedstocks were added, nitrogen was introduced into the fermenter for 1 min, and then the fermenter was sealed. The AD process was conducted in a constant temperature incubator at a medium temperature of 35.0 ± 1.0 °C for 30 days. Three parallel groups were set up for each experiment. Biogas and methane yield was recorded regularly on a daily basis, and liquid digestate (were centrifuged at 6000 r/min for 20 min, and the supernatants were withdrawn for use) was taken every 3 days to measure VFAs. At the end of digestion, the total and morphological contents of HMs in solid digestate were determined. Solid digestate and liquid digestate were also used as culture substrates for seed germination trials. A schematic diagram of the experimental process is shown in Fig. 1.
2.3 Test equipment and sample measurement
TS was measured by drying to constant weight at 105 °C. VS was measured after searing at 550 °C for 2 h in a muffle furnace. TOC and TN were determined with an elemental analyser (vario MACRO cube, Elementar, Germany). Biogas yield was read by a trace gas flow metre (MGC-1-V3, Ritter, Germany). The methane content was determined by gas chromatography coupled with a thermal conductivity detector (TCD) (GC-2010, Shimadzu, Japan). The contents of VFAs were determined by gas chromatography with a flame ionization detector (FID) (7890A, Agilent, USA). The total amount of HMs was detected by inductively coupled plasma mass spectrometry (ICP-MS) (7700x, Agilent, USA) after digesting samples in a mixture of HNO3/HF/HClO4 (5/5/3, V/V/V) [16]. The morphological amounts of HMs were detected using ICP-MS after extracting the samples by the Tessier five-step method [20]. Thirty lettuce seeds were evenly placed on filter paper moistened with culture solution. The solid digestate culture solution was extracted by maceration of air-dried samples at a solid to liquid ratio of 1:10. The liquid digestate culture solution was obtained by diluting the original liquid digestate 10 times. Seed germination and seedling root length were determined after 60 h of incubation at 20.0 ± 1.0 °C. Seed germination and seedling root length of plants moistened with ultrapure water were also measured and used as the control to calculate the seed germination index (GI) [16, 21].
2.4 Quality assurance and quality control
All the test methods involved in our work were operated in strict accordance with the relevant national standards. The reagents were at least of analytical grade, and the water reached ultrapure levels. All glass instruments used for heavy metal determination were immersed in a solution with a concentration of 10% HNO3 for more than 48 h. To ensure the plausibility of the experimental data, three parallels were set up for each sample, and a blank control group was run to exclude solvent interference. The optimum test concentration of ICP-MS was 10–1000 μg L−1. Cu, Zn, Cd, and As were recovered by the national standard soil substance (GBW07403) with spiked recoveries ranging from 80 to 120%.
2.5 Data processing and statistical analysis
IBM SPSS Statistics 23.0 (SPSS Inc., Chicago, USA) was used to process the data, and significant differences between groups were mainly analysed using one-way analysis of variance (ANOVA) and Tukey's test, with p < 0.05 confirmed as a significant difference. All data graphs were produced using Origin 2018 (Origin Lab Corp., MA, USA) and GraphPad Prism 8.0 (GraphPad Software Inc, CA, USA).
Biogas yield kinetics were analysed by a modified Gompertz model [22], which is expressed as follows:
where y(t) is the cumulative biogas yield at time t (mL g−1 VS); Hm is the potential maximum cumulative biogas yield (mL g−1 VS); Rm is the maximum daily biogas yield rate (mL g−1 VS day−1); λ is the lag phase (d); t is the duration of the assay (d); and e is exp(1) = 2.71828.
The bioavailability of HMs can be assessed by the mobility factor (MF) [23], and the specific formula for MF is as follows:
where F1, F2, F3, F4, and F5 represent five fractions of the exchangeable state, carbonate state, Fe–Mn oxide-bound state, organic combination state, and residual state in Tessier, respectively [20].
GI can provide an intuitive picture of the phytotoxicity of solid digestate and liquid digestate [21]. It can be determined using the following equation:
where SG, SLT, and SLC represent the seed germination, seedling root length of treatment, and seedling root length of the control, respectively.
Energy balance calculation referred to the method of Mohamed Farghali et al. [24]. All weight units in the energy conversion were calculated as VS weight.
