Abstract
This work presents a new and simple approach to prepare Dialdehyde Starch (DAS) in one step under microwave irradiations and using, a mild and safer oxidizing agent, potassium iodate. Aldehyde content was evaluated to compare the synthesis results with DAS prepared using potassium periodate as an oxidizing agent for starch. To optimize the synthesis parameters of the new approach, the effect of the quantity of oxidizing agent and the effect of reaction time on the content of aldehyde in DAS were evaluated. According to the results, the optimized time was 10 min at the power of 300 W, and the number of moles of oxidizing agent was 0.014 mol per 2 g of starch. After that, DAS was used to prepare two Schiff bases by reaction with urea (DASU) and thiourea (DASTU), respectively. DAS, DASU and DASTU were characterized by FTIR, XRD, and SEM. Furthermore, DAS, DASU and DASTU were investigated for removing Cu(II), Pb(II), Hg(II), Cd(II), and Cr(III) ions from water. DAS showed the highest removal efficiency towards Pb(II) ions, whereas DASTU exhibited excellent ability for removing the Hg(II) ions. The removal efficiencies of DAS for Pb(II) ions and DASTU for Hg(II) ions are 95.25% and 89.45%, respectively from aqueous solutions containing 100 ppm of respective ions. Adsorption isotherm study suggests that adsorption follows Langmuir isotherm model, (correlation factors (R2) for Langmuir and Freundlich models for DAS/Pb are equal to 0.984 and 0.799, respectively, and for DASTU/Hg they are 0.995 and 0.813, respectively). The theoretical maximum adsorption capacity for DAS/Pb and DASTU/Hg are 245.09 and 180.83 mg/g, respectively.
Similar content being viewed by others
1 Introduction
Over the last few decades, the world has confronted a serious environmental problem related to the pollution of water bodies by heavy metal ions and organic pollutants. This problem started to exacerbate as a result of large activity of industries such as mining, electroplating, pharmaceutical, fertilizer manufacturing industries, and others [1, 2]. Toxic wastes that the industries leave behind (pigments, pesticides, toxic chemicals, ions of heavy metals,…) are disposed of in waterways [3, 4]. Over the years, the concentrations of these toxic metal ions or organic pollutants accumulate in water and become larger than the maximum permissible limits set by the Environmental Protection Departments in each country. The impact of these wastes becomes negative on all forms of life in the ecosystem, especially on human life health, as they cause serious health issues. Studies conducted in China and Cambodia showed high concentrations of arsenic and lead in groundwater [1, 5], and another study conducted in Pakistan on bottled mineral water containers showed a concentration of arsenic that exceeded the permissible limits [6]. Another study conducted on groundwater in the Kingdom of Saudi Arabia showed that the levels of lead, cadmium and copper ions exceeded the permissible limits as well [7]. It was also reported that the average level of mercury in certain water bodies, rich with aquatic species suitable for human consumption, exceeds the maximum level set by the World Health Organization, 0.002 mg/L [8]. According to US Environmental Protection guidelines, arsenic and lead ions in drinking water should be less than 10 parts in billion and 0.08 mg/L respectively, whereas the highest permissible concentration of copper ions in drinking water is 1.3 mg/L, and for chromium ions is 0.1 mg/L [1, 9, 10].
To overcome this serious problem related to water bodies pollution, an extensive research has been conducted to investigate novel approaches to remediate polluted water [2, 4, 5, 11,12,13]. In this respect, novel ecofriendly materials were designed, and their ability to adsorb pollutants was explored. Polysaccharides borne hydrogels, metal-organic frameworks, carbon quantum dots and membranes based on biodegradable polymers are some examples of newly prepared materials [2, 4, 14,15,16,17,18,19]. Materials based on starch derivatives are no exception. They have been prepared and investigated as potential efficient bio-sorbents [20,21,22,23].
Starch is the principal source of dietary calories to the world’s human population. It has also many chemical and physical characteristics that set it apart from other food components and involve it in numerous applications. Native starch is widely used in foods, textile sizing, oil field applications, paper, and pharmaceutical industry [24]. However, starch is not soluble in water and its shear properties and thermal stability are poor. All these limitations hinder the use of starch in industrial applications. In order to cope with the above-mentioned constraints, starch must be modified. The most common techniques for starch modification include physical, chemical, and enzyme alterations [25].
