Abstract
The wetting behavior in subsurface reservoirs plays a crucial role in storage, migration and liberation of fluid phases that are especially relevant to the energy sector. Further, the three-phase contact angle is important for implementing safe and successful subsurface storage of hydrogen and carbon dioxide. For reliable statements on the in situ wetting, it is important to be aware of the decisive factors of influence in order to design and perform the respective experiments in an appropriate way. This paper discusses the most important effects that shall be considered when determining contact angles experimentally, like drop size, surface roughness, aging process, dynamic behavior, and the pH, giving some valuable guidance to guarantee significant results. A drop base diameter of no less than 5 mm is found to be appropriate to minimize the impact of gravity on the contact angle under reservoir conditions. It is further confirmed that surface roughness contributes to better water wetting when the contact angles are below 90°. The versatility of contact angle measurements is shown through the dual-drop dual-crystal method that can be applied to estimate the adhesion forces present at the rock-brine interface and that need to be overcome by the flooding liquid to effectively displace hydrocarbons from the pores.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Reliable data on wetting characteristics are crucial for a large number of industrial applications and systematic investigation of naturally occurring phenomena. Efficiency of fluid separation processes in columns depends on the wetting of the packing surfaces [1]. Water condensation on the inner walls of gas pipelines and subsequent corrosion is triggered by low solid–liquid interfacial energies represented by low contact angles [2]. Good wetting of the inner surface of a pipeline results from favorable adhesion energies that help overcome the energetic barrier for (heterogeneous) nucleation of condensate droplets. Safe storage of gases like hydrogen and carbon dioxide in depleted oil and gas reservoirs as well as aquifers is only possible under favorable wetting conditions, i.e. a capillary pressure positively adding to the hydrostatic pressure for enabling mechanisms such as structural trapping. Migration of hydrocarbons in porous media is also closely related to the interaction of the respective fluid phases with the rock surfaces under prevailing reservoir conditions [3]. Last but not least, efficiency of enhanced hydrocarbon recovery techniques depends on favorable relative permeabilities of the fluids present in oil and gas reservoirs that on their turn are influenced by the competitive wetting of aqueous and hydrocarbon phases on the inner pore surface; the phase showing the higher rock-fluid interactions has lower contact angles (higher wetting), thereby displaying less mobility [4] and lower relative permeability [5]. In all of the aforementioned applications, experimental determination of the wetting behavior in terms of the three-phase contact angle has become an essential approach in order to quantify the multiple interactions between all present phases under real conditions.
Several methods are employed to assess wettability such as the Amott and the United States Bureau of Mines (USBM) tests which are somewhat qualitative in nature and only measure the average wettability [6]. Further, they are performed at atmospheric conditions and do not consider the presence of gases in the system. In recent years, the optical sessile drop or captive bubble method gained increasing interest, since this method best represents the situation within the formation and high-pressure optical cells that allow simple and easy performance of respective measurements are well developed [7,8,9,10]. In the context of carbon capture and storage (CCS), wettability is investigated in systems composed of three components, namely rock samples, brines and CO2. Mostly, the contact angle is determined by creating sessile brine drops on a surface of interest in compressed CO2 environment [11,12,13]. In analogy, the efficiency of methods of enhanced oil recovery (EOR) where gas is present also depends on the wetting behavior, but up to four phases (rock-brine-oil–gas) need to be considered depending on the recovery technique in question. To name a few: alternating gas–water flooding [14], carbonated water flooding [4],or a combination of these techniques, e.g. during foam flooding [15].
Contact angle measurements are usually studied at reservoir conditions, where brine salinity, oil composition, rock mineralogy as well as pressure and temperature are the most investigated parameters [16,17,18,19,20,21]. While the aforementioned indeed influence the wettability, there exist other factors that impact the measurements and their interpretation from a methodological perspective. Any experimentalist who is dedicated to determining the contact angle on a plane solid surface under real conditions faces a series of factors that influence the result, among those are impurities and surface roughness/porosity which are two of the most important. Alnoush et al. investigated the impact of surface roughness on the wettability of calcite and concluded that increasing the surface roughness increases water wetting [22]. Sari et al. investigated the impact of calcite surface roughness on contact angle in high and low salinity brines [23]. Similar to Alnoush et al., they reported that contact angle decreases with increasing surface roughness in both high and low salinity brines, where the surface roughness impacted the contact angle in a subtler manner in the low salinity brine. Other important aspects to be considered are preconditioning of the solid surface [24, 25] and ion interaction specifically with mineral surfaces [26]. Additionally, the drop size dependance of the contact angle needs to be considered. Sakai and Fujii investigated the influence of the drop size on the contact angle [27]. They state that the classical Young equation relating the participating interfacial energies must be corrected by the addition of a term that includes the contribution of the line tension as a characteristic value of the 3-phase system including the radius of the contact line (assuming an axi-symmetric drop) into an overall force balance. Sakai and Fujii include a gravity term in the definition of a relative free energy based on the interfacial energies of the contacting phases and calculate the deviation from a (virtual) case where gravitational forces are absent [28]. The conclusions seem somewhat confusing, as they refer to voids in the surface that are filled with adsorbed gas and these voids tend to “smoothen”, which—according to the authors-justifies the deviation from Wenzel´s law [29]. Ren et al. also try to describe the effect of gravity defining a “k-value” that represents an inverted Bond number and conclude that at k > 30 the effect of gravity on the drop shape is negligible [30]. Seo et al. reported that the contact angle of water drops on a superhydrophobic surface is not affected by the volume for a minimum drop size of 5 µl and above [31]. On the other hand, Cansoy did not find a clear tendency regarding the impact of drop volume on the contact angle on superhydrophobic surfaces [32]. Drelich et al. [33] tried to explain the drop size dependency with the help of a hypothetical quantity called line tension originally defined by Good and Koo [34] as the one-dimensional tension in analogy to the surface tension as a correction term in Young´s equation taking influence especially in case of small drops.