3 Results and discussion
3.1 Effects of Fe2(SO4)3 on biogas yield during AD
Adding different doses of Fe2(SO4)3 had a significant effect on biogas yield. As shown in Fig. 2, daily biogas and methane yield of CK and 0.5%-Fe both peaked on the 4th day. The CK group was 36.28 mL g−1 VS and 18.71 mL g−1 VS, and the 0.5%-Fe group was 39.30 mL g−1 VS and 21.49 mL g−1 VS, respectively. However, daily biogas and methane yield of 3%-Fe and 9%-Fe peaked on the 7th day. The 3%-Fe group was 24.94 mL g−1 VS and 15.83 mL g−1 VS, and the 9%-Fe group was 14.78 mL g−1 VS and 6.44 mL g−1 VS, respectively. It can be inferred that high-dose Fe2(SO4)3 will delay peak biogas yield and decrease its level. Suanon et al. [17] added zero-valent iron during AD of sludge and found a similar phenomenon of a delayed biogas yield peak.
The cumulative biogas yield (Fig. 3a) revealed that 3%-Fe had the highest level of 21.46 L, followed by 18.46 L of 0.5%-Fe, which increased by 26.91% and 9.17%, respectively, compared with 16.91 L of CK. The 9%-Fe produced the lowest cumulative biogas yield with 15.81 L, which decreased by 6.51% in comparison with CK. The cumulative methane yield is shown in Fig. 3b, in 0.5%-Fe and 3%-Fe, their individual values were 8.52 L and 11.69 L, which increased by 21.54% and 66.76%, respectively, compared with 7.01 L in CK. In 9%-Fe, its value was 5.59 L, which was 20.26% lower than CK. This was probably because, on the one hand, iron is an essential nutrient for methanogenic bacteria [25], and exogenous iron can provide electrons for the metabolism of methanogenic bacteria and reduce the redox potential in the system, providing a favorable anaerobic environment for methanogenic bacteria [26]. On the other hand, iron increases the activity of enzymes such as hydrolase and methanogenic enzymes, which in turn increases the efficiency of AD [25]. The accumulation of VFAs, especially propionic acid, is a major cause of deteriorating anaerobic systems. Propionic acid accumulation was observed before many AD experiments failed. The proper amount of SO42− can stimulate the activity of sulfate-reducing bacteria, thus promoting the conversion of propionic acid to methane and improving the stability of AD [18]. Therefore, supplementation with an appropriate amount of Fe2(SO4)3 can appreciably stimulate methane yield (p < 0.05). However, more iron-based additives do not represent a better efficiency of AD. 9%-Fe had the lowest cumulative biogas yield, and cumulative methane yield. Because when Fe2(SO4)3 was added at high concentration, excess iron can disrupt the function and structure of enzymes by replacing original metals in the enzyme cofactor, bacteria may be subject to toxic effects [13, 25, 27]. In addition, when SO42− reached a certain concentration, sulfate-reducing bacteria had an advantage over methanogenic bacteria and competed with methanogens for hydrogen and acetic acid, while the sulfide produced by SO42− reduction also inhibited the growth of methanogens [28]. It has been shown that methane yield was restrained when the cumulative mass concentration of SO42− exceeded 5000 mg L−1 [29]. These factors will lead to changes in microbial community structure and organic matter metabolic pathways. Overall, the effect of Fe2(SO4)3 on AD efficiency was promoted at low concentrations and inhibited at high concentrations (p < 0.05). It has been found that adding Fe3O4/fly ash significantly stimulated AD; however, the biogas yield decreased as its addition was increased [16]. A similar conclusion was drawn by Yang et al. [15] about adding nanoscale zero-valent iron during the AD of glucose. Therefore, the amount of Fe2(SO4)3 additive should be considered.
3.2 Kinetic analysis of cumulative biogas and methane yield
To further investigate the performance of Fe2(SO4)3 enhanced methanogenesis, the AD process was analyzed by using of the modified Gompertz model. Both the cumulative biogas and methane yield of each group are fitted well with the modified Gompertz model (R2 > 0.99). The specific parameters obtained from this model are shown in Table 2. The λ is the lag phase, which can reflect the adaptability of microorganisms to the new environment to a certain extent. The smaller the value, the shorter the adaptation time required [30]. The λ for biogas and methane yield in all groups were between 1 and 2 days, indicating that the microorganisms were able to adapt quickly to the digestive environment. In addition, the Hm fitted for the different treatments was close to the experimental cumulative biogas and methane yield. In this model, the 3%-Fe group showed the highest biogas and methane yield rates, reaching 22.13 and 13.64 mL g−1 VS day−1, respectively, and which were 13.08% and 53.25% higher than the CK group. However, both biogas yield and methane yield rates are decreased with the addition of excess Fe2(SO4)3. To summarize, supplementing appropriate amount of Fe2(SO4)3 to AD of PM can improve the digestion quality and enhance the methane yield efficiency.