Dialdehyde starch (DAS), as one of the main derivatives of starch, exhibits excellent physical, chemical, and biochemical characteristics such as alkaline solubility, strong adhesivity, safety, biodegradability [26]. DAS is also used to prepare stable molecules loaded with anti-cancer drug (doxorubicin), where this compound showed high efficacy and good drug release time [27]. In other research, starch was modified to dialdehyde and used as a crosslinking agent in collagen-based wound dressing materials [28].
Usually, DAS is prepared by periodate oxidation [29]. Periodic acid is a highly selective oxidizing agent, which cleaves the C2–C3 linkage of anhydroglucose units with the formation of dialdehyde groups [30]. However, periodates are known for their high toxicity and irritability to the skin and eyes [31].
Due to the diversity of DAS potential applications, intensive research was conducted on the preparation and characterization of DAS and its derivatives. Almost all of the reported preparation methods used sodium periodate in acidic medium at temperature between 5 and 35 °C for 3–24 h [30, 32,33,34,35].
The main objective of the current research is to prepare DAS and two of its Schiff bases DASU and DASTU in order to explore their capacity as adsorbents for water remediation processes. To this end, a novel approach to prepare DAS in one pot under microwave irradiations, using a mild oxidizer is reported. The novel method saves time and energy in comparison with conventional ones. For the first time, potassium iodate, as a mild oxidizing agent, was used. It is less dangerous and less toxic than the commonly used oxidizer of starch, sodium periodate [36]. To the authors' knowledge, there is no study dealing with the preparation of DAS using a mild oxidizing agent, such as potassium iodate, under microwave irradiation. The characterization of the prepared DAS and its derivatives will also be presented. The use of DAS, DASU and DASTU for water treatment is also investigated.
2 Materials and methods
2.1 Chemicals
Starch (GR), Potassium Iodate (99.5%), Urea (99%), Thiourea (98%), Sulfuric acid (98%), Copper Nitrate (99%), Cadmium Nitrate (100%), Mercury Nitrate (98%), and Lead Nitrate (97%) are purchased from Merck. Sodium Periodate (99.8%) is purchased from Riedel, whereas Acetone (99.5%), Hydrochloric acid (37%), and Sodium Hydroxide (99.5–100%) are purchased from Panreac and Ethanol (99.8%) from Chem-Lab. All chemicals are used as purchased.
2.2 Preparation of dialdehyde starch (DAS)
A mass of 2.9 g of potassium iodate was dissolved in 50 mL HCl (0.6 mol/L). Then, 2 g of starch was added and the mixture was stirred for 5 min. The mixture was treated in a domestic microwave oven at a power of 300 W, and allowed to react for 10 min. A volume of 25 mL of acetone was added to the reactive mixture after cooling it. Then, the mixture was kept in a refrigerator at 5 °C for 2 h. After that, the obtained DAS was filtered and washed three times with acetone. Finally, the sample was kept in a drying oven at 50 °C for 24 h. The same procedure was used, but with sodium periodate, to prepare the sample (DAS-P) for comparison reasons.
2.3 Preparation of DAS schiff bases with Urea (DASU) and thiourea (DASTU)
A mass of 6 g of DAS was dispersed in 70 ml of distilled water in a flask with a round-bottom which was submerged in a water bath. Then, 2 g of urea was slowly added into the flask. The mixture was stirred for 4 h at 55 °C. The slurry was filtered and washed with distilled water and ethanol three times, and the product was then dried at 50 °C for 24 h. The same procedure was repeated for the preparation (DASTU) by using thiourea instead of urea.
2.4 Aldehyde group content (Da%) determination
To determine the (–CHO) group content, the rapid quantitative alkali consumption method was used [37]. First, standardized sodium hydroxide (0.2 mol/L) and sulfuric acid (0.2 mol/L) solutions were prepared. After that 0.2 g of DAS powder was added into a 250 mL conical flask, and 20 mL of standardized sodium hydroxide. Then, the flask was kept for 4 min in a water bath at 70 °C. After the mixture was rapidly cooled down, 20 mL of standardized sulfuric acid, 100 mL of distilled water, and 2 mL of phenolphthalein were added. Finally, the acid solution was titrated using the standardized sodium hydroxide [38].
where C1 is the concentration of NaOH (mol/L), C2 is the concentration of H2SO4 (mol/L), W is the mass of the used DAS, V1 is the volume of NaOH (mL), V2 is the volume of H2SO4 (mL), and 161 represents the average molecular weight of the repeating unit in DAS [38].