One basic question is whether the drop liquid is in direct contact with the solid surface at the moment of determining the contact angle [35]. It was found that droplets bounce off of a surface if the surface happens to be non-wetted by the droplet phase, however, it has also been reported that there exists a fluid cushion which appears also when the surface is wetted by the droplet [36]. This is confirmed by Fig. 1b where a water film separates the hydrocarbon drop phase and the solid surface during the first contact while a direct contact exists between the wetting oil droplet and the surface after the drop stabilizes (f).
Based on several years of laboratory and industrial experience, this work serves as a concise guide that summarizes the main aspects to be considered during contact angle measurement from a methodological point of view, especially when non-atmospheric conditions are applied. These considerations should be accounted for in order to obtain meaningful and objective interpretation of the results, isolate any artefacts that are likely to impact the measurement and facilitate the derivation of generalized statements regarding wettability especially in terms of underground gas storage and enhanced oil recovery.
2 Materials and methods
2.1 Solids
Shale rock samples originating from Al-Sultani deposit located south of Amman, Jordan have been utilized for the captive bubble test. The samples were subject to supercritical carbon dioxide extraction to remove any hydrocarbons. Full details on the extraction procedure are reported in detail elsewhere [37]. No surface treatment was performed on the shale samples to preserve the native characteristics of the natural rock. Moreover, a limestone sample in addition to a calcite crystal -described in [38]- representing a quasi ideal smooth surface were used. X-ray Diffraction (XRD) was performed using an X-ray diffractometer (Empyrean – Malvern Panalytical, UK) to verify the mineralogical composition of the Sultani shale and limestone samples. Powdered samples were loaded into the diffractometer with Cu Kα radiation and a nickel filter, in a 2θ range of ≈ 10°–80°. Figure 2 shows the XRD pattern for both rocks.
2.2 Fluids
Alkanes {n-hexadecane (C16), eicosane (C20) and tetracosane (C24)} were purchased from Fischer Scientific, GmbH, Germany at analytical grade. A model oil composed of 45 wt% C16, 45 wt% C20 and 10 wt% C24 was synthesized based on gas chromatography analysis of oil extracted from the shale using supercritical carbon dioxide as described in [37]. The complete analysis of the extracted oil can be found in [17]. West Texas sour crude oil was also used. The characteristics of the aforementioned crude oil are listed in details in reference [38]. Deionized water as well as brine resembling sea water were used. Sea water composition is shown in Table 1. Carbon dioxide with 99.99% purity was supplied by Westfalen AG, Germany.
2.3 Methods
For determining the contact angle, the sessile drop and captive bubble methods were performed in a high-pressure drop analysis system. The system is composed of a high-pressure (HP) view cell (Pmax 70 MPa, Tmax 200 °C, Eurotechnica GmbH, Germany) coupled with a drop shape analyzer (Kruss GmbH, Germany). The view cell has two high pressure-resistant sapphire windows opposing each other. One window faces a light source that illuminates the back of the view cell and the other faces a charge-coupled device (CCD) camera for measurement observation. A heating jacket is used to heat the view cell to the desired temperature. Within the view cell, a movable capillary extends from either the bottom of the view cell or the top depending on the measurement configuration.
2.3.1 Sessile drop
The capillary extends from the top for a sessile drop configuration. The capillary is connected to a drop dispensing device that is used to dispense the drop of liquid through the capillary and onto the solid surface. A thermocouple with an accuracy of ± 0.1 °C is located 10 mm away from the capillary and is connected to a digital readout device for temperature observation. A pressure transducer with an accuracy of ± 0.1 bar is used to measure the pressure inside the view cell. The CCD camera is connected to a computer to record high quality images in a timely manner. Using a drop shape analysis software (Advance- Kruss GmbH, Germany) the axisymmetric drop shape analysis (ADSA) technique is employed for the evaluation of the contact angle. The software fits a baseline to the solid where the drop meets the surface, detects the drop profile as well as the 3-phase contact point where the contact angle is evaluated. Further information about this technique can be found elsewhere [39]. A syringe pump (D100, Teledyne ISCO, USA) is used to pressurize the CO2 and charge it into the view cell at designated pressure conditions. The schematic diagram of the sessile drop method is depicted in Fig. 3a.