3.3 Changes of VFAs in liquid digestate
As a class of important intermediates during AD, the variation in VFAs reflects the balance between hydrolytic acidification and methane yield, and can directly evaluate the operation of the AD system [31]. High concentrations of VFAs will lead to a reduction in pH and a significant accumulation of undissociated acids, both of which inhibit the activity of methanogenic bacteria [32]. Furthermore, the accumulation of VFAs will restrain the activity of methyl coenzyme M (COM) reductase, thereby inhibiting the metabolic pathway of methane [25]. Several studies have shown that iron can promote the degradation of excess VFAs [9, 11].
As shown in Fig. 4, on the 4th day, the VFAs concentrations in CK, 0.5%-Fe, and 3%-Fe reached peak values of 6122.24 mg L−1, 4267.29 mg L−1, and 4197.18 mg L−1, respectively. The peak in 9%-Fe occurred on the 7th day with a value of 6413.03 mg L−1. It has been shown that the growth of methanogens is limited at VFAs concentrations exceeding 6000 mg L−1, which has a hindering effect on AD [33]. Xu et al. [32] also revealed that methane yield was restricted at VFAs concentrations of 5800–6900 mg L−1. Combined with Figs. 2 and 3, it can be inferred that a moderate amount of Fe2(SO4)3 is conducive to digesting VFAs by microorganisms, while high-dose Fe2(SO4)3 leads to an excessive accumulation of VFAs and restricts methanogenic bacteria from decomposing organic matter. In the middle and late stages of digestion, the concentration of VFAs decreased and levelled off in each group. This was because the acid production rate decreased with the consumption of materials; accordingly, the amount of methane also decreased.
3.4 Total concentrations of HMs in solid digestate
The addition of Fe2(SO4)3 not only affects the biogas yield characteristics but also affects the distribution of HMs in solid digestate. It has been confirmed that HMs are mainly (about 90%) present in the solid digestate after AD, with only a small proportion present in the liquid digestate [17], therefore, this article only addresses HMs in the solid digestate. As shown in Fig. 5, the total contents of Cu, Zn, Cd, and As in the solid digestate of the four experimental groups decreased by 3.22–39.35%, 17.96–28.60%, 77.07–94.40%, and 53.13–75.02%, respectively, compared with PM. This was probably due to the AD process, which was accompanied by material degradation, posing certain leaching and releasing effects on HMs. Moreover, VFAs had a strong binding ability with HMs, which increased the content of HMs in liquid digestate. In addition, compared with Cu and Zn, the total concentrations of Cd and As decreased more prominently. It has been suggested that Cd and As are more readily dispersed in liquid digestate than Cu and Zn [34].
In treatment groups, the highest concentrations of Cu, Zn, and Cd in the solid digestate occurred in 9%-Fe, reaching 455.38 mg kg−1, 1945.95 mg kg−1, and 2.34 mg kg−1, respectively. Since liquid digestate contains a large amount of active HMs, along with the increase in iron and sulfur content, it is prone to various coprecipitations in a complex anaerobic environment [35]. Thus, it can be tentatively concluded that a high dose of Fe2(SO4)3 has a significantly positive effect on the accumulation of Cu, Zn, and Cd in solid digestate (p < 0.05). Conversely, the high concentration of Fe2(SO4)3 restrained the precipitation of As. This may be because the As compounds were very sensitive to pH value, and the SO42− reduction process produced certain alkaline conditions that caused As compounds to dissolve and release.
