Abstract
This work presents a practical strategy for fabrication of transparent, robust and environmentally friendly superhydrophobic surfaces for toys and games by a one-step spray coating method. A type of commercial stringed silica nanoparticles (NPs) is chemically modified by a mixture of two fluorine-free silanes, tetraethyl orthosilicate (TEOS) and dodecyltrimethoxysilane (DDTMS) via a sol–gel process with the aid of ammonia as a basic catalyst and a small amount of water in ethanol, resulting in the formation of an amphiphilic solution, suitable for coating a variety of substrate materials such as glass, ceramics, wood, metal, plastics and paper and so on. Polyarylic acid (PAA) is used as a binder to improve the mechanical robustness of the superhydrophobic coating. Effects of silica NPs concentration, mixing order, TEOS/DDTMS ratio, PAA amount and catalyst on the transparency, uniformity, mechanical robustness and superhydrophobicity of the resultant coatings deposited on the glass slides are investigated. The mechanisms for the superhydrophobicity and water-resistance as well as the effects of catalyst and mixing order are discussed. Furthermore, an example of the superhydrophobic surfaces as toys is presented. This work will pave the way for expanding wide applications of the superhydrophobic surfaces towards toys and games.
Article Highlights
-
A one-step spray coating method is developed to fabricate transparent, robust and environmentally friendly superhydrophobic surfaces on various substrates
-
Polyacrylic acid (PAA) plays an important role in improv-ing the uniformity and mechanical robustness of the superhydrophobic coating.
-
An example of practical application is presented for the superhydrophobic surfaces as toys and game
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Children and adults love to play soap bubbles and have also done for centuries due to the various esoteric properties such as the bubble’s fragile nature, flexibility, transparency, beautiful rainbow colors, the ability to soar through the sky and so on. They often evoke happy childhood memories of blowing and chasing the mystical orbs with our friends. The fun experiments with soap bubbles have also been widely used to teach and explore a wide variety of science concepts such as surface tension, chemistry, elasticity, light and even geometry, to children and even adults at school, television, artistic performances and so on. A soap bubble is just air wrapped in a thin film of soapy water resulting from the surface tension. Soap is available in everyone’s daily life. Every young child can enjoy playing soap bubbles by simply dipping a straw into a soap solution and then blowing the magic bubbles. The ease of playing and availability of the soap solution in daily life can be considered as one major reason why the soap bubbles have become one of the most popular enjoyments as a children’s toy. In order to increase the fun and safety of soap bubble toys, a large number of inventions have been made concerning the bubble-making solutions [1,2,3,4], and bubble-making devices [5,6,7].
In our daily lives in addition to the mystical phenomenon of man-made soap bubble generation as mentioned above, we can also observe another mystical natural phenomenon that a water drop forms or rolls on a lotus leaf when watching lotus flowers nearby the lotus pond. Lotus flower has been regarded as a symbol of purity in many different cultures, especially in the eastern regions. The lotus always remains clean and untouched by the dirt even when growing from the muddy waters due to the self-cleaning of the lotus leaves, the so-called Lotus-effect. The secret behind the Lotus effect is attributed to the unique dual-scale microstructure of a lotus leaf composed of the microstructural epidermal cells on its rough surface covered with nanoscopic wax crystals [8]. A surface which shows the Lotus effect is often referred as superhydrophobic with a water contact angle of greater than 150° and a very small roll-off angle of less than 10° [9].
Due to the fascinating self-cleaning capabilities, superhydrophobic surfaces have attracted extensive attention by mimicking the hierarchical surface morphologies exhibited in natural systems for potential applications, including water repellency, self-cleaning fabrics, containers, windows and buildings, anti-corrosion, anti-biofouling, drag-force reduction, water transport in microfluidics [10], anti-frost/anti-icing [11], antibacterial [12], microdroplet manipulation [13]. In addition, the superhydrophobic surfaces have potential applications as children’s toys and games for enjoyment, similar to the soap bubbles. A recent study has shown that the science education using the Lotus effect led to a significant improvement in the scientific conception of approximately 14-year-old students in the short-term test, and furthermore, one group confronted with their alternative conceptions (AC) showed significantly higher knowledge than the other group being not confronted with their alternative conceptions (NAC) over a long-term test [14]. Several inventions have been made to develop toys and games using the superhydrophobic surfaces [15,16,17]. However, these inventions have had difficulty in finding out the practical applications as toys and game due to two major issues: (1) because the superhydrophobic surfaces have already been applied onto the playboards of the toys, the players can not enjoy super water-repellent phenomenon by making lotus leaf–like surfaces by themselves; (2) the coating solutions for making superhydrophobic surfaces are toxic to the human body as well as the environment because of the use of toxic organic solvents such as cyclohexanone [16] or trichloroethylene [17].
To expand wide applications as toys and games, the design and fabrication of the superhydrophobic surfaces should meet the following requirements: (1) the use of fluorinated substances should be avoided, including perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) with carbon chains greater than six, because they are difficult to be degraded within the human body and thus could cause health issues; (2) the constituent materials should be non-toxic to the human body as well as the environment; (3) the coating process should be able to be not only simply completed in one-step coating but also suitable for the substrates with a variety of shapes such as planer and curved shapes; (4) the coating should be versatile so that it can be applicable to a wide variety of substrate materials such as glass, metal, plastic, wood and paper and so on; (5) the coating should be robust enough to not lose its super water-repellency when played; (6) the coating can be easily removed or reapplied when needed, which allows the well-designed playing devices to be used repeatedly.