2.3.2 Captive bubble
In the captive bubble configuration, the capillary extends from the bottom. This configuration is prevalent in systems where the drop forming fluid has a lower density than the surrounding fluid. As the name implies, the drop forming liquid is trapped between the surrounding fluid and the rock surface. The experimental setup for the captive bubble is otherwise identical to that mentioned in the previous section (see Sect. 2.3.1 Sessile drop). The schematic diagram of the captive bubble configurations is depicted in Fig. 3b.
2.3.3 Dual-drop dual-crystal
Although the previous methods are also used to measure the advancing and receding contact angle, another way to measure the latter in a well-defined way is called the dual-drop dual-crystal (DDDC) method [40]. Basically, a drop is captured between two plates where one is moved laterally to stretch the drop and produce advancing and receding contact angles at the three-phase contact of both plates. In order to guarantee similar starting conditions at both solid surfaces, the final oil drop -of which advancing and receding contact angles are of interest- is created following the procedure presented in Fig. 4: two small oil drops are deposited on two identical plates in a captive bubble configuration where a drop of oil is surround by an aqueous phase. Due to buoyancy forces, the oil drops are pushed towards the surface and in this way the aging of the two surfaces is ensured to be identical. By tilting the lower plate 180°, both drops are brought to coalesce, and the new single larger drop is captured between the plates. Afterwards, the composition of the surrounding aqueous phase may be changed by replacing the originally used aqueous phase with a new solution that either contains surfactants or dissolved gas. So far, measurements have only been carried out in liquid–liquid–solid systems containing either dead oils or live oils above the bubble point pressure [41]. One advantage of this method is that the horizontal movement can be carried out in a precise and defined way for adjusting the velocity to a reproducible value.
2.4 Experimental procedure
Prior to measurement, the entire setup is cleaned to ensure an environment free of contamination. The rock sample is introduced into the view cell and is supported by means of a sample holder. The glass windows are closed and tightened to seal the system against any leakage and the heating of the view cells is initiated. The syringe pump is pre-pressurized to reduce the time required for the CO2 to reach the designated pressure. The valve connecting the view cell to the syringe pump is opened and CO2 is pumped into the view cell until the designated pressure is reached. After the desired temperature is also reached, the drop dispensing device is used to form a drop of brine on the solid surface for contact angle measurement in a rock-brine-CO2 system using the sessile drop configuration. For measurements in a system composed of rock-brine-oil-CO2 using the captive bubble configuration, after the placement of the rock sample, the view cell is filled with brine that is pumped from the brine reservoir as seen in Fig. 3B. A waiting time of 30 min is sufficient for heating the newly introduced brine until it reaches the desired temperature. Once this is achieved, a drop of oil is placed on the sample surface via the moveable capillary. After a drop is created, the valve connecting the view cell to the syringe pump is opened and CO2 is pumped into the view cell until the designated pressure is achieved. The ADSA software evaluates and records the contact angle as a function of time.
3 Results and discussion
Below the factors impacting contact angle measurements are presented and discussed based on experimental data.
3.1 Drop size
Different opinions exist in literature regarding the influence of the drop size on experimental values of the contact angle. As a drop becomes bigger, the influence by gravity on the drop shape increases. This fact is accounted for by the Young Laplace equation of axi-symmetric drop shapes regardless of whether they are attached to a needle or resting on a solid surface. From the Young–Laplace relation, the interfacial tension may be deduced based on the balance between gravitational and interfacial forces. In terms of the contact angle at the three-phase contact of a drop resting on a solid surface, the gravity may distort the macroscopic (optically detectable) value. While a spherical drop is rather unfavorable for measuring the interfacial tension because the interfacial forces dominate over the gravitational forces, it may be preferable for measuring the contact angle. The ratio of gravitational to interfacial forces is given by the so-called Eötvos or Bond number as per Eq. (1):
where g is the gravity constant in m/s2, Δρ is the density difference between two adjacent phases in kg/m3, d is the drop diameter in m and σ is the interfacial tension in mN/m. In case of very small drops and relatively high interfacial tension, Bo is smaller than unity leading to rather spherical drops. As a consequence, small drops are preferred because the impact of gravity on the contact angle itself is reduced. A threshold value of Bo is rather arbitrarily set to unity. In our experiments, a moderate dependency of the contact angle is found in a system comprising model oil drops on an extracted and cleaned shale surface in deionized water where the system is pressurized with CO2 at 10 MPa and 60 °C. This tendency is depicted in Fig. 5.
