Abstract
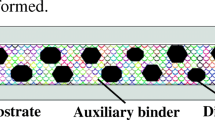
Metal-bonded grinding tools are commonly based on copper as bond material and possess low porosity. The powder metallurgic fabrication and the applied process parameters have a high influence on the mechanical properties of these grinding layers. In this study, Cu–diamond composites are fabricated through Field Assisted Sintering Technology with a variation of holding time, temperature, pressure, and chromium powder particle size. The addition of chromium to these composites can ensure a higher adhesion of the diamonds through carbide formation within the interface of the diamonds and the copper bonding matrix. The coating of diamond with chromium-carbide is mainly controlled by the chromium powder particle size, which leads to a higher critical bond strength with decreasing particle size. Maximum critical bond strength of 463 N/mm2 is reached using chromium with an average particle size of 10 µm. Increasing holding time decreases porosity and increases the critical bond strength of the composites. An increase of sintering temperature from 900 to 1040 °C leads to a decrease of porosity due to local melting of the copper. The interlocking of diamonds due to their high concentration of 50 vol% within the composites results in a relatively high porosity above 7%.
Article Highlights
-
Modelling of the influence of sintering temperature, sintering time and chromium particle size on the critical bond strength
-
Addition of chromium results in an in-situ formed carbide-layer when sintering above a temperature of 900 °C
-
Smaller chromium particle sizes significant increase the mechanical stability of CuCr–diamond composites
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
1 Introduction
A productive fabrication of tungsten carbide tools in a deep feed grinding process requires wear-resistant grinding tools. Metal-bonded or hybrid-bonded diamond grinding tools based on bronze as bond material are commonly used to machine cemented carbides [1]. These grinding tools are produced through sintering at high temperatures and high pressures. The grinding layer, consisting of the bonding and the abrasive material, is sintered together with a tool base unit [2]. High mechanical stability is important for the resulting grinding layer to withstand the high process forces and ensure a stable grinding behaviour. The grain retention of diamond within the grinding layer matrix, which can be derived from the mechanical stability, is an important factor influencing tool wear. To ensure sharp cutting edges, the release of diamonds that have been flattened due to grain wear out of the bonding matrix is necessary [3, 4]. This self-sharpening effect is desirable, and the right balance between mechanical stability and the release of the flattened grains from the matrix needs to be evaluated. The inert behaviour of bronze or copper towards diamond results in an only form-fit connection of the grains within these bonding matrixes. A chemical bonding of diamond grains within the bonding matrix that forms an adhesive connection can be achieved through addition or coating with carbide forming elements, such as chromium [5], titanium [6], or cobalt [7]. Sintered at high temperatures and pressures the resulting interfacial carbide layer enables to adjust the mechanical stability of the grinding layer and therefore the grain retention. For addition or coating with chromium, various researchers [8,9,10,11] have proved the formation of the carbide phases Cr3C2 or Cr7C3 within the interface of diamond and surrounding copper matrix. In none of these studies, the effect of chromium particle size and the interaction effect of the sintering parameters, temperature, sintering time, or pressure on the carbide formation and the mechanical properties has been systematically studied. These enhanced copper diamond composites possess high strength, low expansion coefficient, and reduced thermal resistance across the interface [12]. The interfacial phase does not only increase the mechanical stability of the composite material but can close the gap between the diamond and the metallic bonding matrix. This interfacial layer can reduce the interfacial thermal resistance and, therefore, increase the thermal conductivity of the composite material. Besides the bonding and abrasive material, the porosity of the grinding wheel is significant for the grinding process. High porosity can increase the coolant transportation within the abrasive layer, ensuring better cooling and lubrication and chip removal from the grinding zone [13]. A larger chip storage space due to an increase of porosity without a reduction of mechanical stability can increase the grinding performance of the tool through the ability to grind at higher process parameters [14]. A systematic investigation of how the mechanical properties derived from the carbide formation and the sintering parameters can be adjusted is necessary to characterize the grinding layer. The characterization can then be used to study the effect of the grinding layer properties on the grinding behaviour in the machining of cemented carbides. Therefore, the effect of the sintering parameters and the particle size of chromium on the porosity is investigated in Sect. 3.1, followed by the investigation on the mechanical properties of the composites in Sect. 3.2. Finally, the interfacial carbide formation is investigated and characterized in Sect. 3.3.