Recently, fluorine-free trialkoxysilanes containing long-chain alkyl group with low surface free energy such as dodecyltrimethoxysilane (DDTMS), have been widely used to prepare the superhydrophobic surfaces by surface modification of silica nanoparticles (NPs) through a sol–gel process [18,19,20,21], which is a facile and low-cost method for creating the superhydrophobic surfaces on the substrates with complexed shapes. In this work, we report a practical strategy for the fabrication of transparent, robust and environmentally friendly superhydrophobic surfaces for toys and games by a one-step spray coating method. A kind of cheap and non-toxic ethyl alcohol was used as a solvent. A type of commercial stringed silica NPs is used to create the dual-scale micro/nanostructure required for attaining the Lotus effect because it has been reported to be effective in fabricating the transparent and superhydrophobic surfaces by forming a fractal-like nanoporous network structure [22]. A mixture of two fluorine-free silanes, tetraethyl orthosilicate (TEOS) and DDTMS are used to chemically modify the surface of the stringed silica nanoparticles via a sol–gel process with the aid of ammonia as a base catalyst and a small amount of water in ethyl alcohol, resulting in the formation of an amphiphilic solution. A kind of environmentally friendly polymer binder, water-soluble polyarylic acid (PAA) is used to improve the mechanical robustness of the superhydrophobic coating. The fabrication process of the transparent, robust and environmentally friendly superhydropobic surfaces by a one-step spray coating method is schematically illustrated in Fig. 1. The Effects of silica nanoparticle concentration, mixing order, amounts and hydrolysis condition of two silanes, addition of polymer binder and the coating conditions on the transparency, uniformity, robustness and superhydrophobicity of the resultant coatings onto the glass substrate are investigated. The applicability to a variety of other substrate materials, including ceramics, wood, metal, plastics and paper is demonstrated. Furthermore, we present an example of a practical and potential use of superhydrophobic surfaces for toys and games.
In the next section, we describe the processing and characterization methods. Section 3 shows the experimental results. In Sect. 4, we discuss the mechanisms for the hydrophobicity, water-resistance and the effects of the catalyst and mixing order. In Sect. 5, we draw a conclusion for this work.
2 Experimental procedure
2.1 Raw materials
The raw materials used in this work were ethanol (Mix ethanol NP, ethanol 85.5 wt%, IPA 4.9 wt%, NPA 9.6 wt%, Yamaichi Chemical Industries Co., Ltd.), ammonia (28 wt%, Taiseikakou Co., Ltd.), Nitric acid (62 wt%, Juzen Co. Ltd.), a type of elongated silica NPs dispersed in isopropanol (IPA) (IPA-ST-UP, 15 wt%, diameter of 10 ~ 15 nm, length of 40 ~ 100 nm, Nissan Chemical Corp.), a hydrophobic type of spherically shaped silica NPs powders (QSG 30, average particle size of 30 nm, Shin-Etsu Chemical Co., Ltd.), TEOS and DDTMS (Tokyo Chemical Industry Co., Ltd.), PAA (Average molecular weight 25,000, FUJIFILM Wako Pure Chemical Corp.).
2.2 Processing
Table 1 shows the starting compositions of the coating solutions containing stringed silica NPs used for fabricating the superhydrophobic coating by one-step coating. The total amount of 76 g was used for preparing each sol solution with a 200 ml triangular beaker. Prior to the preparation of the coating solutions, the stock solutions of 5 wt% PAA was prepared by mixing PAA with deionized water in a beaker by magnetic stirring at room temperature. 1 M nitric acid (HNO3) was also prepared by the dilution of concentrated nitric acid in deionized water. To investigate the effect of mixing order on the coating properties of the sol solutions, three different mixing orders were used, as shown in Fig. S1. At each mixing step, the raw materials were slowly added, followed by magnetic stirring at 800 rpm for 10 min at room temperature. After all the materials were added, the stirring was kept at 800 rpm for 24 h as the final mixing step, resulting in the formation of an amphiphilic coating solution containing the silica NPs with surface modification. Glass slides were used as the substrate to investigate the effects of composition and processing method on the formation and hydrophobic properties of the coating. Prior to the coating, the glass slides were cleaned by ultrasonication in ethanol and subsequent drying. The coating solution of 0.5 ml was deposited onto the glass slides (75 mm × 25 mm) by sliding of the syringe, followed by drying at 40 °C for 30 min. The spray coating was also conducted using a 30 ml glass atomizer at a distance about 10 cm between the atomizer and the substrate, and the coating solution about 0.13 ml was sprayed at a time. After spray coating, the drying could be done at room temperature and the drying time was about 3 ~ 24 h, depending on the substrate and ambient temperature. After the spray coating, the drying could also be done with a hair dryer in several minutes. In addition to the glass, various other substrates such as tile, SUS304, acrylic resin, corrugated fiberboard and wood, were used to fabricate superhydrophobic surfaces by spray coating, followed by drying using a hair dryer.