In Fig. 5, the contact angle is measured using the captive bubble method (Sect. 2.3.2 Captive bubble) and the contact angle values are depicted as a function of the bond number. The principal findings of previous authors could be confirmed, i.e. a diminishing dependence of contact angle on the drop size as volume increases. For heptane—water—quartz and tetradecane—water—quartz systems, Drelich found contact angle variations in a range of 2–3 degrees, especially for drops of baseline diameters beyond 2 mm [33]. Only in case of heterogeneous and rough surfaces some model approaches are presented in order to describe the influence that becomes apparent for small drop volumes and for explaining the influence on the contact angle hysteresis, but the lack of a consistent theory must indeed be acknowledged. In general, it is recommended to apply sessile or captive bubble drops with base diameters of no less than 5 mm.
3.2 Drop deposition velocity
Lyklema states that the proper (advancing) contact angle for determining the interfacial energies is the one at zero velocity of the three-phase contact line [42]. In order to meet both requirements—a stationary interface yet an advancing angle—the contact angle may be determined at different sessile drop (Sect. 2.3.1 Sessile drop) deposition rates on a solid surface as shown in Fig. 6.
The corresponding contact angle is depicted as a function of the velocity of three-phase contact moving across the solid surface in Fig. 7.
According to Lyklema [42] and based on the values in Fig. 7, the designated contact angle is determined by extrapolating the line towards u = 0 mm/s, i.e. where the resultant CAu→0 = 78°. The application of the DDDC method (Sect. 2.3.3 Dual-drop dual-crystal) to a system composed of crude oil–water-calcite at elevated pressure is presented in Fig. 8.
In Fig. 8a, the drop is already extended but the interface has not moved yet whereas in the next image (b) the contact line has “shifted”, i.e. it moved away from the area originally exposed to the oil during the aging process due to which erroneous results may be obtained. Hence the decisive contact angle will be retrieved from the image just before this happens (a). In (c) the drop is divided into the two drops again that originally had been aged separately and brought to coalesce for the experiment. From the experiments on the DDDC as illustrated in Fig. 8, adhesive forces may be estimated based on the information on cohesion: As seen in Fig. 8c, the adhesive forces appear to be stronger than the cohesion which on its turn can be determined through the interfacial tension and geometrical considerations. From the geometrical situation in Fig. 8b, a minimum force of adhesion can be deduced from Eq. 2 where σ oil–water is the interfacial tension between water and oil in mN/m, d is the diameter in m and F is the adhesive force in mN:
Based on an interfacial tension of around 20 mN/m between the water and the oil [38], the adhesion force between the oil and rock results in a value of at least 0.15 mN on a surface area of 32 mm2. This force can be translated directly to the flow force in the pore required to displace the oil (Fig. 9).
3.3 Preconditioning of solid surface
Especially for applications related to oil/gas reservoirs as well as subsurface gas storage, relevance of laboratory experiments depends on the extent to which the solid surface under investigation resembles the real situation. In case of shales, the impact by preparing the sample is minimal, because it is laminated and fissile, i.e. it breaks easily leaving smooth surfaces on which the measurement can be directly performed without the need for surface preconditioning. Calcite crystals, which are representative of ideal and clean carbonates, also possess naturally smooth surfaces that are useful for obtaining reproducible results. When using core samples from real carbonate or sandstone reservoirs, the situation becomes more complicated: First, these rock samples are exposed to a cutting process that induces unnatural thermal and chemical conditions. Secondly, the rock samples undergo a cleaning procedure after which the original reservoir conditions shall be restored as much as possible prior to further investigation of the wetting behavior. Based on many years of experience, both industrial and academic, research laboratories developed standard procedures so at least internally comparable results from wetting tests are guaranteed. Hoeiland et al. propose to age the rock samples in acids extracted from crude oil, since these compounds are assumed to be decisive for changing wettability [43]. For this reason, Jaeger et al. applied naphthenic acid for aging carbonate surfaces prior to measuring the contact angle under reservoir conditions [38]. Other labs suggest the following procedure:
-
1.
Vigorous cleaning renders the core water-wet.
-
2.
Saturate in simulated formation brine and age.
-
3.
Flood with crude oil (dead oil).
-
4.
Age for 40 days at reservoir temperature and pressure.
This procedure can also be varied with help of an Amott-type equipment [44]:
-
1.
Expose the rock to vacuum.
-
2.
Saturate rock with crude oil.
-
3.
Place the oil-saturated rock in brine.
-
4.
The release of oil will occur spontaneously until original conditions are restored.
In Fig. 10, the contact between an oil drop and the rock surface is schematically depicted depending on the preconditioning of the rock.