2.5 Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra of starch, DAS, DASU, and DASTU were recorded in the range 500–4000/cm using IR spectrophotometer (Bruker, Vector 22). The FTIR samples were prepared by mixing the powder of the sample with an appropriate amount of KBr powder. Then, the mixture was grinded to obtain a very fine powder. After that, the powder was placed in a cylindrical mold, and a compressive force was applied, using a hydraulic press of 5 tons, to obtain a very thin film.
2.6 X-ray diffractometry (XRD)
A PHILIPS-PW3710XRAY diffractometer was used to record the XRD patterns of the samples, equipped with Cu-Kα radiation (λ = 0.154 nm), operating at 60 kV, and the range of 2θ scan angle was between 10 and 70°.
2.7 Scanning electron microscopy (SEM)
The morphology of the samples was examined using scanning electron microscope VEGA II TESCAN, SEM instrument. The samples were coated with graphite.
2.8 Adsorption experiments
Adsorption experiments were carried out by batch method [38], at room temperature to determine the adsorption capacity (Q) and the percentage of removal efficiency (R.E.) of each DAS, DASU, and DASTU for Cu(II), Pb(II), Hg(II), Cd(II), and Cr(III).
For each heavy metal ion, an aqueous solution of 100 ppm was prepared. In order to investigate the competitive adsorption of ions, another aqueous solution, containing all the studied metal ions, at a concentration of 100 ppm for each ion was also prepared. Then, a mass 0.05 g of DAS or DASU or DASTU was added into 50 cm3 of aqueous solutions containing 100 ppm of each investigated heavy ion. After stirring for 24 h at a temperature of 25 °C to make sure that the adsorption process reaches the equilibrium, the mixture was filtrated, and the final concentrations of metal ions Ct (mg/L) were determined using Atomic Absorption Spectrophotometer, and the adsorption capacity (Q) was calculated using Eq. (2), and the percentage removal efficiency (R.E.) was calculated using Eq. (3):
where C0 (mg/L) is the initial concentration of the metal ions in the aqueous solution, Ct (mg/L) is the final concentration of the metal ions after the adsorption experiment, V is the volume of the aqueous solution (mL), m (g) is the mass of the adsorbent (DAS, DASU or DASTU).
3 Results and discussions
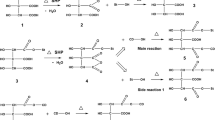
Dialdehyde starch was prepared using potassium iodate under microwave irradiation. The oxidation leads to the cleavage of C2–C3 bonds of anhydrous glucose units. Then, DAS was further reacted with urea and thiourea to form Schiff bases (DASU and DASTU) as illustrated in Scheme 1.
3.1 Parameters optimization
3.1.1 Effect of the quantity of oxidizing agent
For comparison reasons, DAS was prepared using either potassium iodate or sodium periodate as an oxidizing agent under microwave irradiation. Table 1 shows the aldehyde group content, and the number of moles of oxidizing agent. The synthesis was performed under the following conditions for this set of experiments: mass of starch was 2 g, power of microwave was 300 W, and reaction time was 10 min.
From Table 1, it can be observed that aldehyde group content is greater for DAS-P samples compared with DAS samples. This result is expected, since periodate is a more powerful oxidizer. Nevertheless, aldehyde group contents for all the samples are relatively close. It is worthy to mention that increasing the quantity of periodate may result in decreased reaction yield or even product deterioration. On the contrary, increasing the quantity of iodate oxidizer enhances the reaction yield to some extent without deterioration of the product. This confirms the mild role of iodate as an efficient starch oxidizer.
3.1.2 Effect of reaction time
Synthesis was performed at different reaction times using a mass of starch equals to 2 g, number of moles of oxidizing agent was 0.014 mol, and the power of microwave was at 300 W. The results are listed in Table 2. Both oxidizing agents were also used to compare for comparison reasons.