2.3 Characterization
The uniformity and transparency of the coatings were evaluated using a digital camera. The mechanical robustness was characterized using the adhesive tape method. The change in the coating before and after robustness test was observed and water contact angle (WCA) before and after robustness test was measured. The 10 μL water droplets were gently dropped onto the coating surface using a micro-pipette. The images of water droplets formed on the coating surface were captured using a digital camera. The image files were transferred to a computer and static contact angle (SCA) of water was determined using the software of Image J with the contact angle plugin. The contact angle hysteresis (CAH), which is the difference between the advancing contact angle (ACA) and receding contact angle (RCA), was measured by the needle-in-the sessile-drop method using the initial drop, advancing drop and receding drop volume of 5 μL, 10 μL, and 5 μL, respectively [23]. Water resistance at ambient temperature was evaluated by dropping water droplets onto the coating. If the water droplets can hold its shapes for 10 min, the coating is considered to be water resistant, otherwise the coating is considered to have poor water resistance because the coating is dissolved by the water droplet. The hot water resistance was also evaluated by immersing the other half of the sample coating into hot water at 40 ℃ for 68 h. Fourier transforms infrared ray (FTIR) spectra were measured by a FTIR spectrometer (FTIR-8400S, Shimadzu Corp.,) for the raw silica NPs solution (IPA-ST-UP) and the prepared coating solution. FTIR spectra of the sprayed coatings on glass were measured using the powder sample obtained from the peeling of the sprayed coating. The transmittance of bare and coating glass slides was measured by a UV–Vis spectrophotometer (U-1500, Hitachi High-Tech Corp.,). Morphology of the coatings were observed using an electron microscope (TM3030Plus Miniscope, Hitach High-Tech Corp.,)
3 Results
3.1 Effects of silica NPs concentrations, processing methods and PAA addition
Figure 2a–c show the appearance and hydrophobicity of the coatings deposited on the glass slides with the coating solutions containing IPA-ST-UP concentrations of 0.65, 1.30 and 2.60 wt% by the processing method I, corresponding to the silica NPs concentrations of 0.10, 0.20 and 0.39 wt% in the starting compositions, respectively. All three coatings were highly transparent but the uniformity was poor. The increased WCA illustrated an improvement in hydrophobicity with increased silica NPs concentration from 0.10 to 0.39 wt%. However, the superhydrophobicity was not successfully obtained among those three coatings. As shown in Fig. 2c–e, there was no significant change in the uniformity between the coatings deposited by the coating solutions prepared by the processing method I (Fig. 2c) and II (Fig. 2d), but the processing method III (Fig. 2e) resulted in an improvement in the uniformity compared with those prepared by the processing I and II with the same composition No. 3. Despite this, the highly uniform coating was not obtained from the coating solutions without the addition of PAA.
Optical photographs of the coatings deposited on the glass slides by sliding of syringe of 0.5 ml coating solutions prepared from various contents of silica NPs through different processing methods. Processing method I: Compositions (a) No. 1, b No. 2 and c No. 3; Processing method II: Compositions (d) No. 3 and f No. 5; Processing method III: Compositions (e) No. 3 and g No. 5. The inserts were optical photographs of a 10 μL water droplet on the coating surface showing the water repellency
Figure 2d–g show that compared to the processing method II, the processing method III led to an improvement in the coating uniformity and an increase in the WCA from 125 to 140°, 149 to 158°, respectively, regardless of the addition of PAA as a binder. In particular, the coating deposited from the coating solution with the addition of PAA prepared by the processing method III (Fig. 2g) was highly uniform, transparent and superhydrophobic. Here, it should be mentioned that a water droplet on the superhydrophobic coating shown in Fig. 2g can be movable as long as the glass slide is slightly shaken horizontally, and the water droplet can immediately roll down along the coating surface if the other side of the glass slide is slightly lifted even at a tilt angle of less than 5° (Supplementary Video 1). This indicates that the superhydrophobic surface showed a very small roll-off angle of less than 5°. In this case, these results indicate that the highly uniform coating on the glass slide with transparency and superhydrophobicity could be obtained by the combination of increased silica NPs concentration, PAA addition, and optimized mixing order.
3.2 Effect of PAA amount and TEOS/DDTMS ratio
Figure 3a shows that when the amount of PAA was increased to 50 wt% (No. 6) and 100 wt% (No. 7) against the dry weight of silica NPs, the coating on the glass slide became non-uniform and poorly transparent. Compared with the addition of 50 wt% PAA, the addition of 100 wt% PAA led to higher uniformity and less transparency. In the case of the addition of 100 wt% PAA, the coating showed no improvement in the uniformity with decreased TEOS/DDTMS ratio (No. 7 ~ 9) at a given total amount of 0.83 wt% of TEOS and DDTMS, and became the worst in the uniformity when the amount of DDTMS was much more than that of TEOS (No. 10), resulting in the increased total amount of 1.94 wt% of TEOS and DDTMS.
Optical photographs of the coatings deposited on the glass slides by sliding of syringe of 0.5 ml coating solutions using the coating solutions prepared with various amounts of PAA (compositions No. 6 and 7) and various TEOS/DDTMS ratios (Compositions No. 7 ~ 10) by the processing method III: a before and (b) after water repellent test using 10 μL water droplets. The lower images of Fig (a) were optical photographs of a 10 μL water droplet on the coating surface showing the water repellency
As shown in Fig. 3b, at a given total amount of 0.83 wt% of TEOS and DDTMS, after the 10 μL water droplets were dropped onto the coating surface, they were initially repelled regardless of the amount of PAA and TEOS/DDTMS ratio, but the water repellency appeared to become weaker with the decreased TEOS/DDTMS ratio. At a given total amount of 1.94 wt% of TEOS and DDTMS, the coating surface became hydrophilic. Despite this, the water droplets showed a change in their shapes with time and finally lost its nearly spherical shapes and left craters on their places after being removed by tissue paper in all cases. In this case, these results indicate that the amount of PAA plays an important role in controlling the uniformity and superhydrophobicity of the coatings deposited from the coating solution containing stringed silica NPs with surfaces modified by a mixture of silanes TEOS and DDTMS.