3.4 Surface roughness
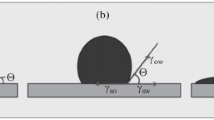
Particularly for investigation of wettability effects in reservoirs, one has to deal with rough or porous surfaces. Wenzel’s statement shown in Eq. 3 is commonly used as the starting point for describing the effect of surface roughness on the macro contact angle [29] where θr is the micro contact angle of the rough surface and θ is the macro contact angle:
implying that for \(\theta > 90^\circ\) the contact angle increases for increasing roughness while the inverse happens for \(\theta < 90^\circ\). This effect is made use of for technical “self-cleaning” surfaces by increasing hydrophobicity through enhanced roughness under the condition of extremely small adherence forces, also known as Cassie´s state [45]. In spite of high contact angles, adherence can still be high which is associated with large contact angle hysteresis, i.e. large differences between the advancing and receding contact angle [46]. Figure 11 shows the effect of a porous surface on the dynamics of the contact angle of a water drop on calcium carbonate surfaces in a CO2 environment measured using the sessile drop method (Sect. 2.3.1 Sessile drop) where the calcite crystal represents a smooth surface, and the limestone represents a rougher surface.
Comparing the values at 0.1 and 5 MPa it can be seen that the initial contact angle increases as the pressure is increased due to competitive wetting between the water and the compressed CO2-phase. An initial contact angle of just below 90° (water wet) as seen for the limestone sample at 0.1 MPa results in a rapidly decreasing contact angle over time due to the permeation of the droplet inside the sample pores, while the initial value on the smooth crystal surface only results in a slightly diminishing value over time at the same pressure. Increasing the contact angle (due to increasing the pressure) to slightly above 90° leads to fairly stable values in both samples as seen at 5 MPa. This is in line with the findings of Ge et al. [47] who stated that at contact angles larger than 90°, i.e. lower surface wetting, the permeation is inhibited. These results indicate that the contact angle may be measured on smooth surfaces as a first indication and for comparison but when transferring the results to real (porous) systems the effect strongly depends on whether the contact angle is below or above 90°.
3.5 Zeta potential and pH
The pH itself is only a measure of free H+ ions that on their turn reflect the degree of dissociation of acids being present in the solution. Dissolved minerals can buffer the acidity as demonstrated in reference [26] for carbonic acid in the presence of carbonate rocks. Due to the existence and nature of ionized sites on rock surfaces, the effect of pH on the wettability is different for carbonates and sandstones. Silica surfaces are negatively charged above a pH of about 2, so positively charged ions (base chemical species) can adsorb. Conversely, calcite surfaces are commonly positively charged, especially below pH 9.5 [48] so negatively charged ions (acidic species) can adsorb. In case anions like SO42− are present in the brine, these may compete with carboxylic acids in crude oils rendering the surface more water wet [49]. Carbonate wettability is also influenced by the reactivity of carbonate minerals. Starting out from a chemical thermodynamic equilibrium initially existing in the reservoir at a pH of 5–6, clay minerals act as ion exchange materials on which acidic and protonated basic components from the crude oil are adsorbed as well as cations from formation water especially those that are divalent like Ca2+, which are known as potentially determining ions (PDI) [50]. The subsequent low pH environment enhances the adsorption of both acidic and basic components of the oil on the surface of the clay. However, the proton H+ has the strongest affinity towards the clay surface (negatively charged), leading to an increase in pH in the bulk. This increase in pH helps release more of the organic material and renders the system more water wet. In other terms, an increase in pH impacts the surface charge of the rock and consequently the overall zeta potential at a rock-brine interface.
The zeta potential is used to measure the electrical potential in the shear plane separating the stern layer (inner layer of the so-called electric double layer (EDL)) and diffusive layer (outer layer of the EDL that forms at the rock-water interface) [51], and will therefore determine the electrostatic forces acting between the rock-water and oil–water interfaces in an oil reservoir. When the zeta potential at each interface has the same polarity, repulsive electrostatic forces act between the two interfaces and stabilize the water film on the mineral surface promoting higher water wetting. The more negative the surface charge in the case of the sandstone, the higher the repulsive forces towards the negative component of the crude oil (carboxylic acid). When the repulsive forces are dominant, the EDL expands and the water film surrounding the rock is said to be stabilized [52].
The zeta potential at the rock-water interface is highly dependent on the concentration and the type of ion (monovalent or divalent). The absolute value of Zeta potential is reported to increase with decrease in salinity and increase in pH [53]. The so-called low salinity water flooding is believed to be favored as an EOR method over high salinity water injection because the presence of high salt concentrations shields the surface charges leading to a reduced repulsion at the solid-oil interfaces [54]. As a conclusion for the experimentalist it may be stated that investigation on wettability in oil reservoirs shall be carried out using model systems not only comprising model brines but also contain carboxylic and other surface active compounds such as asphaltenes [55, 56] in the organic phase in order to gain significant conclusions on the ion interactions that govern wettability [18].
4 Conclusions
The wetting behavior is crucial for a number of applications under reservoir conditions. Quantifying wettability using sessile drop and captive bubble methods has shown great potential. Whether or not the resulting contact angles are significant depends on the correct interpretation for which a number of influencing factors need to be considered, especially in order to exclude artefacts. In this regard, standard operating procedures that lead to formation of uniform drop sizes may support the reproducibility of the experiments. Especially with respect to the drop size, neither too small nor too large drops shall be considered. According to the findings of this study:
-
The drop base diameter is advised to be no less than 5 mm.
