Abstract
In this work, laser-induced breakdown spectroscopy (LIBS) was applied to qualitatively evaluate lead adsorbed from industrial wastewater by nano-CaCO3. Eggshell as a natural source of CaCO3 has been used as a sorbent owing to its low cost and unrivalled adsorption capacity to remove Pb from contaminated water. The structure and morphology of CaCO3 nano-powders were investigated using scanning electron microscopy (SEM), transmission electron microscope (TEM) and Fourier transforms infrared (FTIR). LIBS results were experimentally validated by the results obtained using portable X-ray fluorescence spectroscopy (pXRF) and energy dispersive X-ray (EDS), which confirmed the feasibility of using LIBS to detect traces of Pb ions, while the adsorption process is applied under governing parameters. Langmuir and Freundlich isotherm models were used to model the experimental data. The kinetics of adsorption mechanisms were studied using Lagergren's pseudo-first-order and McKay and Ho's pseudo-second-order. The obtained results demonstrated that bio-CaCO3 nanoparticles could be used as an effective lead-sorbent from wastewater. Accordingly, it is possible to utilize this adsorption technique as a promising practical approach for the treatment of lead-contaminated industrial wastewater and its recirculation.
Graphical abstract

Highlights
-
Natural nano CaCO3 from eggshell was prepared mechanically as a low-cost adsorbent and characterized by SEM, TEM, and FTIR.
-
The capacity of removing Pb (II) by nano-CaCO3 was dependent on pH, metal concentration and contact time .
-
LIBS was used for qualitative analysis of adsorbed Pb (II) and the results were validated with those obtained by EDX and pXRF spectrometry; in addition, isotherm models and kinetics of adsorption mechanisms were investigated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The development of abundant natural multifunctional nano-biosorbents represents extreme importance in remediating polluted water systems [1, 2]. Heavy metals are an intended class to be removed from the water environment due to their harmfulness. Among these heavy metals, Lead ions are not degradable in water, cannot be easily removed and cause acute and chronic damage to aquatic life [3, 4]. Lead is widely prevalent in the industries of batteries, electroplating, metal refining, pesticides, pigments, etc. In order to remove the dissolved heavy metals from polluted water, numerous strategies have been proposed, such as adsorption, electrocoagulation, degradation, membrane filtration, ion exchange, chemical precipitation, photocatalysis, and layered double hydroxide precipitation [5]. Adsorption is considered the superior low-cost mechanism used for the treatment of wastewater, owing to its simplicity, high loading capacity, possible regeneration, and sludge-free operation [6, 7].
Many researchers presented calcium carbonate (CaCO3) as a feasible inorganic sorbent. Eggshell (ES) provides a distinctive biological natural source of CaCO3. It is chemically structured of (94% calcium carbonate, 1% magnesium carbonate, 1% calcium phosphate and 4% organic matter) [8,9,10]. The importance of using such sorbent is exemplified in containing a high number of pores, and hence, a good attraction ability to adsorb such heavy metals [8, 9].
Nanotechnology provides a successful rearrangement in numerous fields of research over the last decade. Its application in purifying aquatic systems from heavy metals exhibits a fast-growing and exciting topic of interest for environmental scientists [11, 12]. Nanosization is the process of preparing materials on a nanoscale that has unique features like high surface area [13, 14]. The nanostructure adsorbents have shown substantially higher efficiency and faster rates of water treatment in comparison to traditional materials.
Traditional analytical techniques such as atomic absorption spectroscopy (AAS) and UV–Visible spectrophotometry [15, 16], flame atomic absorption spectrometry (FAAS) [17], inductively-coupled plasma optical emission spectrometry (ICP-OES) [18], and inductively-coupled plasma mass spectrometry (ICP-MS) are commonly used to analyze heavy metals in industrial effluents. These analytical methods require a lot of time-consuming sample preparation procedures [15, 16].