2 Materials and methods
To investigate the influence of sinter process parameters on the material properties, bond composition, and carbide formation, cylindrical abrasive layer specimens with a diameter of 22 mm and a height of 5 mm are fabricated by the Field Assisted Sintering Technology (FAST) using a Dr. Fritsch GmbH & Co. KG DSP 510 sintering press in a vacuum. Dr. Fritsch GmbH & Co. KG DIACU4500 copper powder with a particle size of < 45 µm and Ceratonia GmbH & Co. KG CNF3080 diamond powder with a grain size of 38–45 µm are used. To investigate the influence of the particle size of chromium on the composites, Dr. Fritsch GmbH & Co. KG DIACHROME4500 is used as purchased for a particle size of < 45 µm and sieved through a sieve with a grid distance of 25 µm to obtain a < 25 µm fraction. Furthermore, ABCR GmbH chromium powder with an average particle size of 10 µm is used (Fig. 1).
Powder mixtures of 50 vol% diamond and 50 vol% bond mixture of 95 wt% copper and 5 wt% chromium are given to a Turbula T2F powder blender mixer for 30 min at a cycle rate of 101 rpm. The bond mixture is not mixed in advance. Afterward, the mixture is filled in and is pre-compacted in a graphite die with an applied load of 5 MPa. During sintering, a load of 35 MPa or 70 MPa is applied. Samples are sintered with a heating rate of 100 °C/min and a holding step at 900 °C and 1040 °C for 900 s and 1200 s. An example of the course of temperature and pressure during the sintering process is illustrated in Fig. 2. To study the effect of diamond concentration on the porosity, the diamond concentrations are varied in 5 steps from 0 to 50 vol%, as shown in Fig. 3. Here, a load of 35 MPa, a holding step of 1200 s, and a sintering temperature of 900 °C is applied. The mixing process, pre-compaction, and heating rate are chosen as described above.
All specimens are uniaxial loaded until they collapse in a 3-point flexural test to explore the mechanical properties of the composites. The vertical force Fz that results in the collapse of the sample is measured. Afterward, the critical bond strength (CBS) is calculated. The CBS is calculated by considering the dimension of the specimen (diameter d, height h) and the distance between the bearings l, which are here set to 14 mm. With the assumption from beam theory that Iy = (d·h3)/12, the CBS σ can be calculated following formula 1:
The density ρ of the specimens is measured based on Archimedes’ principle using an EMB 200-3 V density scale from Kern & Sohn GmbH. The fractured surface of the collapsed Cu-xCr specimens and the morphology of the carbide phase is investigated by scanning electron microscopy (SEM) with a Joel JSM-7610FPlus. EDS-element-mappings and line scans are obtained using two Bruker XFlash 6-60 detectors and the EDS-software Quantax. X-ray diffraction patterns in a range of 20°–99.95° with a step length of 0.05°, are obtained by a two-circle diffractometer system XRD 3003 TT in a Θ/Θ setup, using Cu Kα radiation.
3 Results and discussion
3.1 Porosity
Porosity is one of three main components of a grinding tool crucial for grinding behaviour. The porosity (ф) of the investigated composites is derived using the theoretical density of the composite calculated by the rule of the mixture and the measured density of the composites (2). The formation of the Cr3C2 phase described below is therefore not been considered because it cannot be quantified.
Usually, metal bonded grinding wheels are dense due to the sintered metal-matrix and therefore possess a low porosity below 2% [15]. In the present study, the high diamond grain concentration of 50 vol% causes increased porosity due to the interlocking of diamond particles during the compression within the sintered body. Figure 3 shows the porosity at various diamond concentrations. Above a diamond concentration of 37.5 vol%, the porosity of the composites sharply increases. A possible explanation for this is that the diamonds tend to interlock with neighboured diamonds at higher diamond concentrations and, therefore, locally prevent further compression of the bonding material. This could result in a lower reduced load on the bonding particles during sintering, resulting in a higher microporosity of the copper-chromium matrix with an average pore size of 2–5 µm (Fig. 3).
Composites sintered at 900 °C exhibit an increased porosity compared to the composites sintered at 1040 °C. The decrease in particle size of chromium and an increase of holding time results in a decrease of porosity at both sintering temperatures (Fig. 4).