3.5 Analysis of the change in heavy metal fractions in solid digestate
The migration and transformation of HMs in the environment are closely correlated with their existing states, and the chemical fractions of HMs warrant more attention than their total amount [36]. The chemical fractions of Cu, Zn, Cd, and As in the solid digestate of different experimental groups are shown in Fig. 6. Each heavy metal had its own unique distribution characteristics: Cu existed mainly in organic matter, and its levels increased from 61.07% (in PM) to 73.81–83.11% (after AD). Zn was mainly distributed in the Fe–Mn oxide-bound state at a level of 64.02%, and was present at levels of 56.28%, 67.12%, 63.03%, and 51.64% in PM, CK, 0.5%-Fe, 3%-Fe, and 9%-Fe, respectively. Cd and As were also mainly present in the Fe–Mn oxide-bound state, and their levels decreased from 61.64% (in PM) to 27.42–33.96% (after AD) and 62.86% (in PM) to 29.94–43.98% (after AD).
When comparing CK with PM, the exchangeable and carbonate states of Cu were notably reduced, and the organic combination state was slightly increased. For Zn, the carbonate state was reduced, while the organic combination state was increased. Since AD decomposed organic matter into strongly adsorbed and highly stable humic substances, they could combine with Cu and Zn to form stable metal–humic substance complexes [36]. For Cd, the exchangeable and carbonate state increased prominently (the latter reached 709.21%), probably because Cd formed weak complexes or soluble chelates with humic substances and had little affinity for solid surfaces, which increased the extractability and mobility of Cd [37]. For As, the residual state was increased, along with the exchangeable and carbonate states, but a passivating effect was still exerted on As.
In an AD environment, Fe2(SO4)3 underwent various biochemical reactions with HMs distributed in liquid and solid digestate, which participated in and promoted the interconversion of heavy metal states. When each treatment group was compared with CK, the most obvious changes in exchangeable and residual states of Cu were observed, with the former decreasing by 73.01–81.62% and the latter increasing by 65.75–194.02%, respectively. For Zn, the exchangeable state decreased by 80.48–84.76%, and the residual state increased by 44.12–118.92%. On the one hand, as reported previously, iron can interact with HMs to form stable chemical forms. In addition, iron can react with water and rapidly form a layer of hydroxyl oxides on the surface of the particles, and the formed oxide layer provides a site for the adsorption and co-precipitation of HMs [17]. On the other hand, during AD, under the action of sulfate-reducing bacteria, it stimulated the reduction of sulfate reduction, and the decomposition of sulfur-containing proteins into S2−, and S2− could react with HMs to produce stable metal sulfides. The strong adsorption of these metal sulfides would further promote the conversion of HMs from the bioavailable state to the stable state [38]. The exchangeable state of As decreased by 65.71–69.82%, and the residual state increased by 12.85–35.59%. The immobilization of As by iron compounds is mainly through the formation of amorphous or crystalline iron (III) arsenate compounds with As or by adsorbing As on the surface of the iron oxyhydroxides [39]. However, the distribution rate of Cd in each state did not change significantly.
3.6 Effect of Fe2(SO4)3 addition on MF value
The value of MF (Fig. 7) can evaluate the bioavailability and reflect the passivation effect of HMs [40], which can further estimate the environmental risks of applying solid digestate as the organic fertilizer to the soil. When comparing CK with PM, the MF values of Cu, Zn, and As decreased by 60.28%, 19.13%, and 13.67%, respectively, suggesting the best passivation effect of Cu. This was because organic matter had a stronger affinity for Cu, suggesting that Cu more easily underwent adsorption and complexation reactions with organic matter, thereby creating states that were not readily available to plants [41]. In contrast, Cd increased by 4.77% because the accumulation of humic substances led to the activation of Cd [37].
When comparing each treatment group with CK, for Cu, the MF value decreased by 10.45–25.65%, with the lowest level occurring in 0.5%-Fe. For Zn, bioavailability decreased the most at 9%-Fe because of the stronger affinity of iron-rich humic acid compared to humic acid. The MF value of As was reduced by 13.40–43.02%, and it was previously found that the application of Fe2(SO4)3 to contaminated soils was effective in reducing As mobility and plant availability [39]. When the four experimental groups were compared with PM, no significant differences were found in the MF value of Cd (p < 0.05). In summary, the addition of Fe2(SO4)3 during AD notably reduced the bioavailability of Cu, Zn, and As, while the effect on Cd was not obvious (p < 0.05).
3.7 Evaluation of methane yield and heavy metal passivation
As shown in Table 3, current research on AD is still focused on biogas yield. All of the following additives have a more or less stimulating effect on AD. The stimulating effect of Fe2(SO4)3 on AD is the most pronounced of these additives, resulting in a 66.76% increase in methane yield. Meanwhile, Fe2(SO4)3 has a passable ability to passivate HMs. Therefore, this method can be considered for the treatment of livestock manure.