To acquire a high degree of precision in the results, some spectroscopic techniques are used for large-scale laboratory examination. Laser-induced breakdown spectroscopy (LIBS) and portable X-ray fluorescence (pXRF) are two of these approaches.
Laser-induced breakdown spectroscopy is a well-known multi-elemental spectrochemical analytical technique that can be used with solids, liquids, and gases. LIBS has unique advantages. Specifically, it requires no or minimal sample preparation and is quasi-nondestructive, simple, and cost-effective. Therefore, LIBS exhibits excellent performance in real-time elemental analysis at atmospheric pressure including remote analytical applications [19, 20]. However, LIBS, in its basic form, sometimes has a relatively high limit of detection (LOD), i.e., in the ppm range. Several methods have been proposed to overcome this drawback and improve available LOD [21].
Furthermore, pXRF, which is a well-established multi-element analytical technique, provides an entirely non-destructive quantitative analysis of elemental composition without a need for sample preparation. Given the simplicity of the pXRF technique, it is an acceptable method for several environmental applications, though there are limitations in the detection of low atomic number elements [22].
The ultimate objective of this study is to evaluate the adsorption efficiency of mechanically synthesized CaCO3 nanoparticles contained in the eggshell as a discriminatory adsorbent for Pb (II) from wastewater. In addition, the influence of physical governing parameters such as pH, contact time, and adsorbate concentration on the removal capacity of lead ions has been studied. The obtained data were verified using different adsorption isotherms. The LIBS results were validated by pXRF measurements, which demonstrated the potential of LIBS as an environmental diagnostic technique.
2 Experimental
2.1 Synthesis of eggshell nanoparticles
Chicken eggshells were obtained from raw eggs bought at the supermarket in Cairo, Egypt. The inner shell membrane was manually removed. Then, the eggshells were washed several times with hot double distilled water to remove impurities before drying in an electric oven at 40 °C for 1 h. Dried eggshells were mechanically crushed and sieved through a metal sieve to obtain nanoparticle size. Then, obtained nano-powder was stored at room temperature in an airtight glass desiccators (Fig. 1).
2.2 Preparation of Pb-contaminated water
A stock of Pb (II) solution with a 1500 mg L−1 lead concentration was obtained by dissolving 2.76 g of crystalline lead acetate trihydrate Pb(CH3COO)2·3H2O (Sigma-Aldrich, 99.9%) in 1 L of double-distilled water. Desired concentrations (700, 900, 1100, 1300, and 1500 ppm) were prepared by stock dilution with double distilled water. All chemical reagents were used as received without further purification.
2.3 Characterization of the nanoparticles
Fourier transforms infrared (FTIR) spectrum was obtained by FTIR-ATR Brucker Vertex 80V with resolution 4 cm−1 in the range 0f 4000–400 cm−1. Energy dispersive X-ray (EDX) pattern was scrutinized by a Zeiss Axiovert optical microscope used for microstructure investigation. A scanning electron microscope (SEM, JEOL, JSM 5410, Japan) equipped with energy-dispersive spectroscopy (EDS) unit was used for surface morphology investigation. The nanostructure and surface morphology were characterized by a scanning electron microscope (SEM, FEI Quanta FEG 250 series, Japan) operating at 30 kV accelerating voltage and a transmission electron microscope, JEOL (TEM, JEM-1230, Japan). The pXRF spectrum was measured using a portable pXRF system (Thermo Scientific, NITON/XLt 8138, 592 GKV, USA) with a 40 kV X-ray tube with a gold anode excitation source.