3.3 Effect of catalyst type
In order to investigate the effect of catalyst type used for TEOS and DDTMS on the preparation and coating properties of the coating solution, a basic type, ammonia, and an acidic type, nitric acid, were chosen in this work. As shown in Fig. 4a, the ammonia led to the formation of a cloudy coating solution, but the nitric acid led to the formation of a clear coating solution. It is interesting to see that the coating deposited on the glass slide by the cloudy coating solution showed poor uniformity and also a certain degree of transparency, but the one obtained by the clear coating solution showed improved uniformity and poor transparency, as shown in Fig. 4b. Furthermore, the uniform area of the coating surface obtained from the cloudy coating solution showed superhydrophobicity with a WCA of 152°, but the coating surface obtained from the clear coating solution showed weak hydrophobicity with a WCA of 104°. This indicates that the acidic catalyst could not lead to the formation of the coating solution for fabricating the superhydrophobic surface.
Optical photographs of (a) the coating solutions prepared by the processing method II with the addition of ammonia and nitric acid as a catalyst of the hydrolysis and condensation of TEOS and DDTMS, and (b) the coatings formed on the glass slides by sliding of syringe of 0.5 ml coating solutions (Left: black background only, Right: black background with white Japanese characters). The lower images of Fig (b) were optical photographs of two 10 μL water droplets on the coating surface showing the water repellency
3.4 Spray coating, mechanical robustness, water resistance, and applicability on various substrates
Spraying is one of the most widely used methods to artificially create hydrophobic or superhydrophobic coating for our daily necessities such as car body, shoes, shoulder bag, umbrella, kitchen and so on, due to its versatility, easiness of use and low-cost. Aerosol spray and spray bottle are two major forms of waterproof products. Although it has been most widely used in our daily lives, recently it has been recognized that aerosol spray can be dangerous to the environment and human body, particularly children. From health and safety point of view, a spray bottle without any propellant is the most preferable method to create superhydrophobic surfaces for children’s toys and games. In this case, 30 ml glass atomizers, widely used for cosmetics were chosen to investigate the feasibility of one-step spray coating method to fabricate superhydrophobic surfaces on various substrates using the coating solutions studied in this work.
Figure 5a shows that the coatings deposited on the glass slides exhibited high uniformity and a certain degree of transparency within the number of sprays from 3 to 12. It is evident that the bare glass slide without spray was highly transparent and was completely hydrophilic. The coatings became superhydrophobic when the number of sprays was increased to 6 over the glass slide. When the number of sprays was increased further, the superhydrophobicity showed no significant change but the coatings became less transparent. As shown in Fig. 5b, the spray coating led to a significant decrease in transparency of the glasses. It is interesting to see that when the number of sprays was increased from 6 to 12, the transparency showed almost no change.
As the superhydrophobic surfaces for toys and games, mechanical robustness should be enough to some extent to not fail during the enjoyment of the toys and games. Taking into account that the player usually does not touch the superhydrophobic surface as toys and games using water droplets, the adhesive tape method was used to evaluate the mechanical robustness of the superhydrophobic coating. As shown in Fig. 6a, in the case of no PAA addition, the coating surface apparently got damaged and some coating was removed, thus resulting a large reduction in WCA from 140 to 110°, whereas in the case of 10 wt% PAA addition, the coating surface exhibited almost no change and exhibited the WCAs of higher than 150°, that is, the coating still remained superhydrophobic after peel test. Figure 6b illustrate that the mechanical robustness of the superhydrophobic coatings could be enhanced by increased number of sprays. The coatings sprayed by 6 times could remain superhydrophobic within the peel test of 5 times, whereas the those sprayed by 12 times could remain superhydrophobic within the peel test of 9 times. Moreover, Within the peel test of 5 times, in both cases the coating surfaces exhibited SCAs of higher than 150°and CAHs of less than 5°, indicating that both they are superhydrophobic. In addition, such superhydrophobic coating was found to still remain superhydrophobic even after being kept for more than six months at room temperature. In this case, we think that the addition of PAA as a binder allows the superhydrophobic coating to attain adequate mechanical robustness for the application as toys and games.
Evaluation results of mechanical robustness of the coatings deposited on the glass slides by the one-step spray coating using the adhesive tape method. a Optical photographs of the coatings sprayed from the coating solutions without (Composition No. 3) and with the addition of 10 wt% PAA (Composition No. 5) by the processing method III before and after peel test. Insets in (a): Optical photographs of a 10μL-water droplet and SCAs. b SCAs and CAHs of water on the superhydrophobic coatings sprayed with different times on the glass slides using the coating solution with the addition of 10 wt% PAA after different numbers of peel test
As toys and games, the superhydrophobic surfaces should also be highly resistant to the water. The superhydrophobic coating was found to remain superhydrophobic even after being immersed in water at room temperature (~ 25 °C) for one day. In order to investigate the effect of water temperature, the water resistance in hot water at 40 °C was also conducted. Figure 7 shows that the coating surface showed almost no change in the uniformity even after being immersed for 68 h, but the WCA showed a reduction from 151 to 135°, indicating the loss of superhydrophobicity. It is worth mentioning that the coating surface still remained superhydrophobic within 12 h-immersion in hot water at 40 °C during our test.
Optical photographs and water repellency of the coating deposited on the glass slide by one-step spray coating of the coating solution with the addition of 10 wt% PAA based on the dry weight of silica NPs (Composition No. 5) (Number of spray: 6 times) by the processing method III before and after water resistance test by immersing it into hot water at 40 °C for 68 h
Figure 8 shows that in addition to the glass, the coating solution prepared from the composition No. 5 by the processing method III can be used to fabricate superhydrophobic surfaces on various other substrates such as tile, SUS304, acrylic resin, corrugated fiberboard and wood, by one-step spray coating using commercial spray bottle. This indicates that the coating solution successfully obtained in this work makes the superhydrophobic coating applicable to a variety of substrates as playing devices for toys and games. Furthermore, the superhydrophobic coating can be easily removed by ethyl alcohol solution or household washing detergent, and the substrate can be repeatedly used when it is necessary.