3.8 Effects of Fe2(SO4)3 addition on seed GI
The end products of AD were applied to the soil and ultimately manifested themselves in the form of effects on plant growth. The inhibition or toxicity of organic materials was evaluated using the GI ratios, and a higher GI indicated that the substrate had lower bioinhibitive ability. When the GI > 50%, the toxicity of the substrate is at a low level or has fallen to a level that plants can tolerate; if the GI is > 80%, the substrate is completely nontoxic to plants [44].
As shown in Fig. 8a, the GI of the PM group was only 32.68%, indicating that seed germination and growth were strongly curbed. This was due to the presence of HMs, antibiotics, pathogens, and other substances in PM that have inhibitory effects on plant growth. Compared with PM (32.68%), the GI of solid digestate in CK was higher (72.85%) (p < 0.05). This was because AD could reduce the bioavailability of HMs. In addition, as anaerobic microorganisms decompose organic matter, the sorption sites for antibiotics are reduced, which has a degrading effect on some antibiotics [45]. Furthermore, solid digestate contains abundant organic matter, humic acid, nitrogen, phosphorus, potassium, and other nutrients, as well as amino acids, vitamins, enzymes, trace elements, and other important active substances [46]. However, this result is slightly lower than the GI measured using the final product of aerobic composting of pig manure as a substrate (approximately 80%) [47]. The GI of the three groups with Fe2(SO4)3 ranged from 81.57 to 107.59%, which was higher than that of CK and PM. This finding was similar to a previous study in which the addition of Fe3O4/fly ash reduced the toxicity of solid digestate to plants [16]. Meanwhile, liquid digestate is abundant in nutrients and trace elements and effectively stimulates the activity of enzymes in the seeds, thus promoting embryo cell division. The GI of CK liquid digestate reached 98.20%, and the GI of liquid digestate of the three groups with Fe2(SO4)3 ranged from 84.08 to 131.20%, of which 9%-Fe was lower than CK. This may be due to the toxic effects of the sulfate and VFAs remaining in the liquid digestate of this group on the plants (Fig. 8b). The maximum GI of solid digestate and liquid digestate appeared at 3%-Fe. Such results indicated that the addition of an appropriate amount of Fe2(SO4)3 could improve digestion quality and reduce the toxicity of the substrates to plant seeds (p < 0.05).
3.9 Estimation of benefits
In addition, we carried out corresponding economic analysis of the addition of Fe2(SO4)3 in the AD of PM. Results indicated that, with the increase of Fe2(SO4)3 addition, the total cost showed in an increase trend (Table 4). The laboratory-scaled net incomes of CK, 0.5%-Fe, 3%-Fe and 9%-Fe groups were 0.32, 0.36, 0.43, and 0.15 USD/kg VS, respectively. Meanwhile, the environmental benefits also should be considered alongside the economic benefits. The addition of 3% Fe2(SO4)3 can resulting in a net income of 0.43 USD/kg VS, as well as had a potential environmental benefit as it possessed a blunting effect on HMs and showed less toxic effects on seed germination. In the considering of the comprehensive economic and environmental benefits, the optimal dosage of Fe2(SO4)3 was 3% in this current study.
4 Conclusions
The addition of different doses of Fe2(SO4)3 during AD promoted biogas yield at low doses and inhibited it at high doses. 3%-Fe had the best AD efficiency, with increases of 26.93% and 66.76% in cumulative biogas yield and methane yield, respectively. Moreover, AD effectively passivated Cu, Zn, and As, and the addition of Fe2(SO4)3 made this passivation effect more pronounced. However, the passivated Cd performance during this process can be negligible. Furthermore, the addition of Fe2(SO4)3 can reduce the toxicity of the end products of AD to plants. Taking the economic and environmental benefits into account, the optimal dosage of Fe2(SO4)3 was 0.5%. In conclusion, Fe2(SO4)3-assisted AD is feasible to treat PM. These findings provide useful data for the rational treatment of livestock manure. However, there were still some limitations in this study, e.g., the addition of sulfate increased the concentration of hydrogen sulfide; therefore, relative environmental risk assessments are required to be carried out. In addition, consider the prevalently presence of HMs in livestock manure, new HMs passivation methods should be developed and their mechanisms are also need to be elucidated in future studies.