Experimental and testing equipment mainly includes a conventional single pulse LIBS setup was used. The laser source was an Nd: YAG laser (BRIO, Quantel, France) with the laser pulse energy of 90 mJ at λ = 1064 nm, a repetition rate of up to 10 Hz, and pulse duration of 5 ns. All measurements were carried out in air at room temperature and normal atmospheric pressure. The laser beam was focused onto the target surface by a plano-convex fused silica lens with a focal length of 10 cm. Laser-induced plasma plume emission was collected by an optical fiber with a 600-µm core diameter placed at 45° relative to the target surface and a 2-cm distance from the laser spot. Then, the collected plasma emission is fed through an optical fiber to an echelle spectrometer (Mechell 7500, multichannel, Sweden). A UV-intensified ICCD camera (DiCAM-PRO, PCO-computer optics, Germany) coupled to a spectrometer was used to record dispersed light in the wavelength range of 200–700 nm, and the identification of spectral emission lines was achieved by the LIBS++ software. The lens-to-sample-surface distance is controlled by a micrometric translational stage to achieve accurate focusing just below the target surface to avoid a breakdown in air. Each LIBS spectrum is the average of 25 spectra collected as five spectra from each of the different five spots on each target sample. The experimental conditions, i.e., the delay time (td), the time interval between firing the laser and triggering the ICCD camera, and gate width (Dt) [time during which ICCD is sensitive) were optimized. Optimized td and Dt values, providing the best signal-to-noise ratio, were 1500 ns and 2500 ns, respectively.
2.4 Adsorption procedures
Adsorption experiments were conducted by mixing 1.0 g of dry nano-eggshell (mainly CaCO3) with 150 mL of lead-contaminated water of predetermined concentration at room temperature (25 ± 2 °C) in 300-mL Erlenmeyer flasks. The effect of pH values on adsorption capacity was investigated in the range of 2.0–9.0 for a 1500 mg L−1 Pb (II) solution. The pH values higher than nine were not studied because at pH higher than 9, lead precipitates as insoluble lead hydroxide. 1 M HCl or 1 M NaOH solutions were used to adjust the desired pH values. The mixture was agitated at 200 rpm for 30 min and filtrated with a 0.7-mm filter paper (Whatman, Cat No. 1001 125). The effect of contact time for Pb (II) of different concentrations (700, 900, 1100, 1300, and 1500 mg L−1) was studied in the time range of 2–20 min under the same experimental conditions at optimum pH. The solid samples of nano- CaCO3 with adsorbed Pb ions were filtered and dried. The dry samples were pressed into tablets (1.5-cm diameter and 0.4-mm thickness) using a hydraulic 15 t compressor and then analyzed via LIBS, pXRF and, EDX techniques.
Adsorption kinetic is studied to estimate the time required to obtain an equilibrium concentration of adsorbate for the adsorption of heavy metals. Kinetic behavior can be quantitatively explored by pseudo-first-order [23] (Eq. 1) and pseudo-second-order [24] (Eq. 2) adsorption models .
where \(k_{1}\) (min−1) and \(k_{2}\) (g mg−1 min−1) are the rate constants of pseudo-first and pseudo-second-order models, respectively; \(q_{e}\) (mg.g−1) and \(q_{t}\) (mg.g−1) are the adsorption capacity at equilibrium and time t respectively.Furthermore, the equilibrium data for Pb were tested by Langmuir [25] and Freundlich [26] models, which are given by Eqs. 3 and 4, respectively.
where \(q_{e}\) (mg g−1) is the equilibrium capacity of metal ion on the adsorbent; \(q_{max}\) (mg g−1) is the monolayer adsorption capacity of the adsorbent; b (L mg−1) is the Langmuir adsorption constant related to the free energy of adsorption; \(k_{f}\) (mg1−1/n g−1 L1/n) and n are the Freundlich constants, which measure the capacity and intensity of adsorption, respectively.
3 Results and discussion
3.1 Fourier transforms infrared analysis (FT-IR)
FT-IR spectroscopy is an important instrument used to identify different phases of organic and inorganic compounds. The FTIR analysis given in Fig. 2 demonstrated that the eggshell nano-powder consists of CaCO3. The hallmark broadband at wavenumber 1406.61 cm−1 is attributed to the \({\text{CO}}_{3}^{ - 2}\) a moiety of calcium carbonate corresponding to the symmetric stretching mode of the C–O bond. Two other sharp peaks of calcite appeared at 876 cm−1 and 712 cm−1 were referred to as bending vibrations of the C–O bond. These observations are found to be consistent with prior studies [27].