For iodate ions, the aldehyde group content increases with increasing time from 5 to 10 min. The, it doesn’t change. This indicates that 10 min is an optimum time for synthesis. As for periodate ions, an induction time of at least 5 min is necessary to ignite the reaction. This result seems surprising, since periodate is more powerful oxidizer. One could contribute this to a higher activation energy for the reaction with periodate. However, once the reaction is initiated, the content of aldehyde groups using periodate ions is higher than that using iodate ions.
According to the results of the above-mentioned experiments, the optimized DAS will be prepared using the following parameters: reaction time is 10 min at the power of 300 W, and the number of moles of oxidizing agent is 0.014 mol per 2 g of starch.
3.2 FTIR spectra
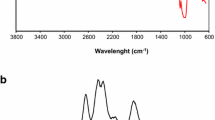
The FTIR spectra of starch, DAS, and DAS-P is shown in Fig. 1A. FTIR spectrum of starch showed a band at 1089/cm is attributed to the stretching vibration for cyclic ether group of glucose molecule, while the band at 3437/cm corresponds to stretching vibration of hydroxyl group.
FTIR spectra of DAS-P and DAS show a new peak at 1702/cm and 1683/cm respectively, and it is attributed to the stretching vibrations of the carbonyl group. This peak doesn’t appear in the FTIR spectrum of starch. This is an indicator of the successful synthesis of DAS.
FTIR spectra of DASU and DASTU are exhibited in Fig. 1B, and show that the peak at 1683/cm of the C=O groups disappear and an another peak at 1650/cm appears, and it is related to stretching vibration of imine groups (C=N). The characteristics of IR bands are in good match with the findings reported in scientific literature [39,40,41].
3.3 XRD patterns
X-ray diffraction patterns of starch and DAS are depicted in Fig. 2. They show that starch microstructure contains crystalline and amorphous regions. The positions of XRD diffraction peak were around 14°, 18° and that refers to monoclinic (A-type) crystalline structure [42, 43]. The percentage of the crystalline structure is decreased after oxidization process, and it is converted into an amorphous structure. This decrease in crystallinity is also proved by the increase of the halo of the amorphous phase in the diffraction pattern.
3.4 Scanning electron microscopy (SEM)
Scanning electron microscopy (SEM) was used to study the morphology of the starch and DAS (Fig. 3). The starch particles are oval-shaped granules and showed smooth surfaces. After oxidation, DAS particles appeared fibrillary and had fabric morphology with high specific surface area. This suggests better adsorption abilities of the DAS.
3.5 Adsorption results
The adsorption capacity of DAS, DASU, and DASTU for Cu(II), Pb(II), Hg(II), Cd(II), and Cr(III) was studied by conducting batch adsorption experiments. The results are listed in Table 3.
The results in Table 3 shows the DAS has a higher ability for removing the Pb(II) ions than other ions, while the DASU and DASTU have excellent ability for removing the Hg(II).
In order to simulate real situation, the competitive adsorption experiments are performed for testing the adsorption capacity of the previous compounds towards Pb(II) and Hg(II) ions at 100 ppm when they are in aqueous solution with other heavy metal ions. The results depicted in Table 4 showed a good match with the previous results from the adsorption experiments of each ion being alone in the aqueous solution. The results also showed that the DAS has still higher adsorption ability and selectivity towards Pb(II) than the Hg(II) ions, while the DASU and DASTU have always excellent selectivity towards Hg(II) ions. Furthermore, DASTU has higher adsorption selectivity towards Hg(II) ions than the DASU.
These results could be explained due to the relatively big ionic volume of Pb(II) and Hg(II) which would be suitable for the gaps in the DAS, DASU, and DASTU structures. Furthermore, the higher removal efficiencies towards Pb(II) and Hg(II) for DAS and DASTU, respectively could be related to highly stable coordination complexes of those ions with the binding sites on the bio adsorbents.