Data availability
All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
References
Luan H, Liu F, Long SD et al (2021) The migration, transformation, and risk assessment of heavy metals in residue and bio-oil obtained by the liquefaction of pig manure. Environ Sci Pollut Res 28:15055–15069. https://doi.org/10.1007/s11356-020-11748-2
Chen ZQ, Fu QQ, Cao YS et al (2021) Effects of lime amendment on the organic substances changes, antibiotics removal, and heavy metals speciation transformation during swine manure composting. Chemosphere 262:128342. https://doi.org/10.1016/j.chemosphere.2020.128342
Yang XP, Li Q, Tang Z et al (2017) Heavy metal concentrations and arsenic speciation in animal manure composts in China. Waste Manage 64:333–339. https://doi.org/10.1016/j.wasman.2017.03.015
Wan YN, Huang QQ, Wang Q et al (2019) Accumulation and bioavailability of heavy metals in an acid soil and their uptake by paddy rice under continuous application of chicken and swine manure. J Hazard Mater 384:121293. https://doi.org/10.1016/j.jhazmat.2019.121293
Wang H, Dong YH, Yang YY et al (2013) Changes in heavy metal contents in animal feeds and manures in an intensive animal production region of China. J Environ Sci 25:2435–2442. https://doi.org/10.1016/S1001-0742(13)60473-8
Zheng XR, Wu KH, Sun PJ et al (2021) Effects of substrate types on the transformation of heavy metal speciation and bioavailability in an anaerobic digestion system. J Environ Sci 101:361–372. https://doi.org/10.1016/j.jes.2020.08.032
Chen GY, Dong JZ, Wu P et al (2022) Effect of different storage methods on physic-chemical properties of pig slurry. J South China Agric University 43:38–46. https://doi.org/10.7671/j.issn.1001-411X.202109031 (in Chinese)
Ye M, Liu JY, Ma C (2018) Improving the stability and efficiency of anaerobic digestion of food waste using additives: a critical review. J Clean Prod 192:316–326. https://doi.org/10.1016/j.jclepro.2018.04.244
Choong YY, Norli I, Abdullah AZ et al (2016) Impacts of trace element supplementation on the performance of anaerobic digestion process: a critical review. Bioresour Technol 209:369–379. https://doi.org/10.1016/j.biortech.2016.03.028
Ugwu SN, Biscoff RK, Enweremadu CC (2020) A meta-analysis of iron-based additives on enhancements of biogas yields during anaerobic digestion of organic wastes. J Clean Prod 269:122449. https://doi.org/10.1016/j.jclepro.2020.122449
Feng YH, Zhang YB, Quan X et al (2014) Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res 52:242–250. https://doi.org/10.1016/j.watres.2013.10.072
Zhang HY, Tian YL, Wang LJ et al (2016) Effect of ferrous chloride on biogas production and enzymatic activities during anaerobic fermentation of cow dung and Phragmites straw. Biodegradation 27:69–82. https://doi.org/10.1007/s10532-016-9756-7
Zhang ZS, Guo L, Wang Y et al (2020) Application of iron oxide (Fe3O4) nanoparticles during the two-stage anaerobic digestion with waste sludge: impact on the biogas production and the substrate metabolism. Renew Energy 146:2724–2735. https://doi.org/10.1016/j.renene.2019.08.078
Amen TWM, Eljamal O, Khalil AME et al (2018) Methane yield enhancement by the addition of new novel of iron and copper-iron bimetallic nanoparticles. Chem Eng Process 130:253–261. https://doi.org/10.1016/j.cep.2018.06.020
Yang Y, Guo JL, Hu ZQ (2013) Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res 47:6790–6800. https://doi.org/10.1016/j.watres.2013.09.012
Liu CR, Tong Q, Li YC et al (2019) Biogas production and metal passivation analysis during anaerobic digestion of pig manure: effects of a magnetic Fe3O4/FA composite supplement. RSC Adv 9:4488–4498. https://doi.org/10.1039/c8ra09451a
Suanon F, Sun Q, Mama D et al (2015) Effect of nanoscale zero-valent iron and magnetite (Fe3O4) on the fate of metals during anaerobic digestion of sludge. Water Res 88:897–903. https://doi.org/10.1016/j.watres.2015.11.014