3.2 Structure, surface morphology and particle size distribution of the eggshell nanoparticles
Scanning electron microscopy (SEM), transmission electron microscope (TEM) were used to characterize the structure, surface morphology, and particle size distribution of pure eggshell nanoparticles before adsorption of Pb (II), respectively (Fig. 3).
Since the CaCO3 nano-powder is a non-conducting material, the SEM pictures were obtained at a low vacuum setting in order to acquire sharp images. SEM micrographs of a nano eggshell at various magnification ratios were shown in Fig. 3a, b. It also demonstrated particle nanosization in the 32–40 nm average diameter region. Furthermore, the nanoparticles agglomerate in a roughly spherical form [28].
The high-resolution TEM micrograph is shown in Fig. 3c–g; clearly displays a spherical shaped pure eggshell nanoparticle with 8–28 nm sization. The revealed CaCO3 nanoparticles are highly agglomerated in lattice planes which assures the high crystallinity of the bio-CaCO3 nanoparticles [11].
3.3 Effect of pH
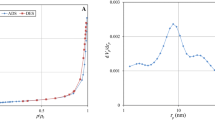
A solution hydrogen ion concentration (pH value) is an essential process parameter in the adsorption of heavy metal ions from aqueous solutions. Figure 4 shows the effect of pH on the adsorption capacity of nano- CaCO3 of Pb (II) at the concentration of 1500 ppm using LIBS and pXRF techniques. Owing to its unique characteristics, such as being a non-resonant line, the spectral line of Pb I at 261.3 is suitable for the qualitative analysis of the sample. The results show that with an increase in pH from 2.0 to 6.0, the ion exchange capacity of Pb (II) increases until it levels off at the pH range of 6.0–9.0. When pH is 6.0, nano-CaCO3 was effective in attenuating lead ions in aqueous solutions, and the highest Pb (II) adsorption in nano-CaCO3 was obtained. Thus, the sorbent surface is not positively charged, and its increase is less favourable for complexing Pb (II) on the sorbent surface than the net negative charge sites. Thus, the increased net positive charge of the CaCO3 surface in the pH range of 2–3 causes reduced Pb (II) removal from wastewater [29].
3.4 Effect of contact time
Figure 5 exhibits the effect of contact time on adsorption uptake of Pb (II) onto eggshell (mainly CaCO3) from synthetic wastewater at different concentrations using LIBS, pXRF, and EDX analysis. The results indicate that both LIBS intensity and adsorption uptake increase with increasing contact time until reaching the equilibrium point at 8 min for the Pb (II) concentrations of (700, 900 ppm), 12 min for 1100 ppm and at 16 min for (1300, 1500 ppm) as presents in Fig. 5a. The rate of metal removal was determined to be higher, in the beginning, owing to the greater surface area available for adsorption [30]. Reaching the point of equilibrium means that the adsorbent occupies all active sites. This adsorption process is accentuated by making use of pXRF and EDX analysis under the same experimental conditions as shown in Fig. 5b, c. The attitudes of both the pXRF and EDX curves indicate a substantial consistency; that lends confidence to the LIBS results.
3.5 Adsorption kinetics and isotherm
Figure 6 depict a linear relationship between ln (qe − qt) versus t and between \(\frac{t}{{q_{t} }}\) versus t regarding the adsorption of Pb (II) into nano-CaCO3 with pseudo-first-order (a) and pseudo-second-order adsorption models (b) for different concentrations. The rate constants \(k_{1}\) (min−1) and \(k_{2}\) (g mg−1 min−1) are obtained from the slope and \(q_{e}\) (mg.g−1) and \(q_{t}\) (mg.g−1) from the intercept. The relative kinetic parameters of both models are summarized in Table 1. Based on the calculated constants shown in Table 1, the adsorption of Pb (II) into nano-CaCO3 was fitted better with the pseudo-second-order kinetics. This result indicates that the adsorption of Pb (II) into nano- CaCO3 is dominated by physical adsorption [31].