3.6 Adsorption isotherms
Langmuir and Freundlich’s models are applied to demonstrate the adsorption equilibrium between the adsorbate and adsorbent surface area. To obtain the adsorption experimental data, Langmuir and Freundlich’s models were studied [44]. Langmuir model assumes that the absorption occurs on a single homogeneous layer of the absorbent’s surface, and the absorption sites have the same energy. Langmuir model is usually presented as a relation between the equilibrium adsorption capacity (q), and the equilibrium concentration of the metal ions (Ceq) [45, 46]:
where kL (L/mg) is the Langmuir constant which expresses the interaction between adsorbate and the surface of the absorbent. Qmax refers to the maximum adsorption capacity.
While Freundlich model supposes that the adsorption takes place at heterogeneous sites on the adsorbent surface, and it is expressed by the following equation [45, 46]:
where kF is the Freundlich constant and it is a measure of the sorption capacity, while n is an empirical constant. Figures 4 and 5 exhibit Langmuir and Freundlich curves of DAS/Pb and DASTU/Hg, respectively, and the constants of each isotherm model are listed in Table 5.
From Figs. 4, 5, and Table 5, the correlation factors for Langmuir and Freundlich models of DAS/Pb are 0.984 and 0.799, respectively. As for DASTU/Hg, they are 0.995 and 0.813, respectively. By comparing these values, it is readily noticed that Langmuir model is better to explain the adsorption process of heavy metal ions on both DAS and DASTU. The values of the Qmax from Langmuir model for DAS/Pb and DASTU/Hg are 206.47 and 160.54 mg/g, respectively. According to Table 5, one can notice the good match between the maximum adsorption capacities evaluated experimentally, and the maximum adsorption capacities evaluated using Langmuir model.
According to these results, one can infer that the adsorption follows Langmuir adsorption model, which means that the adsorption occurs at specific binding sites that are localized on the surface of the adsorbent and all adsorption sites on the surface of the adsorbent are identical. Lead and mercury ions form strong coordination bonds with carbonyl, hydroxyl and imine groups present at the surface of the bio-adsorbents.
Gibbs free energy ΔGo is the thermodynamic state function that indicates the spontaneity of the interaction of between the adsorbent and the adsorbate when it has negative value [44]. To calculate ΔGo for the adsorption of the heavy metal, the following equation was used:
where K0 = kL × Atomic mass of the metal × 103 × 55.5.
In fact, since kL in the Langmuir isotherm has a dimension, and the recommendations of the International Union of Pure and Applied Chemistry (IUPAC) indicate that the standard equilibrium constant \(\left( {K_{0} } \right)\) for calculating ΔGo must be a dimensionless parameter, K0. The equation of K0 is suggested by Zho et al. [47].
The estimated ΔGo(Pb) = − 30.1 kJ/mol, and ΔGo(Hg) = − 30.8 kJ/mol. This indicates that the adsorption process occurs spontaneously because ΔGo values are negative.
3.7 Comparison with other adsorbents
In order to assess the adsorption performance of DAS and DASTU, Table 6 shows a comparison of the adsorption capacity of Hg(II) and Pb(II) with other bio adsorbents based on starch, recently reported in the literature. According to the results of Table 6, one can deduce that both DAS, and DASTU show a good adsorption performance in comparison with other adsorbents. Table 7 also presents the maximum molar adsorption capacity of DAS and DASTU compared to other adsorbents. The current DAS and DASTU show higher molar adsorption capacity than the other mentioned adsorbents with a value of 1.183 mmol/g for DAS/Pb and 0.901 mmol/g for DASTU/Hg.
4 Conclusion
Starch dialdehyde was successfully prepared by a new and simple approach using microwave irradiation in a short time (10 min) in presence of a mild oxidizing agent, potassium iodate. The prepared dialdehyde was used to prepare the Schiff bases by reacting it with urea and thiourea. The adsorption ability of the prepared compounds (DAS, DASU, and DASTU) towards Cu(II), Pb(II), Hg(II), Cd(II), and Cr(III) were investigated. The results showed that DAS has higher ability for removing the Pb(II), while the DASU and DASTU have excellent ability for removing the Hg(II) ions. The removal efficiency of DAS and DASTU were 99.25% and 79.5%, respectively from solutions containing 100 ppm of Pb(II) and Hg(II) ions respectively. The adsorption isotherm studies showed that the adsorption process is spontaneous, and follows Langmuir Model for DAS and DASTU. The values of the theoretical maximum adsorption capacities for DAS/Pb and DASTU/Hg are 245.09 and 180.83 mg/g, respectively. Therefore, the prepared materials are very promising adsorbents for removal of heavy ion metals from wastewater. The simple preparation method, and the excellent performance of the bio-adsorbents encourage to investigate the up- scaling of the preparation process.