-
Sophisticated techniques like the dual-drop dual-crystal method give valuable insight especially into the adhesion-cohesion phenomena but due to the complex experimental setup, measurements under reservoir conditions, especially at elevated pressures, are very time-consuming resulting in a relatively small number of comparable experiments.
-
The impact of the drop deposition speed shall be accounted for. It was found that performing the measurements at different velocities of the three-phase contact across a solid surface yields different results, however, the extrapolation towards zero velocity can yield a meaningful outcome.
-
The wetting behavior changes depending on surface roughness. For rough surfaces such as limestone, it is found that the contact angle decreases over time due to drop permeation into the rock pores when the contact angle is less than 90°, however, as water wetting decreases (contact angle just above 90°), the permeation process is inhibited. This is represented by a stable value of water contact angle on limestone at 5 MPa.
-
The pH and zeta potential should be accounted for during contact angle measurement as they impact the adsorption of brine ions on the rock surface and the polarity of rock- brine and oil-brine interfaces which ultimately influences electrostatic forces.
In general, it is advisable to investigate the multiple factors of influence separately making use of model systems and draw conclusions regarding the original system based on the dominating mechanisms.
References
Eggers R, Jaeger P (2004) Interfacial phenomena in countercurrent and spray processing using supercritical fluids. In: Supercritical fluids as solvents and reaction media. Elsevier, pp 363–377. https://doi.org/10.1016/B978-044451574-2/50015-8
Knauer S, Quynh-Hoa L, Baessler R, Jaeger P (2019) Contact angle and corrosion of a Water – CO2 system on X70 and S41500 at 278 K and pressures up to 20 MPa. Int J Greenh Gas Control 89:33–39. https://doi.org/10.1016/j.ijggc.2019.06.021
Wang J, Ryan D, Samara H, Jaeger P (2020) Interactions of CO2 with hydrocarbon liquid observed from adsorption of CO2 in organic-rich shale. Energy Fuels 34(11):14476–14482. https://doi.org/10.1021/acs.energyfuels.0c02774
Drexler S, Hoerlle F, Godoy W, Boyd A, Couto P (2020) Wettability alteration by carbonated brine injection and its impact on pore-scale multiphase flow for carbon capture and storage and enhanced oil recovery in a carbonate reservoir. Appl Sci 10(18):6496. https://doi.org/10.3390/app10186496
Chang YC, Mohanty KK, Huang DD, Honarpour MM (1997) The impact of wettability and core-scale heterogeneities on relative permeability. J Pet Sci Eng 18(1):1–19. https://doi.org/10.1016/S0920-4105(97)00006-5
Anderson W (1986) Wettability literature survey—part 2: wettability measurement. J Pet Technol 38(11):1246–1262. https://doi.org/10.2118/13933-PA
Saraji S, Goual L, Piri M, Plancher H (2013) Wettability of supercritical carbon dioxide/water/quartz systems: simultaneous measurement of contact angle and interfacial tension at reservoir conditions. Langmuir 29(23):6856–6866. https://doi.org/10.1021/la3050863
Seyyedi M, Sohrabi M, Farzaneh A (2015) Investigation of rock wettability alteration by carbonated water through contact angle measurements. Energy Fuels 29(9):5544–5553. https://doi.org/10.1021/acs.energyfuels.5b01069
Siddiqui MAQ, Ali S, Fei H, Roshan H (2018) Current understanding of shale wettability: a review on contact angle measurements. Earth Sci Rev 181:1–11. https://doi.org/10.1016/j.earscirev.2018.04.002
Zhang Y, Zeng J, Qiao J, Feng X, Dong Y (2018) Investigating the effect of the temperature and pressure on wettability in crude oil–brine–rock systems. Energy Fuels 32(9):9010–9019. https://doi.org/10.1021/acs.energyfuels.8b01404
Iglauer S (2017) CO2–water–rock wettability: variability, influencing factors, and implications for CO2 geostorage. Acc Chem Res 50(5):1134–1142. https://doi.org/10.1021/acs.accounts.6b00602
Arif M, Abu-Khamsin SA, Iglauer S (2019) Wettability of rock/CO2/brine and rock/oil/CO2-enriched-brine systems: critical parametric analysis and future outlook. Adv Colloid Interface Sci 268:91–113. https://doi.org/10.1016/j.cis.2019.03.009
Yekeen N, Padmanabhan E, Sevoo TA, Kanesen KA, Okunade OA (2020) Wettability of rock/CO2/brine systems: a critical review of influencing parameters and recent advances. J Ind Eng Chem 88:1–28. https://doi.org/10.1016/j.jiec.2020.03.021