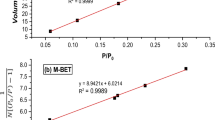
The adsorption isotherm constants are determined from the plot of \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {c_{e} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${c_{e} }$}}\) versus \({\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 {q_{e} }}}\right.\kern-\nulldelimiterspace} \!\lower0.7ex\hbox{${q_{e} }$}}\) and the plot of \(\ln c_{e}\) versus \(\ln q_{e}\) for Langmuir and Freundlich models as shown in Fig. 7a, b, respectively. The relevant parameters are listed in Table 2.
Usually, a high correlation coefficient, 0.91002, indicates that the application of the Langmuir equation supports monolayer formation on the surface of the adsorbent. The Langmuir isotherm constants for the adsorption of lead ions are given on the corresponding figure. The values of b of 0.02 and \(q_{max}\) of 180.5 for Pb (II) indicate that the adsorption process depends on both the concentration and contact time.
Figure 7b shows the fitting plot of Freundlich isotherm for Pb (II). The constant values obtained from Freundlich adsorption isotherm, and its correlation coefficient R2 are summarized in the figure. The regression value of 0.983 for Pb (II) is acceptable to describe the adsorption of heavy metal on n-CaCO3. The constant obtained from (1/n) is 0.27622, indicating favorable and high-affinity adsorption of nano-CaCO3 for metallic ions. By comparing all parameters of both isotherms, it has been found that the equilibrium data confirm Freundlich isotherm, which suggests it is applicable for non-ideal adsorption on heterogeneous adsorbent surfaces [32].
4 Conclusion
In this work, LIBS (a spectrochemical analytical technique) was exploited to monitor the removal of Pb (a toxic heavy metal) from contaminated water via bio-CaCO3nanoparticles. The economically natural source eggshell, was dried and ground to nanosize to be used as a discriminative sorbent to remove Pb from water. The efficiency of eggshells in the adsorption of heavy metals is due to the presence of CaCO3 as the main component, which has unrivaled adsorption capacity to remove heavy metals through ion exchange reactions with calcium ions. Optimum adsorption parameters values were obtained by studying different ranges of pH values and contact times at various concentrations of lead ions. All LIBS results were confirmed using the pXRF and EDX techniques. The pronounced agreement between LIBS, pXRF and EDX results emphasize the feasibility of using LIBS as a powerful spectrochemical analytical technique for water pollution analysis, which develops an appropriate technology regarding the removal of heavy metals from contaminated industrial effluents. The adsorption data for nano-CaCO3 were in good compactness with the pseudo-second-order kinetic and Freundlich isotherm models. Consequently, natural nano-CaCO3 has been proved as a promising adsorbent for removing lead ions from synthetic wastewaters, which makes its real-life application practical and cost-effective. The recyclability of nano-CaCO3 adsorbents, visibility of scaling-up on real industrial wastewater will be considered in the future study.