Data availability
Data availability sharing is not applicable to this article.
References
Ahmad K, Shah HUR, Nasim HA, Ayub A, Ashfaq M, Rauf A, Hussain E (2021) Synthesis and characterization of water stable polymeric metallo organic composite (PMOC) for the removal of arsenic and lead from brackish water. Toxin Rev. https://doi.org/10.1080/15569543.2021.1919902
Shah HUR, Ahmad K, Naseem HA, Parveen S, Ashfaq M, Rauf A, Aziz T (2021) Water stable graphene oxide metal-organic frameworks composite (ZIF-67@ GO) for efficient removal of malachite green from water. Food Chem Toxicol 154:112312
Bădescu IS, Bulgariu D, Ahmad I, Bulgariu L (2018) Valorisation possibilities of exhausted biosorbents loaded with metal ions–a review. J Environ Manag 224:288–297
Ahmad K, Parveen S, Aziz T, Naseem HA, Ashfaq M, Rauf A (2021) Metal Organic Framework (KIUB-MOF-1) as efficient adsorbent for cationic and anionic dyes from brackish water. J Mol Struct 1242:130898
Ahmad K, Ashfaq M, Nawaz H (2021) Removal of decidedly lethal metal arsenic from water using metal organic frameworks: a critical review. Rev Inorg Chem. https://doi.org/10.1515/revic-2021-0005
Nazir MA, Yasar A, Bashir MA, Siyal SH, Najam T, Javed MS et al (2020) Quality assessment of the noncarbonated-bottled drinking water: comparison of their treatment techniques. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1846732
Alothman ZA, Bahkali AH, Khiyami MA, Alfadul SM, Wabaidur SM, Alam M, Alfarhan BZ (2020) Low cost biosorbents from fungi for heavy metals removal from wastewater. Sep Sci Technol 55(10):1766–1775
Logan N, McVey C, Elliott C, Cao C (2020) Amalgamated gold-nanoalloys with enhanced catalytic activity for the detection of mercury ions (Hg2+) in seawater samples. Nano Res 13(4):989–998
Singh N, Chandra R (2021) A naked-eye colorimetric sensor based on chalcone for the sequential recognition of copper (II) and sulfide ions in semi-aqueous solution: spectroscopic and theoretical approaches. New J Chem 45(23):10340–10348
Kar A, Dey S, Burman D, Santra S, Guha PK (2021) RGO/Ni2O3 heterojunction-based reusable, flexible device for Cr(VI) Ion Detection in water. IEEE Trans Electron Devices 68(2):780–785
Mohammad N, Atassi Y (2021) Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes. Water Sci Eng 14(2):129–138
Rahal M, Atassi Y, Alghoraibi I (2021) Quenching photoluminescence of carbon quantum dots for detecting and tracking the release of minocycline. J Photochem Photobiol A: Chem 412:113257
Tally M, Atassi Y (2016) Synthesis and characterization of pH-sensitive superabsorbent hydrogels based on sodium alginate-g-poly (acrylic acid-co-acrylamide) obtained via an anionic surfactant micelle templating under microwave irradiation. Polym Bull 73(11):3183–3208
Ahmad K, Nazir MA, Qureshi AK, Hussain E, Najam T, Javed MS et al (2020) Engineering of zirconium based metal-organic frameworks (Zr-MOFs) as efficient adsorbents. Mater Sci Eng: B 262:114766
Shah HUR, Ahmad K, Bashir MS, Shah SSA, Najam T, Ashfaq M (2022) Metal organic frameworks for efficient catalytic conversion of CO2 and CO into applied products. Mol Catal 517:112055
Mohammad N, Atassi Y, Tally M (2017) Synthesis and swelling behavior of metal-chelating superabsorbent hydrogels based on sodium alginate-g-poly (AMPS-co-AA-co-AM) obtained under microwave irradiation. Polym Bull 74(11):4453–4481
Ghanem A, Al-Marjeh RAQB, Atassi Y (2020) Novel nitrogen-doped carbon dots prepared under microwave-irradiation for highly sensitive detection of mercury ions. Heliyon 6(4):e03750. https://doi.org/10.1016/j.heliyon.2020.e03750