Pancholi S, Negi GS, Agarwal JR, Bera A, Shah M (2020) Experimental and simulation studies for optimization of water-alternating-gas (CO2) flooding for enhanced oil recovery. Pet Res 5(3):227–234. https://doi.org/10.1016/j.ptlrs.2020.04.004
Szabries M, Jaeger P, Amro MM (2019) Foam analysis at elevated pressures for enhanced oil recovery applications. Energy Fuels 33(5):3743–3752. https://doi.org/10.1021/acs.energyfuels.8b03088
Lu Y, Najafabadi NF, Firoozabadi A (2017) Effect of temperature on wettability of oil/brine/rock systems. Energy Fuels 31(5):4989–4995. https://doi.org/10.1021/acs.energyfuels.7b00370
Samara H, Jaeger P (2021) Driving mechanisms in CO2-assisted oil recovery from organic-rich shales. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.1c01525
Xie Y, Khishvand M, Piri M (2020) Wettability of calcite surfaces: impacts of brine ionic composition and oil phase polarity at elevated temperature and pressure conditions. Langmuir 36(22):6079–6088. https://doi.org/10.1021/acs.langmuir.0c00367
Arif M, Abu-Khamsin SA, Zhang Y, Iglauer S (2020) Experimental investigation of carbonate wettability as a function of mineralogical and thermo-physical conditions. Fuel 264:116846. https://doi.org/10.1016/j.fuel.2019.116846
Alqam MH, Abu-Khamsin SA, Sultan AS, Okasha TM, Yildiz HO (2019) Effect of rock mineralogy and oil composition on wettability alteration and interfacial tension by brine and carbonated water. Energy Fuels 33(3):1983–1989. https://doi.org/10.1021/acs.energyfuels.8b04143
Shaik IK, Song J, Biswal SL, Hirasaki GJ, Bikkina PK, Aichele CP (2020) Effect of brine type and ionic strength on the wettability alteration of naphthenic-acid-adsorbed calcite surfaces. J Pet Sci Eng 185:106567. https://doi.org/10.1016/j.petrol.2019.106567
Alnoush W, Sayed A, Solling TI, Alyafei N (2021) Impact of calcite surface roughness in wettability assessment: interferometry and atomic force microscopy analysis. J Pet Sci Eng 203:108679. https://doi.org/10.1016/j.petrol.2021.108679
Sari A, Al Maskari NS, Saeedi A, Xie Q (2020) Impact of surface roughness on wettability of oil-brine-calcite system at sub-pore scale. J Mol Liq 299:112107. https://doi.org/10.1016/j.molliq.2019.112107
Drexler S, Hoerlle FO, Silveira TMG, Cavadas LA, Couto P (2019) Impact of rock aging time on the initial wettability of minerals and analogue rocks using pre-salt fluids under reservoir conditions. Presented at the offshore technology conference Brasil. https://doi.org/10.4043/29909-MS
Al-Mahrooqi SH, Grattoni CA, Muggeridge AH, Jing XD (2005) Wettability alteration during aging: the application of NMR to monitor fluid redistribution. International Symposium of the Society of Core Analysts
Almeida da Costa A et al (2019) The influence of rock composition and pH on reservoir wettability for low salinity water-CO2 EOR applications in Brazilian reservoirs. In Day 3 Wed, October 02, 2019, Calgary, Alberta, Canada, p D031S040R001. https://doi.org/10.2118/195982-MS
Gaydos J, Neumann AW (1987) The dependence of contact angles on drop size and line tension. J Colloid Interface Sci 120(1):76–86. https://doi.org/10.1016/0021-9797(87)90324-9
Sakai H, Fujii T (1999) The dependence of the apparent contact angles on gravity. J Colloid Interface Sci 210(1):152–156. https://doi.org/10.1006/jcis.1998.5940
Wenzel RN (1936) Resistance of solid surfaces to wetting by water. Ind Eng Chem 28(8):988–994. https://doi.org/10.1021/ie50320a024
Ren S et al (2021) The effect of drop volume on the apparent contact angle of hierarchical structured superhydrophobic surfaces. Colloids Surf Physicochem Eng Asp 611:125849. https://doi.org/10.1016/j.colsurfa.2020.125849
Seo K, Kim M, Ahn JK, Kim DH (2015) Effects of drop size and measuring condition on static contact angle measurement on a superhydrophobic surface with goniometric technique. Korean J Chem Eng 32(12):2394–2399. https://doi.org/10.1007/s11814-015-0034-x
Cansoy CE (2014) The effect of drop size on contact angle measurements of superhydrophobic surfaces. RSC Adv 4(3):1197–1203. https://doi.org/10.1039/C3RA45947K
Drelich J (1997) The effect of drop (bubble) size on contact angle at solid surfaces. J Adhes 63(1–3):31–51. https://doi.org/10.1080/00218469708015212
Good RJ, Koo MN (1979) The effect of drop size on contact angle. J Colloid Interface Sci 71(2):283–292. https://doi.org/10.1016/0021-9797(79)90239-X
Hirasaki GJ (1991) Wettability: fundamentals and surface forces. SPE Form Eval 6(02):217–226. https://doi.org/10.2118/17367-PA