Li Q, Li YY, Qiao W et al (2015) Sulfate addition as an effective method to improve methane fermentation performance and propionate degradation in thermophilic anaerobic co-digestion of coffee grounds, milk and waste activated sludge with AnMBR. Bioresour Technol 185:308–315. https://doi.org/10.1016/j.biortech.2015.03.019
Van Roy S, Vanbroekhoven K, Dejonghe W, Diels L (2006) Immobilization of heavy metals in the saturated zone by sorption and in situ bioprecipitation processes. Hydrometallurgy 83:195–203. https://doi.org/10.1016/j.hydromet.2006.03.024
Tessier A, Campbell PGC, Bisson M (1979) Sequential extraction procedure for the speciation of particulate trace metals. Anal Chem 51:844–851. https://doi.org/10.1021/ac50043a017
Zhou HB, Meng HB, Zhao LX et al (2018) Effect of biochar and humic acid on the copper, lead, and cadmium passivation during composting. Bioresour Technol 258:279–286. https://doi.org/10.1016/j.biortech.2018.02.086
Mu Y, Yu HQ, Wang G (2007) A kinetic approach to anaerobic hydrogen-producing process. Water Res 41:1152–1160. https://doi.org/10.1016/j.watres.2006.11.047
Achiba WB, Lakhdar A, Gabteni N et al (2009) Accumulation and fractionation of trace metals in a Tunisian calcareous soil amended with farmyard manure and municipal solid waste compost. J Hazard Mater 176:99–108. https://doi.org/10.1016/j.jhazmat.2009.11.004
Farghali M, Yuhendra AP, Mohamed IM et al (2021) Thermophilic anaerobic digestion of Sargassum fulvellum macroalgae: Biomass valorization and biogas optimization under different pre-treatment conditions. J Environ Chem Eng 9:106405. https://doi.org/10.1016/j.jece.2021.106405
Wang SN, Yuan RF, Liu CC et al (2020) Effect of Fe2+ adding period on the biogas production and microbial community distribution during the dry anaerobic digestion process. Process Saf Environ Prot 136:234–241. https://doi.org/10.1016/j.psep.2019.12.031
Wei J, Hao XD, Van Loosdrecht MCM et al (2018) Feasibility analysis of anaerobic digestion of excess sludge enhanced by iron: a review. Renew Sustain Energy Rev 89:16–26. https://doi.org/10.1016/j.rser.2018.02.042
Abdelsalam E, Samer M, Attia YA et al (2017) Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 120:842–853. https://doi.org/10.1016/j.energy.2016.11.137
Paepatung N, Songkasiri W, Yasui H et al (2020) Enhancing methanogenesis in fed-batch anaerobic digestion of high-strength sulfate-rich wastewater using zero valent scrap iron. J Environ Chem Eng 8:104508. https://doi.org/10.1016/j.jece.2020.104508
Isa Z, Grusenmeyer S, Verstraete W (1986) Sulfate reduction relative to methane production in high-rate anaerobic digestion: technical aspects. Appl Environ Microbiol 51:572–579. https://doi.org/10.1016/S0769-2609(86)80024-2
Chen YW, Yang ZH, Zhang YR et al (2020) Effects of different conductive nanomaterials on anaerobic digestion process and microbial community of sludge. Bioresour Technol 304:123016. https://doi.org/10.1016/j.biortech.2020.123016
Owusu-Agyeman I, Plaza E, Cetecioglu Z (2020) Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: effect of substrate proportions and long-term operation. Waste Manage 112:30–39. https://doi.org/10.1016/j.wasman.2020.05.027
Xu ZY, Zhao MX, Miao HF et al (2014) In situ volatile fatty acids influence biogas generation from kitchen wastes by anaerobic digestion. Bioresour Technol 163:186–192. https://doi.org/10.1016/j.biortech.2014.04.037
Siegert I, Banks C (2005) The effect of volatile fatty acid additions on the anaerobic digestion of cellulose and glucose in batch reactors. Process Biochem 40:3412–3418. https://doi.org/10.1016/j.procbio.2005.01.025
Ma JQ, Zhu HG, Fan M (2014) Study on distribution rules of the heavy metals in anaerobic fermentation residue of biogas. Anhui Agric Sci 42:193–196. https://doi.org/10.13989/j.cnki.0517-6611.2014.01.102 (in Chinese)
Fu FL, Dionysiou DD, Liu H (2014) The use of zero-valent iron for groundwater remediation and wastewater treatment: a review. J Hazard Mater 267:194–205. https://doi.org/10.1016/j.jhazmat.2013.12.062
Dong B, Liu XG, Dai LL et al (2013) Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour Technol 131:152–158. https://doi.org/10.1016/j.biortech.2012.12.112