The sintering temperature of 1040° C is close to the solidus temperature of copper of 1085 °C. During the sintering process, the temperature is measured and monitored with average fluctuations below 2 °C. However, the temperature is measured using NiCrNi-thermocouples in drillings within the graphite die that reach up to 2 mm to the sintered material. The indirect temperature measurement cannot rule out a possible offset of temperature within the composite material during sintering, with the potential that-at least partly-the melting point has been exceeded. It can be assumed that this would decrease porosity due to the melt that can move within the interlocked composite material and explains the relatively lower porosity compared to sintering at 900 °C.
Besides the sintering temperature, the applied pressure during sintering also affects the porosity and the mechanical properties of the composite. The increase in pressure from 35 to 70 MPa results in a significant decrease in porosity of 50%, whereas the CBS of the composite is not strongly affected. The variation of the CBS with increasing pressure is within standard deviation and can therefore be neglected (Fig. 5).
3.2 Mechanical properties
An adjusted critical bond strength (adj. CBS) is calculated, to neglect the effect of the porosity (ф) on the critical bond strength (Eq. 2).
For all temperature and holding time combinations, an increase of particle size causes a decrease of adj. CBS. For holding times of 900 and 1200 s, a decrease of temperature results in an increase of adj. CBS. An increase of holding time results in an increase of adj. CBS while this increase is also more pronounced at a temperature of 900 °C. The highest adj. CBS of 463 N/mm2 is reached at a temperature of 900 °C and a holding time of 1200 s using chromium particles with an average diameter of 25 µm. To further study the effect of the particle size and further increase the adj. CBS, chromium powder with an average particle size of 10 µm is used. The results show that the adj. CBS can be further increased to 475 N/mm2. Zhang et al. and Xie et al. reported maximum bending strengths of CuCr-diamond composites fabricated by pressure infiltration of 525 N/mm2 at 0.8 wt% chromium and 523 N/mm2 at 0.7 wt% chromium, respectively [10, 16]. Both reports focus on optimizing the interfacial thermal resistance as well as the bonding strength and varied the chromium content below 1 wt% with a particle size of 60–70 µm. Compared to these reports, the here presented research at 5 wt% chromium addition implicates that the bond strength can also be optimized by a decrease in particle size. Within the investigated process parameters, the adj. CBS is most affected by the chromium particle size, as shown in the pareto chart (Fig. 6b), followed by the holding time that affects the adj. CBS positively. The data of the adj. CBS shown in Fig. 6a can be described as an interaction model (3):
where T is the sintering temperature within 900 to 1040 °C, t is the holding time within 900 to 1200 s, and ø is the chromium particle size between 25 and 45 µm. The importance of this empirical model for the ongoing research project is described in the outlook.
3.3 Interfacial characterization
SEM micrographs of the fractured surface of the composite specimens provide information on the in-situ carbide formation at the surface of the diamonds and the morphology of the bonding matrix (Fig. 7). The use of chromium with a particle size of 45 µm results in an only partly coated diamond surface with chromium carbide. The diamond grains appear to be internally intact and not effected bydegradation through graphitization [7]. Diamond grains sintered with copper and chromium with a particle size of 25 and 10 µm appear to be fully coated with chromium carbide. Internally broken diamond grains are apparent within at the fractured surface. It can be assumed that these diamonds shattered due to the load of the 3-point-flexural test and the higher adhesion of the diamonds within the bonding matrix. The diffusion coefficient of Cr in Cu at 1040 °C is generally low with a value of 65.5·10–15 m/s and decreases further with decreasing temperature [17]. Because of the negligible diffusion paths at holding times of 900 s or 1200 s, it can be assumed that the diffusion of Cr in Cu has a minor effect on the reaction of diamond and chromium. However, the local availability of chromium in contact with diamond directly after the mixing process has a major influence on carbide formation. Due to the smaller particle size, the amount of chromium particles within the matrix increases when keeping the mass percentage constant. Therefore, the probability of a chromium particle in contact with a diamond grain increases because it is dependent on the number of particles within the matrix [18]. Furthermore, the decrease in particle size results in a higher specific surface area of the chromium particles. Since surface diffusion between particles is the predominant mechanism during sintering, it can be assumed that the relatively higher surface area with decreasing particle size leads to a higher reactivity and the more pronounced carbide formation and, therefore, the higher adhesion.