3.5 Potential applications as toys and games
Figure S2 shows that although the superhydrophobic coating solution prepared in this work showed two-phase separation of the upper clear solvent and lower cloudy silica NPs suspensions after being kept for one day at ambient temperature, it could be redispersed by hand-shaking. Even after being kept for more than six months, the coating solution could be highly redispersed only by hand-shaking, that is, the sedimentation of silica NPs did not lead to the formation of hard agglomerates during the storage. In particular, the phase-separation during the storage did not lead to the change in the formation and superhydrophobicity of the coating by one-step spray coating regardless of the substrate.
Figure 9 presents a typical example of toys and games to illustrate the potential applications of the superhydrophobic coating solution for toys and games by providing a surface showing the Lotus effect. As shown in Fig. 9a a toy is basically composed of four components: (T1) The superhydrophobic spray solution for creating a superhydrophobic surface prior to playing; (T2) Playing device composed of an open wood box and preset wood blocks in which blocks can be freely set on the bottom board as the player likes; (T3) A syringe used to generate water droplets; (T4) Three primary dye color solutions (yellow, magenta and cyan) used to create droplets with various different colors. Figure 9b shows the three primary colors of paint or pigment or ink.
Optical photographs of a typical example of toys that can be played using the superhydophobic coating developed in our work. a The four components of a toy or a game (T1: The superhydrophobic spray solution for creating a superhydrophobic surface prior to playing; T2: Playing device composed of an open wood box and wood blocks in which blocks can be freely set on the bottom board to the player liking; T3: A syringe used to generate water droplets; T4: Three primary dye color solutions (yellow, magenta and cyan). b Three primary colors of paint. c A play method showing how to play the toy after coating the inner surfaces and all wood blocks: (i) A yellow droplet was dropped at the goal, and the other magenta droplet was dropped at some place outside the goal; (ii) The magenta droplet was moved by shaking the box horizontally or using the tip of the syringe around the blocks to get to the entrance of the goal; (iii) The magenta droplet was moved into the goal to meet the yellow droplet, resulting in the generation of a red droplet
Prior to playing, the superhydrophobic spray solution was used to carefully coat all the inner surfaces of the box and all the surfaces of the blocks, particularly the contacted areas between the bottom surface of the blocks and the bottom board of the box by spray coating, followed by drying using a hair dryer for about 5 min. In order to ensure that all the inner surfaces are superhydrophobic, when the first coating is finished, water repellent test is suggested by moving a water droplet. If the water droplet stops at some place, the spray coating should be done again for those places by the same method. Once the superhydrophobic surface is ready, the play can start, as illustrated in Fig. 9c presented only as an example (Supplementary Video 2–4). For example, firstly (i), a yellow droplet was dropped at the goal, and the other magenta droplet was dropped at some other place outside the goal; secondly (ii), the magenta droplet was moved by shaking box horizontally, or using the tip of the syringe, around the blocks to get to the entrance of the goal; finally (iii), the magenta droplet was moved into the goal to meet the yellow droplet, resulting in the generation of a red droplet. It is worth mentioning that the superhydrophobic coating formed on the playing device T2 still remained superhydrophobic even after over four months, that is to say, the playing device can be played again without recoating.
As illustrated above, using the toy with the superhydrophobic spray solution, children not only can enjoy making the Lotus-like superhydrophobic surface by themselves, but also enjoy rolling motion of water droplets and even magic color change of water droplets by mixing two or more water droplets with any two different colors using the T4 color solutions. Therefore, it is expected that this novel Lotus-effect toy can influence children’s competencies, attitudes, values and conceptions toward science, particularly encourage children’s natural curiosity.
4 Discussion
4.1 Mechanism for superhydrophobicity
In the presence of ammonia, the hydrolysis reactions of TEOS and DDTMS take place as follows, respectively [24]:
During the hydrolysis reaction, the condensation reaction or the silylation reaction between the hydrolyzed silanes of DDTMS and silanol groups of the silica particles take place simultaneously as follows:
During the hydrolysis reaction, the condensation reactions between the hydrolyzed silanes of TEOS and between the hydrolyzed silanes of TEOS and the silanol groups of the silica particles took place as follows, respectively:
Reaction (3) leads to the hydrophobization of silica NP particle surface, whereas Reactions (4) and (5) are believed to lead to the growth of the pre-existed silica NPs to some extent, maintaining a certain number of silanol groups at the surface of silica NPs. The co-existence of both hydrophobic group (CH3(CH2)11-) and hydrophilic group (-OH) gives the amphiphilicity, thereby enabling the coating solution applicable to various substrates whether it is hydrophobic or hydrophilic.
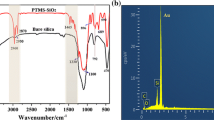
FTIR spectra presented in Fig. 10 illustrates that after hydrolysis, the absorption peak at 2881 cm−1, corresponding to the C–H stretching vibration of –(CH2)n-, became stronger in the coating solution than the raw silica NPs solution. This indicates that the surface of silica NPs was successfully modified with long alkyl chains of DDTMS, making it hydrophobic. The strong absorption peak at 2970 cm−1 was attributed to the C–H stretching vibration of CH3, resulting from the combination of ethanol, DDTMS and TEOS. A broad absorption peak at 3337 cm−1, was attributed to the stretching vibrations of Si–OH on the surface of silica NPs, making it also hydrophilic. Clearly, after spray coating, the broad abruption peak disappeared and only a broad absorption peak at 1092 cm−1was left, corresponding to the stretching vibration of Si–O-Si. Nevertheless, the absorption peak of –(CH2)n- was not observed. In addition, two weak absorption peaks at 2357 and 2330 cm−1, respectively, were observed in both the coating solution and spray coating. Unfortunately, these two peaks are not identified yet. However, during the drying of the coating, the condensation reactions of surface silanol groups (Si–OH) between the silica NPs occur and lead to the disappearance of silanol groups (Si–OH) via formation of Si–O–Si, resulting in the formation of hydrophobic surface. At the same time, the evaporation of solvents allows the surface-modified silica NPs to rearrange themselves spatially due to the long-range interparticle repulsion introduced by the heterogenous surface chemistry, resulting in the formation of a fractal-like, nanoporous network structure. The SEM images presented in Fig. 11 clearly illustrates the formation of a uniform hierarchical structure consisting of networked channels with width of about 1 μm in the spray coating. This is the reason why the superhydrophobic surface was generated in this work.