Zhou T, Wu LH, Luo YM et al (2017) Effects of organic matter fraction and compositional changes on distribution of cadmium and zinc in long-term polluted paddy soils. Environ Pollut 232:514–522. https://doi.org/10.1016/j.envpol.2017.09.081
Kretzschmar R, Schaefer T (2005) Metal retention and transport on colloidal particles in the environment. Elements 1:205–210. https://doi.org/10.2113/gselements.1.4.205
Xenidis A, Stouraiti C, Papassiopi N (2010) Stabilization of Pb and As in soils by applying combined treatment with phosphates and ferrous iron. J Hazard Mater 177:929–937. https://doi.org/10.1016/j.jhazmat.2010.01.006
Singh J, Lee BK (2015) Reduction of environmental availability and ecological risk of heavy metals in automobile shredder residues. Ecol Eng 81:76–81. https://doi.org/10.1016/j.ecoleng.2015.04.036
Rodriguez-Rubio P, Morillo E, Madrid L et al (2003) Retention of copper by a calcareous soil and its textural fractions: influence of amendment with two agroindustrial residues. Eur J Soil Sci 54:401–409. https://doi.org/10.1046/j.1365-2389.2003.00529.x
Zhou H, Brown RC, Wen ZY (2020) Biochar as an additive in anaerobic digestion of municipal sludge: biochar properties and their effects on the digestion performance. ACS Sustain Chem Eng 8:6391–6401. https://doi.org/10.1021/acssuschemeng.0c00571
Tian Y, Zhang H, Chai Y et al (2017) Biogas properties and enzymatic analysis during anaerobic fermentation of phragmites australis straw and cow dung: influence of nickel chloride supplement. Biodegradation 28:1–11. https://doi.org/10.1007/s10532-016-9774-5
Zucconi F, Pera A, Forte M et al (1981) Evaluating toxicity of immature compost. Biocycle 1981(22):54–57. https://doi.org/10.1071/SR9810361
Mohring SAI, Strzysch I, Fernandes MR (2009) Degradation and elimination of various sulfonamides during anaerobic fermentation: a promising step on the way to sustainable pharmacy? Environ Sci Technol 43:2569–2574. https://doi.org/10.1021/es802042d
Abubaker J, Risberg K, Pell M (2012) Biogas residues as fertilisers-effects on wheat growth and soil microbial activities. Appl Energy 99:126–134. https://doi.org/10.1016/j.apenergy.2012.04.050
He MM, Li WH, Liang XQ et al (2009) Effect of composting process on phytotoxicity and speciation of copper, zinc and lead in sewage sludge and swine manure. Waste Manage 29:590–597. https://doi.org/10.1016/j.wasman.2008.07.005
Wang HG, Li N (2018) Benefit analysis and policy recommendations based on biogas engineering. Renew Energy Resources 36:811–819. https://doi.org/10.13941/j.cnki.21-1469/tk.2018.06.004
Funding
This research was financially supported by the National Science and Technology Major Project of Science and Technology of China (No.2017ZX07603002), University Natural Science Research Project of Anhui Province (KJ2021A0081), and the Open Project of Anhui Province Key Laboratory of Wetland Ecosystem Protection and Restoration, Anhui University (Nos. AKLWEPR-K-X-2019-01 and AKLWEPR-K-X-2019-05).
Author information
Authors and Affiliations
Contributions
MZ: performing the experiments, data collection and analysis, writing original draft; YL: performing the experiments, data collection and analysis, review; RS: conducting the experiments, review; XF: performing the experiments, data collection and analysis, review; YL: design of experiments, data analysis, checking errors, review; XZ: design of experiments, data analysis, review and editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Consent for participate
Not applicable.
Consent for publication
Not applicable.
Ethical approval
All authors followed the ethical responsibility of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, M., Li, Y., Sun, R. et al. Fe2(SO4)3-assisted anaerobic digestion of pig manure: the performance of biogas yield and heavy metal passivation. SN Appl. Sci. 4, 278 (2022). https://doi.org/10.1007/s42452-022-05161-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05161-2