Using EDS-analysis, copper, chromium, and carbon can be identified (Fig. 8). Due to the preparation and coating of the specimens, Au can also be identified. The EDS-mapping gives an insight into the composite structure that possessed the highest adj. CBS sintered at 900 °C for 1200 s using a 10 µm chromium particle size. The green visualized areas within the blue visualized copper matrix correspond to the remaining chromium within the bonding matrix that has not reacted with diamond. Within the interface of copper and diamond, an increase of chromium intensity is visible surrounding the entire broken diamond grain. The intensity of Cu drops significantly when reaching the interfacial area. The grey visualized broken surface of the diamond grain is unaffected by chromium and copper. The surrounding carbide layer significantly increases the adhesion of diamond within the copper matrix. This results in an internal breakage of several diamonds due to the 3-point flexural test instead of an outbreak of diamonds from the copper matrix. These internal breaks of diamond were only observed using the 25 and 10 µm particle size chromium powder indicating stronger adhesion through the complete in-situ coating with the carbide that is following the data for the adj. CBS.
Qualitative analysis of the composite sample with energy-dispersive X-ray spectroscopy. Through the spectra analysis, copper, carbon, chromium, and gold (from the coating) can be identified (top). An EDS-Mapping combined with a line scan shows a broken diamond grain (grey) surrounded by an approximated 1 µm thick chromium layer (green) and the copper-chromium matrix (blue/green)
For further analysis, the diamonds are extracted from the CuCr-matrix using nitric acid before XRD-analysis. This chemical leeching procedure leads to the solution of copper but does not influence carbon-containing crystallites [7]. In this way, the in-situ carbide coating and diamonds can be measured with relatively high intensity due to the absence of copper. A single extracted diamond-coated carbide layer can be seen on the right in Fig. 9. Through comparison and the good agreement of the theoretical reflections of Cr3C2 and the pattern of the extracted diamonds, the formation of Cr3C2 can be assumed for all investigated samples. Furthermore, remnant Cu can be detected in all leeched diamond samples indicating an incomplete dissolution through the extraction process. Exemplary, the XRD pattern of one sample is given in Fig. 9. The results verify the formation of the intended chromium carbide layer on the surface of the diamond grains.
4 Conclusions
Cu–Cr–diamond composites with 50 vol% diamond concentration are fabricated by FAST. Using different sintering conditions and powder metallurgical routes, the following conclusions about the properties of the manufactured Cu–Cr–diamond composites can be derived.
The porosity of conventional metal bonded grinding tools is exceeded with a porosity of approximately 7% while still exhibiting high mechanical stability. The high volume of diamond acts as a resistance against the compression during sintering through the interlocking of the different grains within the bonding matrix. Due to a formation of a 1 µm thick chromium-carbide layer within the interface between diamond and the copper-chromium matrix, the grain retention is increased. The particle size of the added chromium powder exhibits a crucial role in the local availability of chromium in contact with diamond grains because diffusion of chromium within the copper matrix is generally low. A decrease in particle size can therefore ensure a higher local availability due to an increased amount of particles within the matrix when keeping the mass percentage of chromium constant. Furthermore, the increase in relative surface area with decreased particle size leads to a higher reactivity between diamonds and chromium particles since surface diffusion is one of the main mechanisms during sintering. This in-situ reaction of diamond and chromium leads to the formation of Cr3C2 at the interface of diamond and bonding matrix, resulting in higher critical bond strength. Higher critical bond strength also emerges by increasing the sintering pressure or time but results in a decrease of porosity. Using sintering temperatures near the melting point result in a decrease of porosity through local melting of the copper matrix.