References
Ahmad I, Siddiqui WA, Ahmad T (2019) Synthesis and characterization of molecularly imprinted magnetite nanomaterials as a novel adsorbent for the removal of heavy metals from aqueous solution. J Matter Res Technol 8:4239–4252. https://doi.org/10.1016/j.jmrt.2019.07.034
Pylypchuk I, Kessler V, Seisenbaeva GA (2018) A simultaneous removal of acetaminophen, diclofenac, and Cd (II) by Trametes versicolor laccase immobilized on Fe3O4/SiO2-DTPA hybrid nanocomposites. ACS Sustain Chem Eng 6:9979–9989. https://doi.org/10.1021/acssuschemeng.8b01207
Liu S, Cui S, Guo H, Wang Y, Zheng Y (2021) Adsorption of lead ion from wastewater using non-crystal hydrated calcium silicate gel. Materials 14:842–854. https://doi.org/10.3390/ma14040842
Dargahi A, Golestanifar H, Darvishi P, Karami A, Hasan S, Poormohammadi A, Behzadnia A (2016) An investigation and comparison of removing heavy metals (lead and chromium) from aqueous solutions using magnesium oxide nanoparticles. Pol J Environ Stud 25:557–562. https://doi.org/10.15244/pjoes/60281
Habte L, Shiferaw N, Khan MD, Thriveni T, Ahn JW (2020) Sorption of Cd+2 and Pb+2 on Aragonite synthesized from eggshell. Sustainability 12:1174–1189. https://doi.org/10.3390/su120311742020
Rezk RA, Galmed AH, Abdelkreem M, Abdel Ghany NA, Harith MA (2018) Detachment of Cu (II) and Co (II) ions from synthetic wastewater via adsorption on Lates niloticus fish bones using LIBS and XRF. J Adv Res 14:1–9. https://doi.org/10.1016/j.jare.2018.05.002
Smiciklas I, Dimović I, Mitrić M (2006) Removal of Co2+ from aqueous solutions by hydroxyapatite. Water Res 40:2267–2274. https://doi.org/10.1016/j.watres.2006.04.031
Jendia AH, Hamzah S, Abuhabib AA, Ashgar NM (2020) Removal of nitrate from groundwater by eggshell biowaste. Water Supply 20:2514–2529. https://doi.org/10.2166/ws.2020.151
Stadelman WJ (2000) Eggs and egg products. In: Francis FJ (ed) Encyclopedia of food science and technology, 2nd edn. Wiley, New York
Tangboriboon N, Unjan W, Sangwan W, Sirivat A (2018) Preparation of anhydrite from eggshell via pyrolysis. Green Process Synth 7:139–146. https://doi.org/10.1515/gps-2016-0159
Dargahi A, Golestanifar H, Darvishi P, Karami A, Hasan SH, Poormohammadi A, Behzadnia A (2016) An investigation and comparison of removing heavy metals (lead and chromium) from aqueous solutions using magnesium oxide nanoparticles. Pol J Environ Stud 25:557–562. https://doi.org/10.15244/pjoes/60281
Hassan TA, Rangari VK, Rana RK, Jeelani S (2013) Sonochemical effect on size reduction of CaCO3 nanoparticles derived from waste eggshells. Ultrason Sonochem 20:1308–1315. https://doi.org/10.1016/j.ultsonch.2013.01.016
Villarreal-Lucio DS, Rivera-Armenta JL, Martínez-Hernández AL, Zednik R, Estrada Moreno IA (2018) Effect of nano CaCO3 particles from eggshell on mechanical and thermal properties in pp/eggshell composites. J Eng Technol 6:456–468
Feng L, Cao M, Ma X, Zhu Y, Hu C (2012) Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J Hazard Mater 217–218:439–446. https://doi.org/10.1016/j.jhazmat.2012.03.073
Liu F, Ye L, Song K, Shen T, Zhang C, He Y (2018) Fast detection of copper content in rice by laser-induced breakdown spectroscopy with uni- and multivariate analysis. Sensor 18:705–720. https://doi.org/10.3390/s18030705
Bukhari M, Awan MA, Qazi IA, Baig MA (2011) Development of a method for the determination of chromium and cadmium in tannery wastewater using laser-induced breakdown spectroscopy. J Anal Methods Chem 2012:1–7. https://doi.org/10.1155/2012/823016