Mohammad N, Atassi Y (2021) TiO2/PLLA electrospun nanofibers membranes for efficient removal of methylene blue using sunlight. J Polym Environ 29(2):509–519
Mohammad N, Atassi Y (2020) Adsorption of methylene blue onto electrospun nanofibrous membranes of polylactic acid and polyacrylonitrile coated with chloride doped polyaniline. Sci Rep 10(1):1–19
Gunawardene OHP, Gunathilake CA, Amaraweera APSM, Fernando NML, Manipura A, Manamperi WA et al (2021) Removal of Pb(II) ions from aqueous solution using modified starch. J Compos Sci 5(2):46
Fu Y, Yang C, Zheng Y, Jiang J, Sun Y, Chen F, Hu J (2021) Sulfur crosslinked poly (m-aminothiophenol)/potato starch on mesoporous silica for efficient Hg(II) removal and reutilization of waste adsorbent as a catalyst. J Mol Liq 328:115420
Naushad M, Ahamad T, AlOthman ZA, Al-Muhtaseb AH (2019) Green and eco-friendly nanocomposite for the removal of toxic Hg(II) metal ion from aqueous environment: adsorption kinetics & isotherm modelling. J Mol Liq. https://doi.org/10.1016/j.molliq.2019.01.090
Liu F, Hua S, Wang C, Qiu M, Jin L, Hu B (2021) Adsorption and reduction of Cr(VI) from aqueous solution using cost-effective caffeic acid functionalized corn starch. Chemosphere 279:130539
Daniel JR, Whistler RL, Röper H, Elvers B (2008) Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Zuo Y, Liu W, Xiao J, Zhao X, Zhu Y, Wu Y (2017) Preparation and characterization of dialdehyde starch by one-step acid hydrolysis and oxidation. Int J Biol Macromol 103:1257–1264
Veeleart S (1996) Properties and applications of dialdehyde starch. Żywn Technol Jakość 7(2):75–83
Yu D, Xiao S, Tong C, Chen L, Ming LX (2007) Dialdehyde starch nanoparticles: preparation and application in drug carrier. Chin Sci Bull 52(21):2913–2918
Ozcan C, Ozgunay H, Marin S, Kaya MGA (2016) Investigation of dialdehyde corn starch as crosslinking agent in collagen-based wound dressing materials (ICAMS). The National Research & Development Institute for Textiles and Leather-INCDTP, Bucharest, pp 299–304
Yang D, Gao L, Zhao W (2008) Synthesis, characterization and catalytic activity of dialdehyde starch-schiff base Co(II) complex in the oxidation of cyclohexane. Catal Lett 126:84–88
Wongsagon R, Shobsngob S, Varavinit S (2005) Preparation and physicochemical properties of dialdehyde tapioca starch. Starch-Stärke 57(3–4):166–172
National Center for Biotechnology Information (2021) PubChem Compound Summary for CID 23667635, Sodium periodate. https://pubchem.ncbi.nlm.nih.gov/compound/Sodium-periodate.APA. Accessed 29 Jun 2021
Salisu AA, Usman AH, Danjani AG (2016) Removal of Co(II), Pb(II) and Zn(II) ions by dialdehyde starch aminopyrazole schiff base from aqueous solution. J Appl Chem (ISOR-JAC) 9(6):61–67
Usman AH, Salisu AA, Danjani AG (2016) Preparation and characterization of dialdehyde starch urea (DASU) and it’s sorption potential for Co(II), Pb(II) and Zn(II) ions from aqueous solution. J Pure Appl Sci 9(2):213–220
Yu J, Chang PR, Ma X (2009) The preparation and properties of dialdehyde starch and thermoplastic dialdehyde starch. Carbohydr Polym 79(2):296–300
Salisu AA, Musa H, Abba H, Kogo AA (2013) Preparation and characterization of dialdehyde starch and its’ cross-linking with copper (II) ion. J Chem Pharm Res 5(5):153–158
National Center for Biotechnology Information (2021) PubChem Compound Summary for CID 23665710, Potassium iodate. https://pubchem.ncbi.nlm.nih.gov/compound/Potassium-iodate. Accessed 29 Jun 2021