4.2 Role of catalyst in the formation of superhydrophobic coating
This work shows that the basic catalyst, ammonia, led to the formation of cloudy coating solution, but superhydrophobic the formation of superhydrophobic coating with high uniformity and transparency, whereas the acidic catalyst, nitric acid, led to the formation of clear coating solution but the superhydrophobicity was not achieved for the coating. The hydrolysis speed of DDTMS is more rapid than the condensation speed at acidic condition, and vice versa at basic condition [25]. In this case, in the presence of nitric acid, the hydrolysis reaction (2) of DDTMS was dominant and the hydrolyzed silanes remained soluble in the ethanol–water system, thus the resultant solution was transparent and no sediment occurred. However, due to the slow condensation reaction (3), the degree of silylation reaction between the hydrolyzed silanes of DDTMS and silanol groups of the silica particles was low, thereby resulting in a low degree of hydrophobicity of the silica NP surface. In this case, the superhydrophobicity was not achieved for the coating. In contrast, in the presence of ammonia, the condensation reaction (3) of DDTMS was more rapid than the hydrolysis reaction (2), the dominant condensation reaction between the hydrolyzed silanes of DDTMS and silanol groups of the silica particles led to the sedimentation of surface-modified silica NPs due to the high degree of surface hydrophobization.
Although the coating solution showed sedimentation or phase separation behavior during the storage, the redispersion was easily regained only by hand-shaking. This indicates that at basic condition the surface-hydrophobized silica NPs are not agglomerated due to the steric effect resulting from the long-chain hydrophobic groups (CH3(CH2)11-) on the surface. Furthermore, this work indicates that to obtain superhydrophobicity, a basic catalytic condition is necessary for preparing the coating solution via sol–gel process of silanes.
4.3 Role of mixing order in the formation of superhydrophobic coating
In this work, the order of the uniformity of the coating deposited on the glass slide using the coating solution prepared in the absence of PAA by the different processing methods is the method III > II > I. As shown in Fig. S3a, right after all the raw materials were added, the sedimentation occurred in the solutions prepared by the method II but did not occur in those prepared by the method III regardless of the addition of PAA. Nevertheless, the addition of PAA led to a reduction in the sedimentation. TEOS and DDTMS are soluble in ethanol but insoluble in water. In addition, IPA-ST-UP is a product of silica NPs dispersed in isopropanol. The sedimentation occurred in the solution prepared by the method II is attributed to the poor dissolution of TEOS and DDTMS in the previously formed mixed solution of ethanol, silica NPs, water and ammonia. In contrast, according to the method III, silica NPs were completely dispersed in ethanol in the sol form in the first step, followed by complete dissolution of TEOS and DDTMS in the second step, resulting in the homogenously mixed solution in the absence of water. In the third step, although all three materials contained a small amount of water, the small amount of water did not lead to the precipitation of TEOS and DDTMS probably due to their solubility in a miscible solution of ethanol and water. After the stirring was completed, all the solutions became cloudy (Fig. S3b). However, with the time, the sedimentation was faster in the solutions prepared by the method II than those prepared by the method III (Fig. S3c and d). This work indicates that in order to enhance the dispersion of the surface-modified silica NPs in the coating solution, the TEOS and DDTMS should be completely dissolved in the miscible solution of ethanol and water prior to the onset of hydrolysis and condensation.
PAA is an anionic polymer with a general formula of [–CH2–CH–COOH–]n. When dissolved in water, the carboxylic acid groups of PAA dissociate to produce negatively charged molecules [–CH2–CH–COO−]n as follows:
As the pH of the solution is increased, PAA molecules are ionized and undergo a conformational transition from a coiled state at pH < 3.5 to stretched state at pH > 7 [26]. At pH > 10, PAA molecules are believed to be completely ionized. In the method III, the improved stability of the solution with the addition of PAA could be attributed to the electrosteric stabilization of the surface-modified silica NPs by the completely ionized PAA molecules with a stretched form in the high pH range of pH > 10 [27]. The enhanced stability of the surface-modified silica NPs in the solution with the addition of PAA prepared by the method III led to the formation of the most homogeneous superhydrophobic coating.
4.4 Role of PAA in the formation of superhydrophobic coating
The addition of PAA was found to play an important role in the formation of the superhydrophobic coating with high uniformity, transparency, mechanical robustness, water resistance and superhydrophobicity using hydrophilically stringed silica NPs by surface modification via sol–gel process of a mixture of silanes TEOS and DDTMS. One of the most interesting results is the reason why the water-soluble PAA as a binder led to the formation of a water-resistant superhydrophobic coating, with which the water-resistance depended strongly on the amount of PAA. As illustrated in Fig. 3, whether the amount of PAA is less than < 10 wt% or higher than 10 wt%, all the coatings deposited on the glass slides showed no water-resistance regardless of the TEOS/DDTMS ratio.