AlOthman ZA, Habila M, Yilmaz E, Soylak M (2012) Solid phase extraction of Cd(II), Pb(II), Zn(II) and Ni(II) from food samples using multiwalled carbon nanotubes impregnated with 4-(2-thiazolylazo) resorcinol. Microchim Acta 177:397–403
Mao XL, Ciocan AC, Borisov OV, Russo RE (1998) Laser ablation processes investigated using inductively coupled plasma atomic emission spectroscopy (ICP-AES). Appl Surf Sci 127:262–268
Legnaioli S, Campanella B, Poggialini F, Pagnotta S, Harith MA, Abdel-Salam ZA, Palleschi V (2020) Industrial applications of laser-induced breakdown spectroscopy: a review. Anal Methods 12:1014–1029. https://doi.org/10.1039/C9AY02728A
Azzeer AM, AI-Dwayyan AS, AI-Salhi MS, Kamal AM, Harith MA (1996) Optical probing of laser-induced shock waves in air. Appl Phys B 63:307–310. https://doi.org/10.1007/BF01833801
Rezk RA, Galmed AH, Abdelkreem M, Abdel Ghany NA, Harith MA (2016) Quantitative analysis of Cu and Co adsorbed on fish bones via laser-induced breakdown spectroscopy. Opt Laser Technol 83:131–139. https://doi.org/10.1016/j.optlastec.2016.02.025
Peinado FM, Ruano SM, González MGB, Molina CE (2010) A rapid field procedure for screening trace elements in polluted soil using portable X-ray fluorescence (PXRF). Geoderma 159:76–82. https://doi.org/10.1016/j.geoderma.2010.06.019
Lagergren S (1898) About the theory of so-called adsorption of soluble substances Kungliga Svenska Vetenskap-sakademiens. Handlingar 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:45–65. https://doi.org/10.1016/S0032-9592(98)00112-5
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295. https://doi.org/10.1021/ja02268a002
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–470. https://doi.org/10.4236/wsn.2009.14040
Laohavisuti N, Boonchom B, Boonmee W, Chaiseeda K, Seesanong S (2021) Simple recycling of biowaste eggshells to various calcium phosphates for specific industries. Sci Rep 11:15143. https://doi.org/10.1038/s41598-021-94643-1
Chilakala R, Shiferaw N, Habte L, Mulatuand D, Ahn JW, Thenepalli T (2019) Synthesis of nano-calcium oxide from waste eggshell by sol-gel method. Sustainability 11:3196–3206. https://doi.org/10.3390/su11113196
Guru PS, Dash S (2014) Sorption on eggshell waste: a review on ultrastructure, biomineralization and other applications. Adv Colloid Interface Sci 209:49–67. https://doi.org/10.1016/j.cis.2013.12.013
Saeed A, Akhter MW, Iqbal M (2005) Removal and recovery of heavy metals from aqueous solution using papaya wood as a new biosorbent. Separ Purif Technol 45:25–31. https://doi.org/10.1016/j.seppur.2005.02.004
Liu R, Guan Y, Chen L, Lian B (2018) Adsorption and desorption characteristics of Cd2+ and Pb2+ by micro and nano-sized biogenic CaCo3. Front Microbiol 9:41–50. https://doi.org/10.3389/fmicb.2018.00041
Anastopoulos I, Kyzas GZ (2014) Agricultural peels for dye adsorption are view of recent literature. J Mol Liq 200:381–389. https://doi.org/10.1016/j.molliq.2014.11.006
Acknowledgements
The authors greatly appreciate the support and facilities offered by the National Research Centre (NRC) Cairo, Egypt, and the National Institute of Laser Enhanced Science (NILES), Cairo University, Egypt.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors of this paper declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rezk, R.A., Abdel-Salam, Z., Abdel Ghany, N.A. et al. LIBS and pXRF validation for the removal of Pb by bio-CaCO3 nanoparticles from contaminated water. SN Appl. Sci. 4, 151 (2022). https://doi.org/10.1007/s42452-022-05014-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-05014-y