Hofreiter BT, Alexander BH, Wolff IA (1995) Rapid estimation of dialdehyde content of periodate oxystarch through quantitative alkali consumption. J Anal Chem 27:1930–1931
Li R, Ding W, Zhao P (2011) Removal of Zn(II) ions by dialdehyde 8-aminoquinoline starch from aqueous solution. J Carbohydr Polym 83(2):802–807
Awokoya KN, Moronkola BA (2013) 2-Amino-4,6-dihydroxylpyrimidine modified corn starches for uptake of Cu(II) ions from aqueous media. J Polym Sci 2:49–55
Ekebafe LO, Ogbeifun DE, Okieimen FE (2012) Removal of heavy metals from aqueous media using native cassava starch hydrogel. Afr J Environ Sci Technol 6(7):275–282
Rahman ML, Yusoff MM, Abd Malik MN, Abu Samah N (2014) Synthesis of polymeric chelating ligands from sago starch for metal removal from electroplating wastewater. Indian J Chem Technol 21:30–37
Hoover R (2001) Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym 45(3):253–267
Dutta H, Paul SK, Kalita D, Mahanta CL (2011) Effect of acid concentration and treatment time on acid–alcohol modified jackfruit seed starch properties. Food Chem 128(2):284–291
Velusamy S, Roy A, Sundaram S, Kumar Mallick T (2021) A Review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec 21(7):1570–1610. https://doi.org/10.1002/tcr.202000153
Wahlström N, Steinhagen S, Toth G, Pavia H, Edlund U (2020) Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr Polym 249:116841. https://doi.org/10.1016/j.carbpol.2020.116841
Al-Marjeh RAQB, Atassi Y, Mohammad N, Badour Y (2019) Adsorption of methyl orange onto electrospun nanofiber membranes of PLLA coated with pTSA-PANI. Environ Sci Pollut Res 26(36):37282–37295
Zhou X, Zhou XIN (2014) The unit problem in the thermodynamic calculation of adsorption using the Langmuir equation. Chem Eng Commun 201(11):1459–1467
Awokoya KN, Oninla VO, Bello DJ (2021) Synthesis of oxidized Dioscorea dumentorum starch nanoparticles for the adsorption of lead (II) and cadmium (II) ions from wastewater. Environ Nanotechnol Monit Manag 15:100440
Fang Y, Lv X, Xu X, Zhu J, Liu P, Guo L et al (2020) Three-dimensional nanoporous starch-based material for fast and highly efficient removal of heavy metal ions from wastewater. Int J Biol Macromol. https://doi.org/10.1016/j.ijbiomac.2020.07.017
Ma X, Liu X, Anderson DP, Chang PR (2015) Modification of porous starch for the adsorption of heavy metal ions from aqueous solution. Food Chem 181:133–139. https://doi.org/10.1016/j.foodchem.2015.02.089
Naushad M, ALOthman ZA, Awual MR, Alam MM, Eldesoky GE (2015) Adsorption kinetics, isotherms, and thermodynamic studies for the adsorption of Pb2+ and Hg2+ metal ions from aqueous medium using Ti(IV) iodovanadate cation exchanger. Ionics 21(8):2237–2245. https://doi.org/10.1007/s11581-015-1401-7
Dong A, Xie J, Wang W, Yu L, Liu Q, Yin Y (2010) A novel method for amino starch preparation and its adsorption for Cu(II) and Cr(VI). J Hazard Mater 181(1–3):448–454. https://doi.org/10.1016/j.jhazmat.2010.05.031
Ding W, Zhao P, Li R (2011) Removal of Zn(II) ions by dialdehyde 8-aminoquinoline starch from aqueous solution. Carbohydr Polym 83(2):802–807. https://doi.org/10.1016/j.carbpol.2010.08.057
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest.
Ethical approval
This research do not involve human participants or animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wannous, A., Milaneh, S., Said, M. et al. New approach for starch dialdehyde preparation using microwave irradiation for removal of heavy metal ions from water. SN Appl. Sci. 4, 133 (2022). https://doi.org/10.1007/s42452-022-05024-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05024-w