In order to investigate the effect of silanes on the water-resistance of the coatings, we conducted an experiment using the compositions with various PAA amounts in the absence of both TEOS and DDTMS as shown in Table S1. As shown in Fig. S4, it is interesting to see that despite the non-uniformity in all cases, the coatings were dissolved in the cases with the PAA amount of 1wt%, 50 wt% and 100 wt%. However, the coating with the 10 wt% PAA was not dissolved in water. This indicates that the reason why the coating with the addition of 10 wt% PAA retained water-resistance could not be attributed to the hydrophobization of the PAA molecules themselves via the silylation reaction between the hydrolyzed silanes (–OH) of DDTMS (or TEOS) and the carboxylic groups (–COOH) of PAA, as suggested in a recent study [28]. PAA molecules are soluble in water but show low solubility in ethanol. The solubility of PAA in a mixed solution of ethanol and water depends on the degree of ionization of PAA and the ethanol/water volume ratio [29]. The fully ionized PAA is insoluble in 40% ethanol, but unionized PAA is soluble.
In this work, the addition of ammonia allowed the mixed solutions of ethanol and water to exhibit higher pH of > 10, so the added PAA should be fully ionized and precipitated from water. Considering the water was a minor phase and the ethanol was a major phase in the mixed solution, the reverse micromicelles of PAA were probably formed at the amount of 10 w%, which is the critical micelle concentration (CMC) [30, 31]. At basic pH, the surface-modified silica NPs should be negatively charged because of the existence of a certain amount of the silanol groups (Si–OH), so the negatively charged PAA molecules exhibit almost no adsorption on the surface of silica NPs because of the electrostatic repulsion [26].
Furthermore, in order to investigate the effect of surface chemistry of silica NPs on the formation of reverse micelles of PAA, we also conducted an experiment with the compositions of various concentrations of ammonia added by using a commercially surface-hydrophobized silica nanoparticles (QSG30) in the presence of 10 wt% PAA, as shown in Table S2. Figure S5 illustrated that regardless of ammonia concentration, when the water droplets were dropped, all the coatings deposited on the glass slides were initially hydrophobic, but were dissolved with the time. All the coatings could be completely removed by rinsing it with water. This indicates that these coatings did not attain the water-resistance with the addition of 10 wt% PAA, that is to say, the reverse micromicelles of PAA were not formed in the solutions containing surface-hydrophobized silica NPs. The surface-hydrophobized silica NPs are not charged in the mixed solution of ethanol and water because of the lack of the surface silanol groups (Si–OH). In this case, at basic pH, the completely hydrophobized surface of silica NPs showed strong adsorption of the negatively charged PAA molecules by extending the hydrophilic groups (–COOH) into the solution. Thus, the reverse micromicelles could not be formed. In a word, this work suggests that if a water-soluble polymer is used as a binder to improve the mechanical robustness of the superhydrophobic coating containing silica NPs, it is necessary to choose a hydrophilic type of silica NPs.
5 Conclusions
A one-step spray coating method has been successfully developed for fabricating transparent, robust and environmentally friendly superhydrophobic surfaces for toys and games by sol–gel process, using stringed silica nanoparticles and a mixture of two fluorine-free silanes, tetraethyl orthosilicate and dodecyltrimethoxysilane. The uniformity and superhydrophobicity of the coatings are dependent on the surface properties and amounts of silica nanoparticles, type of catalyst, mixing order, tetraethyl orthosilicate/dodecyltrimethoxysilane ratio, and polyarylic acid amount. Polyarylic acid is found to play an important role in the formation of the coating with high uniformity, mechanical robustness, water resistance and superhydrophobicity. The water-resistance of superhydrophobic coating could be attained with the addition of about 10 wt% polyarylic acid, whereas below or above which the coatings become water-soluble. In the case of 10 wt% polyarylic acid, the water-resistance could be attributed to the formation of the reverse micromicelles of polyarylic acid. The applicability of the coating solution to a variety of substrate materials such as glass, ceramics, wood, metal, plastics and paper and so on, has been well demonstrated by one-step spray coating method. Spray coating can be dried at room temperature and also can be rapidly dried by a hair dryer. An example of applications as toys and games using the superhydrophobic spray solution is presented for enjoying the Lotus effect. This work will pave the way for expanding wide applications of the superhydrophobic surfaces towards toys and games. However, in the present work there are some important issues that need to be addressed in the future research, including the water-resistant mechanisms with the addition of about 10 wt% polyarylic acid, improvements in transparency and mechanical robustness.