Sharma PK, Dixit HN (2021) Regimes of wettability-dependent and wettability-independent bouncing of a drop on a solid surface. J Fluid Mech 908:A37. https://doi.org/10.1017/jfm.2020.773
Samara H, Ke L, Ostrowski TV, Ganzer L, Jaeger P (2019) Unconventional oil recovery from Al Sultani tight rock formations using supercritical CO2. J Supercrit Fluids 152:104562. https://doi.org/10.1016/j.supflu.2019.104562
Jaeger PT, Alotaibi MB, Nasr-El-Din HA (2010) Influence of compressed carbon dioxide on the capillarity of the gas−crude oil−reservoir water system. J Chem Eng Data 55(11):5246–5251. https://doi.org/10.1021/je100825b
Saad SMI, Neumann AW (2016) Axisymmetric drop shape analysis (ADSA): an outline. Adv Colloid Interface Sci 238:62–87. https://doi.org/10.1016/j.cis.2016.11.001
Rao DN, Girard MG (1994) A new technique for reservoir wettability characterization. In Annual technical meeting, Calgary, Alberta, Canada, p 21. https://doi.org/10.2118/94-48
Rao DN, Karyampudi RS (2002) Application of the dual-drop dual-crystal contact angle technique to characterize heavy oil reservoir wettability. J Adhes Sci Technol 16(5):581–598. https://doi.org/10.1163/156856102760070385
Lyklema J (2000) Fundamentals of interface and colloid science, vol 3. Acedemic Press
Hoeiland S, Barth T, Blokhus AM, Skauge A (2001) The effect of crude oil acid fractions on wettability as studied by interfacial tension and contact angles. J Pet Sci Eng 30(2):91–103. https://doi.org/10.1016/S0920-4105(01)00106-1
Amott E (1959) Observations relating to the wettability of porous rock. Trans AIME 216(01):156–162. https://doi.org/10.2118/1167-G
Cassie ABD, Baxter S (1944) Wettability of porous surfaces. Trans Faraday Soc 40:546. https://doi.org/10.1039/tf9444000546
Feng L, Zhang Y, Xi J, Zhu Y, Wang N, Xia F, Jiang L (2008) Petal effect: a superhydrophobic state with high adhesive force. Langmuir 24(8):4114–4119. https://doi.org/10.1021/la703821h
Ge W-K, Lu G, Xu X, Wang X-D (2016) Droplet spreading and permeating on the hybrid-wettability porous substrates: a lattice Boltzmann method study. Open Phys 14(1):483–491. https://doi.org/10.1515/phys-2016-0055
Rezaei Gomari KA, Hamouda AA (2006) Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface. J Pet Sci Eng 50(2):140–150. https://doi.org/10.1016/j.petrol.2005.10.007
Karimi M, Al-Maamari RS, Ayatollahi S, Mehranbod N (2016) Impact of sulfate ions on wettability alteration of oil-wet calcite in the absence and presence of cationic surfactant. Energy Fuels. https://doi.org/10.1021/acs.energyfuels.5b02175
Pierre A, Lamarche JM, Mercier R, Foissy A, Persello J (1990) Calcium as a potential determining ion in aqueous calcite suspensions. J Dispers Sci Technol 11(6):611–635. https://doi.org/10.1080/01932699008943286
Alotaibi MB, Yousef AA (2017) The role of individual and combined ions in waterflooding carbonate reservoirs: electrokinetic study. SPE Reserv Eval Eng 20(01):077–086. https://doi.org/10.2118/177983-PA
Nasralla RA, Nasr-El-Din HA (2014) Double-layer expansion: is it a primary mechanism of improved oil recovery by low-salinity waterflooding? SPE Reserv Eval Eng 17(01):49–59. https://doi.org/10.2118/154334-PA
Shehata AM, Nasr-El-Din HA (2015) Zeta potential measurements: impact of salinity on sandstone minerals. Presented at the SPE international symposium on oilfield chemistry, The Woodlands, Texas, USA. https://doi.org/10.2118/173763-MS
Farajzadeh R, Guo H, van Winden J, Bruining J (2017) Cation exchange in the presence of oil in porous media. ACS Earth Space Chem 1(2):2. https://doi.org/10.1021/acsearthspacechem.6b00015
Sauerer B, Al Abdulghani AJ, Abdallah MS, Abdallah W (2019) Interfacial activity of characterized middle eastern asphaltenes. Energy Fuels 33(5):3723–3732. https://doi.org/10.1021/acs.energyfuels.8b03085
Mohammed S, Gadikota G (2020) Dynamic wettability alteration of calcite, silica and illite surfaces in subsurface environments: a case study of asphaltene self-assembly at solid interfaces. Appl Surf Sci 505:144516. https://doi.org/10.1016/j.apsusc.2019.144516
Funding
Open Access funding enabled and organized by Projekt DEAL. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing financial or non-financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Samara, H., Jaeger, P. Experimental determination of wetting behavior under non-atmospheric conditions relevant to reservoirs: a practical guide. SN Appl. Sci. 4, 85 (2022). https://doi.org/10.1007/s42452-022-04963-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-022-04963-8