5 Outlook
Future work will focus on the characterization of the thermal conductivity of the CuCr–diamond composites. Knowledge of thermal and mechanical properties combined with the application behaviour enables the formulation of empiric models that predict process forces and wear as a function of grinding layer properties and grinding process parameters. Furthermore, micro CT will be used to unravel the mechanisms that lead to high porosity at diamond concentrations above 37.5%.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Denkena B, Grove T, Bremer I, Behrens L (2016) Design of bronze-bonded grinding wheel properties. CIRP Ann 65:333–336. https://doi.org/10.1016/j.cirp.2016.04.096
Linke BS (2014) Sustainability concerns in the life cycle of bonded grinding tools. CIRP J Manuf Sci Technol 7:258–263. https://doi.org/10.1016/j.cirpj.2014.05.002
Tsuwa H (1964) An Investigation of grinding wheel cutting edges. J Eng Ind 86:371–382. https://doi.org/10.1115/1.3670568
Malkin S, Cook NH (1971) The wear of grinding wheels: part 1—attritious wear. J Eng Ind 93:1120–1128. https://doi.org/10.1115/1.3428051
Zhang H, Zhang J, Liu Y, Zhang F, Fan T, Di Z (2018) Unveiling the interfacial configuration in diamond/Cu composites by using statistical analysis of metallized diamond surface. Scripta Mater 152:84–88. https://doi.org/10.1016/j.scriptamat.2018.04.021
Chang G, Sun F, Duan J, Che Z, Wang X, Wang J, Kim MJ, Zhang H (2018) Effect of Ti interlayer on interfacial thermal conductance between Cu and diamond. Acta Mater 160:235–246. https://doi.org/10.1016/j.actamat.2018.09.004
Tillmann W, Ferreira M, Steffen A, Rüster K, Möller J, Bieder S, Paulus M, Tolan M (2013) Carbon reactivity of binder metals in diamond–metal composites—characterization by scanning electron microscopy and X-ray diffraction: Metal composites? Characterization by scanning electron microscopy and X-ray diffraction. Diam Relat Mater 38:118–123. https://doi.org/10.1016/j.diamond.2013.07.002
Schubert T, Ciupiński Ł, Zieliński W, Michalski A, Weißgärber T, Kieback B (2008) Interfacial characterization of Cu/diamond composites prepared by powder metallurgy for heat sink applications. Scripta Mater 58:263–266. https://doi.org/10.1016/j.scriptamat.2007.10.011
Jia SQ, Bolzoni L, Li T, Yang F (2021) Unveiling the interface characteristics and their influence on the heat transfer behavior of hot-forged Cu–Cr/diamond composites. Carbon 172:390–401. https://doi.org/10.1016/j.carbon.2020.10.036
Zhang X, Guo H, Yin F, Fan Y, Zhang Y (2011) Interfacial microstructure and properties of diamond/Cu-xCr composites for electronic packaging applications. Rare Met 30:94–98. https://doi.org/10.1007/s12598-011-0204-x
Zhao C, Wang J (2013) Enhanced mechanical properties in diamond/Cu composites with chromium carbide coating for structural applications. Mater Sci Eng A 588:221–227. https://doi.org/10.1016/j.msea.2013.09.034
Grzonka J, Kruszewski MJ, Rosiński M, Ciupiński Ł, Michalski A, Kurzydłowski KJ (2015) Interfacial microstructure of copper/diamond composites fabricated via a powder metallurgical route. Mater Charact 99:188–194. https://doi.org/10.1016/j.matchar.2014.11.032
Gviniashvili VK, Woolley NH, Rowe WB (2004) Useful coolant flowrate in grinding. Int J Mach Tools Manuf 44:629–636. https://doi.org/10.1016/j.ijmachtools.2003.12.005
Ding WF, Xu JH, Chen ZZ, Yang CY, Song CJ, Fu YC (2013) Fabrication and performance of porous metal-bonded CBN grinding wheels using alumina bubble particles as pore-forming agents. Int J Adv Manuf Technol 67:1309–1315. https://doi.org/10.1007/s00170-012-4567-4
Tian C, Li X, Li H, Guo G, Wang L, Rong Y (2019) The effect of porosity on the mechanical property of metal-bonded diamond grinding wheel fabricated by selective laser melting (SLM). Mater Sci Eng A 743:697–706. https://doi.org/10.1016/j.msea.2018.11.138
Xie Z, Guo H, Zhang X, Huang S, Xie H, Mi X (2021) Tailoring the thermal and mechanical properties of diamond/Cu composites by interface regulation of Cr alloying. Diamond Relat Mater. https://doi.org/10.1016/j.diamond.2021.108309
Hoshino K, Iijima Y, Hirano K-I (1977) Diffusion of vanadium, chromium, and manganese in copper. Metall Mater Trans A 8:469–472. https://doi.org/10.1007/BF02661758
Coble RL (1973) Effects of particle-size distribution in initial-stage sintering. J Am Ceram Soc 56:461–466. https://doi.org/10.1111/j.1151-2916.1973.tb12524.x
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors would like to thank the German Research Foundation (DFG) for their organizational and financial support within the project DE447/184-1.
Author information
Authors and Affiliations
Contributions
Conceptualization, methodology, formal analysis, investigation, data curation, visualization, writing—original draft preparation: RL; writing—review and editing: BB; supervision: BD. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Denkena, B., Bergmann, B. & Lang, R. Influence of the powder metallurgy route on the mechanical properties of Cu–Cr–diamond composites. SN Appl. Sci. 4, 161 (2022). https://doi.org/10.1007/s42452-022-05048-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05048-2