References
Netherly GP (1971) Bubble compositions. United States patent US3630951
Ehrlich JR (1981) Aqueous bubble blowing composition. United States patent US4284534
Cottone S, Domsky II, Hussain H (2001) Edible bubble making composition and child’s toy. United States patent US6303164B2
Furutaka Y (2020) Soap bubble liquid composition. Japanese Patent Publication No. 2020–130743
Allen RY, Shaw M, Umstead DE (1962) Soap bubble toy. United States patent US3021639
Kainuma S (2008) Soap bubble toy. Japanese Patent Publication No. 2008–295924
Takeuchi N (2018) Soap bubble toy. Japanese examined utility model registration publication No. 321775
Barthlott W, Neinhuis C (1997) Purity of the sacred lotus, or escape from contamination in biological surfaces. Planta 202:1–8. https://doi.org/10.1007/s004250050096
Wang D, Sun Q, Hokkanen MJ, Zhang C, Lin FY, Liu Q, Zhu SP, Zhou T, Chang Q, He B, Zhou Q, Chen L, Wang Z, Ras RHA, Deng X (2020) Design of robust superhydrophobic surfaces. Nature 582:55–59. https://doi.org/10.1038/s41586-020-2331-8
Xue CH, Jia ST, Zhang J, Ma JZ (2010) Large-area fabrication of superhydrophobic surfaces for practical applications: an overview. Sci Technol Adv Mater 11:033002. https://doi.org/10.1088/1468-6996/11/3/033002
Esmeryan KD (2020) From extremely water-repellent coatings to passive icing protection—principles, limitations and innovative application aspects. Coatings 10:66. https://doi.org/10.3390/coatings10010066
Zhan Y, Yu S, Amirfazli A, Siddiqui AR, Li W (2022) Magnetically responsive superhydrophobic surfaces for microdroplet manipulation. Adv Mater Interfaces 9:2102010. https://doi.org/10.1002/admi.202102010
Zhan Y, Yu S, Amirfazli A, Siddiqui AR, Li W (2022) Recent advances in antibacterial superhydrophobic coatings. Adv Eng Mater 24:2101053. https://doi.org/10.1002/adem.202101053
Kubisch F, Heyne T (2016) Students’ alternative conceptions about the lotus effect: to confront or to ignore? J Biol Educ 50:86–100. https://doi.org/10.1080/00219266.2015.1007886
Yamamoto Y (1984) The toy for moving water droplet. Japanese Examined Utility Model Registration Publication No. S59–64195
Mita M (2017) Game toy. Japanese Patent Publication No. 2017–209132
Reick FG (1980) Toys and games using super-hydrophobic surfaces. United States patent US4199142
Guo XJ, Xue CH, Jia ST, Ma JZ (2017) Mechanically durable superamphiphobic surfaces via synergistic hydrophobization and fluorination. Chem Eng J 320:330–341. https://doi.org/10.1016/j.cej.2017.03.058
Zhang ZH, Wang HJ, Liang YH, Li XJ, Ren LQ, Cui ZQ, Luo C (2018) One-step fabrication of robust superhydrophobic and superoleophilic surfaces with self-cleaning and oil/water separation function. Sci Rep 8:3869. https://doi.org/10.1038/s41598-018-22241-9
Pan G, Xiao X, Yu N, Ye Z (2018) Fabrication of superhydrophobic coatings on cotton fabric using ultrasound-assisted in-situ growth method. Prog Org Coat 125:463–471. https://doi.org/10.1016/j.porgcoat.2018.09.026
Zhao L, Du Z, Tai X, Ma Y (2021) One-step facile fabrication of hydrophobic SiO2 coated super-hydrophobic/super-oleophilic mesh via an improved Stöber method to efficient oil/water separation. Colloids Surf A Physicochem Eng Asp 623:126404. https://doi.org/10.1016/j.colsurfa.2021.126404
Ge D, Yang L, Zhang Y, Rahmawan Y, Yang S (2014) Transparent and superamphiphobic surfaces from one-step spray coating of stringed silica nanoparticle/sol solutions. Part Part Syst Charact 31:763–770. https://doi.org/10.1002/ppsc.201300382
Korhonen KT, Huhtamäki T, Ikkala O, Ras RHA (2013) Reliable measurement of the receding contact angle. Langmuir 29:3858–3863. https://doi.org/10.1021/la400009m
Greenwoo P, Gevert B (2011) Aqueous silane modified silica sols: theory and preparation. Pigment Resin Technol 40:275–284. https://doi.org/10.1108/03699421111176171
Brinker CJ (1988) Hydrolysis and condensation of silicates: effects of structure. J Non-Cryst Solids 100:31–50. https://doi.org/10.1016/0022-3093(88)90005-1
Hackley VA (1997) colloidal processing of silicon nitride with poly (acrylic acid): I, adsorption and electrostatic interactions. J Am Ceram Soc 80:2315–2325. https://doi.org/10.1111/j.1151-2916.1997.tb03122.x
Hackley VA (1998) Colloidal processing of silicon nitride with poly (acrylic acid): II, rheological properties. J Am Ceram Soc 81:2421–2428. https://doi.org/10.1111/j.1151-2916.1998.tb02638.x
Shen MW, Chen ST (2020) Fabrication of an organo-fluoride–free hydrophobic film using the sol-gel method by factors screening and poly-acrylic acid addition. Coatings 10:1011. https://doi.org/10.3390/coatings10101011
Eliassaf J (1963) Solution of neutralized poly-(acrylic acid) in mixtures water-ethanol. J Appl Polymer Sci 7:s9–s10. https://doi.org/10.1002/app.1963.070070233
Takai-Yamashita C, Ando M, Noritake M, Razavi Khosroshahi H, Fuji M (2017) Emulsion templating of poly (acrylic acid) by ammonium hydroxide/sodium hydroxide aqueous mixture for high-dispersed hollow silica nanoparticles. Adv Powder Technol 28:398–405. https://doi.org/10.1016/j.apt.2016.10.010
Lin CH, Chang JH, Yeh YQ, Wu SH, Liu YH, Mou CY (2015) Formation of hollow silica nanospheres by reverse microemulsion. Nanoscale 7:9614–9626. https://doi.org/10.1039/C5NR01395J
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Arafumi Kimura declares that he has no conflict of interest. Kentaro Nagashima declares that he has no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 20728 kb)
Supplementary file3 (MP4 15657 kb)
Supplementary file4 (MP4 23488 kb)
Supplementary file5 (MP4 38857 kb)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kimura, A., Nagashima, K. A practical strategy for fabrication of transparent, robust and environmentally friendly superhydrophobic surfaces for toys and games. SN Appl. Sci. 4, 237 (2022). https://doi.org/10.1007/s42452-022-05118-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05118-5